Abstract
To reduce high-salt waste from cucumber fermentations, low-salt fermentations are under development. These fermentations may require the use of starter cultures to ensure normal fermentations. Because potential phage infection can cause starter culture failure, it is important to understand phage ecology in the fermentations. This study investigated the phage ecology in a commercial cucumber fermentation. Brine samples taken from a fermentation tank over a 90-day period were plated onto deMan-Rogosa-Sharpe agar plates. A total of 576 lactic acid bacterial isolates were randomly selected to serve as potential hosts for phage isolation. Filtered brine served as a phage source. Fifty-seven independent phage isolates were obtained, indicating that 10% of the bacterial isolates were sensitive to phage attack. Phage hosts include Lactobacillus brevis (67% of all hosts), Lactobacillus plantarum (21%), Weissella paramesenteroides, Weissella cibaria, and Pediococcus ethanolidurans. Nearly 50% of phages were isolated on day 14, and the majority of them attacked L. brevis. Some phages had a broad host range and were capable of infecting multiple hosts in two genera. Other phages were species specific or strain specific. About 30% of phage isolates produced turbid pinpoint plaques or only caused reduced cell growth on the bacterial lawns. Six phages with distinct host ranges were characterized. The data from this study showed that abundant and diverse phages were present in the commercial cucumber fermentation, which could cause significant mortality to the lactic acid bacteria population. Therefore, a phage control strategy may be needed in low-salt cucumber fermentations.
INTRODUCTION
Like most other vegetable fermentations, cucumber fermentations are driven by a variety of lactic acid bacteria (LAB) naturally present on vegetables. The metabolic activities of LAB determine the quality and safety of the final fermentation products (30). Vegetable fermentation is a dynamic biochemical system in which the chemical composition and microbial ecology are continuously changing. Those changes can be influenced by many physical, chemical, and biological factors such as temperature, pH, salt concentration, and the microbiota (bacteria, fungi, bacteriophages, etc.) present on vegetables (5, 12). The presence of diverse phages against LAB in vegetable fermentations can potentially cause significant mortality to LAB, thereby influencing the number, type, and succession of LAB populations during the fermentation, as well as the type, rate, and extent of carbohydrate metabolism. Phages are ubiquitous in nature. Several studies showed that a diverse phage community was present in vegetable fermentations (2, 19, 20, 36, 37). We previously reported the phage ecology in commercial sauerkraut fermentations (20), and the genome sequences of two LAB phages (ΦJL-1 and Φ1-A4, infecting Lactobacillus plantarum and Leuconostoc mesenteroides, respectively) isolated from vegetable fermentations (17, 18). Although several LAB phages active against L. plantarum and Pediococcus sp. have been isolated from commercial cucumber fermentations (19, 36), phage ecology in commercial cucumber fermentations has not been investigated.
In the pickle industry, cucumbers are typically fermented in a brine containing 6% NaCl in open-top tanks that are about 40,000 liters in volume (5). In cold climates additional salt, up to 12%, is added after fermentation to minimize freezing of the tanks during the winter (25). To make edible pickle products, the excess salt must be washed out of the fermented cucumbers and discarded as waste. Disposal of such waste has become a costly problem for the pickling industry (25). To reduce the amount of brine waste released into the environment, reusing the brine from the previous cucumber fermentation has become a common practice in the industry. Brine recycling can alter the initial pH and microflora, including phages, thereby influencing cucumber fermentations. Knowledge of phage ecology is needed in order to better understand cucumber fermentations, especially those using recycled brine, since this has become a common practice in the pickle industry.
Significant reductions in salt concentration (to ≤4%) in cucumber fermentations may also be possible with fermentation technology under development, using blanched cucumbers to reduce the initial microflora present on the cucumbers (4, 10, 25). For these fermentations, LAB starter cultures may be required to drive the fermentations. Because of the potential for phage infection, which can cause starter culture failure, the phage ecology in commercial cucumber fermentation needs to be investigated.
The objectives of this study were to explore the ecology of phages that attack LAB in a commercial cucumber fermentation that used recycled brine and to characterize several LAB phages isolated from the fermentation. The study may provide new insights into our understanding of the microbial ecology in commercial cucumber fermentations. The data from this study may be valuable for the development of low-salt or salt-free cucumber fermentations that require the use of LAB starter cultures, which may be attacked by naturally present phages. To our knowledge, this is the first study to examine phage ecology in commercial cucumber fermentations.
MATERIALS AND METHODS
Industrial cucumber fermentation and sample collection.
A commercial cucumber fermentation tank was examined in this study. Fresh cucumber samples (500 g) were collected prior to brining to determine the sugar content by high-performance liquid chromatography (HPLC) analysis as described below. A 40,000-liter fermentation tank was packed with two sizes of cucumbers: size 3A (44 to 51 mm in diameter) for the top half of the tank and size 2B (32 to 38 mm in diameter) for the other half of the tank. The recycled brine from previous cucumber fermentations was adjusted with 200 grain vinegar (containing 20% acetic acid) and pickling salt so that the brine contained 50 mM acetic acid and 2.06 M NaCl. After equilibration between whole cucumbers and the brine, the concentrations of acetic acid and NaCl were 25 mM and 1.03 M (6%), respectively. The resulting pH was 4.4, at which the fermentation started. Brine samples from the fermentation were collected on days 1, 3, 7, 14, 30, and 90 during the period from October 2009 to January 2010. On each sampling day, two brine samples (500 ml each) were taken from two independent locations, 2 ft (61 cm) and 8 ft (244 cm) below the surface of the brine in the fermentation tank. The samples were placed in 1.35-liter jars, immediately transported to our laboratory on ice, and processed on the same day.
The treatment of brine samples for host and phage isolations.
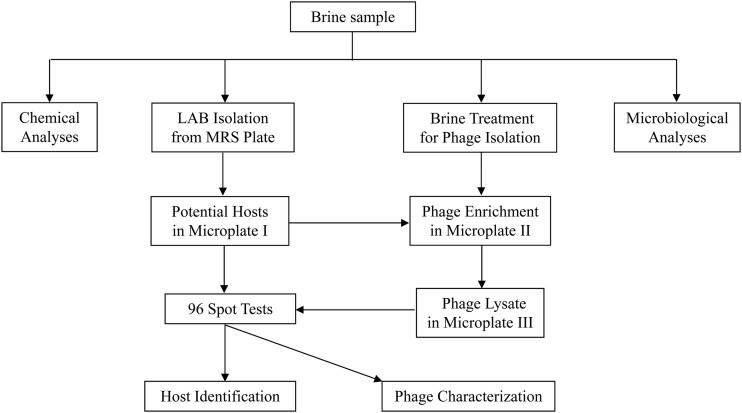
Each brine sample was divided into several portions for microbiological and chemical analyses and for isolation of phages and their hosts (Fig. 1). One milliliter of each brine sample was used for immediate microbiological analysis and phage host isolation. Ten milliliters of each brine sample was stored at −20°C for later chemical analysis. The remaining brine samples were centrifuged at 13,000 × g (Eppendorf 5810R centrifuge; Eppendorf North America, Inc., Westbury, NY) at 4°C for 20 min to remove cells and solid particles. The supernatants were filtered through Nalgene filtration units with a 0.45-μm pore size. The pH of each filtrate was measured and then adjusted to approximately 6.3 with 5.0 N NaOH (Sigma-Aldrich). The pH-adjusted filtrate was stored at 4°C for later use as a potential phage source for phage isolation.
Fig 1.
Flow diagram for the isolation of phages and their hosts in a commercial cucumber fermentation.
Chemical analyses.
The salt (NaCl) content in brine was determined by titration with AgNO3 using dichlorofluorescein as an indicator (11). Sugar and organic acid concentrations were measured by HPLC analysis using a 30-cm HPX-87H column (Bio-Rad Laboratories, Hercules, CA) as described by McFeeters and Barish (24). The column was heated to 37°C and eluted with 0.03N sulfuric acid at a flow rate of 1 ml/min. A Thermo Separations UV6000 diode array detector (Spectra System Thermo Scientific, Waltham, MA) was used to collect data at 210 nm for the analysis of lactic, acetic, propionic, malic, and butyric acids. A refractive index detector (model 410; Waters Corp./Millipore Corp., Billerica, MA) connected in series with the diode array detector was used to measure glucose, fructose, and ethanol. External standards (each with four concentrations) were used to calibrate the system.
Microbiological analysis.
Brine samples were plated on deMan-Rogosa-Sharpe (MRS) plates supplemented with 1% cycloheximide (Difco Laboratories, Detroit, MI) to inhibit the growth of yeasts for the isolation of LAB, using a spiral plater (Autoplate 4000; Spiral Biotech, Inc., Bethesda, MD). The plates were incubated at 30°C for 48 h anaerobically. The colonies on the plates were enumerated with an automated colony counter (Q-Count; Spiral Biotech).
Isolation of LAB hosts and phages.
From each time point, about 96 colonies were randomly selected from the MRS agar plates containing 30 to 300 colonies. Each colony was then streaked onto an MRS agar plate for purification. Frozen stocks of each LAB isolate were prepared in MRS broth containing 15% glycerol as a cryoprotectant and maintained at −80°C. The purified LAB isolates served as potential hosts for phage isolation. Fresh cultures of 96 LAB isolates were prepared in a 96-well microplate (Fig. 1, Microplate I). Each well contained 200 μl of MRS broth and was inoculated with one LAB isolate. After overnight incubation at 30°C, 50 μl of each culture in microplate I was transferred into a new microplate (Fig. 1, Microplate II) where each well contained 150 μl of MRS broth and 50 μl of the pH-adjusted, filtered brine (as a potential phage source). After the incubation at 30°C for 24 h, microplate II was centrifuged (SH-3000 rotor and RC-5B centrifuge; Sorvall, Newtown, CT) at 4,000 rpm and 4°C for 20 min. The supernatants on microplate II were transferred into a new microplate (microplate III). Ninety-six individual spot tests were performed by spotting 10 μl of supernatant (titer of ≥200 PFU/ml) from a well in microplate III onto the corresponding bacterial lawn resulting from 100 μl of an overnight culture from the corresponding well in microplate I. The 96 spot test plates were incubated overnight at 30°C. Primary phage-host relationships were indicated by positive spot test plates. Each host isolate was assigned an identification number (ID) based on the sampling day and location. For example, host 3.2.27 was isolated on day 3 from the sample collected at a 2-ft depth in the fermentation tank, and it was the 27th isolate from the same location on day 3. The corresponding phage isolate was assigned the same ID plus a prefix Φ, such as Φ3.2.27. Theoretically, the detection limit for phages was 20 PFU/ml brine sample because 50 μl of brine sample was used for phage isolation. Most of the resulting phage isolates were purified by one round of single-plaque isolation according to the method described by Lu et al. (21). Isolates selected for characterization underwent two rounds of plaque purification. Phages were propagated on their early-log-phase hosts at a multiplicity of infection of about 0.01 in MRS broth supplemented with 10 mM CaCl2 at 30°C. Additional spot tests were performed to determine phage typing and host range. Phages and their glycerol stocks were stored at −80°C for later use.
Identification of phage hosts.
Based on phage typing, distinct phage hosts were determined. The hosts were identified by partial 16S rRNA gene sequencing. Bacterial chromosomal DNA was obtained using InstaGene Matrix (Bio-Rad, Hercules, CA) or a Wizard Genomic DNA Purification Kit (Promega, Madison, WI). The PCR mix contained 2× Master Mix (Bio-Rad), chromosomal DNA, and forward and reverse primers, which were 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) (2) and U1492r (5′-GGTTACCTTGTTACGACTT-3′) (35), respectively. Primers were obtained from Integrated DNA Technologies (Coralville, IA). The PCR amplifications were carried out using the following conditions: 94°C for 1 min, 25 cycles of 1 min at 94°C, 2 min at 56°C, and 2 min at 72°C, followed by a final cycle of 7 min at 72°C. Amplicons were purified using a Qiagen PCR purification kit and sequenced by Eton Bioscience Inc. (Raleigh, NC). The sequences obtained were subjected to the Basic Local Alignment Search Tool (BLAST 2.2.26) (1) in the GenBank (3) using the 16S microbial database (38) to determine the identity of the isolates. Only alignment matches with 99 or 100% identity, with no gaps, and expect values of 0.0 were considered for identification purposes.
Characterization of phages.
Forty plaque-purified phage isolates (titer of ≥108 PFU/ml), along with 40 host isolates, were used in a cross-infection experiment (Table 1) with spot tests (as described above) to determine host ranges of the phages. Distinct phages were determined based on host ranges. Several phages were selected for further characterization of their morphologies, major structural protein profiles, and restriction endonuclease digestion patterns using the methods previously described by Lu et al. (19) with minor modifications. Briefly, phage lysates were centrifuged at 4,000 × g for 10 min and filtered (0.45-μm pore size). The filtrates were treated with DNase I and RNase A. Phage particles were concentrated by polyethylene glycol (PEG) precipitation and then purified by CsCl density gradient ultracentrifugation at 600,000 × g for 6 h at 15°C. The ultracentrifuge-purified phages were used for electron microscopy analysis, SDS-PAGE, and DNA extraction. Phage samples were negatively stained with 2% (wt/vol) aqueous uranyl acetate and examined by transmission electron microscopy at an accelerating voltage of 80 kV. SDS-PAGE was carried out with boiled phage samples loaded onto NuPAGE precast gradient minigels (4 to 12% bis-Tris; Invitrogen Corporation, Carlsbad, CA). Phage DNA was prepared from the concentrated lysate using the phenol-chloroform extraction method and digested with restriction endonucleases (EcoRV and HindIII) according to the supplier's recommendations (Promega, Madison, WI). The resulting DNA fragments were separated on an 0.8% agarose gel containing 0.001% SYBR Safe DNA gel stain (Invitrogen, CA) by gel electrophoresis in Tris-borate-EDTA (TBE) buffer at 75 V (constant voltage) for 2.5 h.
Table 1.
Phages and their hosts isolated from a commercial cucumber fermentation
| Host |
Phage profile by indicated day(s) of isolationa |
||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Nameb | Day 3 |
Day 7 |
Days 14 and 30 |
|||||||||||||||||||||||||||||||||||||
| Φ3.2.27 | Φ3.8.30 | Φ3.8.43 | Φ3.8.44 | Φ3.8.32 | Φ3.2.31 | Φ3.8.48 | Φ3.8.18 | Φ3.2.35 | Φ7.2.29 | Φ7.2.49 | Φ7.8.33 | Φ7.2.36 | Φ7.8.27 | Φ7.2.50 | Φ7.8.17 | Φ14.2.14 | Φ14.2.18 | Φ14.2.31 | Φ14.8.6 | Φ14.8.24 | Φ14.8.36 | Φ14.8.40 | Φ14.8.44 | Φ14.8.23 | Φ14.2.46 | Φ14.2.20 | Φ14.2.4 | Φ14.2.39 | Φ14.2.12 | Φ14.2.13 | Φ14.2.15 | Φ14.2.48 | Φ14.2.30 | Φ14.8.7 | Φ14.8.43 | Φ14.2.37 | Φ14.8.19 | Φ14.2.33 | Φ30.2.8 | ||
| 3.2.27 | W. cibaria | + | + | + | + | + | |||||||||||||||||||||||||||||||||||
| 3.8.43 | W. cibaria | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||||||
| 3.8.44 | W. cibaria | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||||||
| 3.8.32 | W. cibaria | + | + | + | |||||||||||||||||||||||||||||||||||||
| 3.2.31 | W. paramesenteroides | + | |||||||||||||||||||||||||||||||||||||||
| 3.8.48 | W. paramesenteroides | + | |||||||||||||||||||||||||||||||||||||||
| 3.2.35 | L. plantarum | + | + | ||||||||||||||||||||||||||||||||||||||
| 3.8.30 | L. plantarum | + | + | + | + | ||||||||||||||||||||||||||||||||||||
| 3.8.18 | L. brevis | + | + | + | + | + | |||||||||||||||||||||||||||||||||||
| 7.2.29 | L. brevis | + | + | ||||||||||||||||||||||||||||||||||||||
| 7.2.49 | L. brevis | + | + | ||||||||||||||||||||||||||||||||||||||
| 7.8.33 | L. brevis | + | + | ||||||||||||||||||||||||||||||||||||||
| 7.8.17 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||||
| 7.2.36 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 7.2.50 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 7.8.27 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.4 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.12 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.13 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.15 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.18 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.20 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.30 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.39 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.46 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.2.48 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.8.7 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.8.23 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.8.24 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||
| 14.8.43 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||
| 14.2.14 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||
| 14.8.44 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | + | ||||||||||||||||||||||||||||
| 14.2.31 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| 14.8.6 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| 14.8.36 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| 14.8.40 | L. brevis | + | + | + | + | + | + | + | + | + | + | + | |||||||||||||||||||||||||||||
| 14.2.37 | L. brevis | + | + | ||||||||||||||||||||||||||||||||||||||
| 14.8.19 | L. brevis | + | |||||||||||||||||||||||||||||||||||||||
| 14.2.33 | L. plantarum | + | |||||||||||||||||||||||||||||||||||||||
| 30.2.8 | L. plantarum | + | + | ||||||||||||||||||||||||||||||||||||||
The six phages selected for further characterization are highlighted in bold. +, the host is sensitive to the phage or the phage infects the host.
W. represents Weissella; L. represents Lactobacillus.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were submitted to GenBank under accession numbers JX826519 to JX826575.
RESULTS AND DISCUSSION
The 40,000-liter commercial fermentation tank was filled with two different sizes of cucumbers: size 3A for the top half of the tank and size 2B for the other half of the tank. In the pickle industry, when one size of cucumbers is not enough to pack the entire tank, different sizes of cucumbers are used to pack the same tank. A total of 12 brine samples were taken from two independent locations of the fermentation tank over a 90-day period covering the primary fermentation (usually completed within a month) and 2 months of long-term storage.
Chemical analyses.
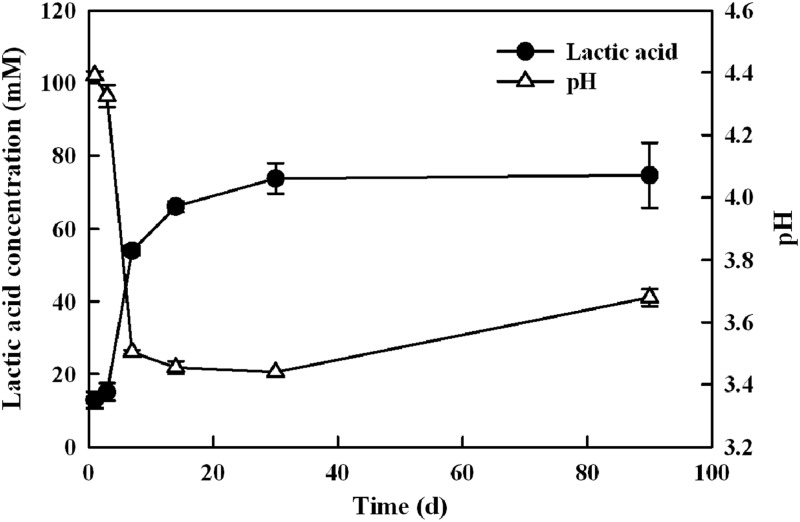
The fresh cucumbers (size 3A) contained 28 mM glucose and 30 mM fructose (data not shown). The sugar content in size 2B cucumbers was not determined in this study but was expected to be similar to that in size 3A cucumbers based on previously reported data (21). Figure 2 shows the changes in lactic acid concentration and pH at the 2-ft depth in the fermentation tank during the cucumber fermentation. As expected, lactic acid production was rapid during the first month of the fermentation, especially during the first week, resulting in the fast acidification of the brine, as evidenced by the rapid decrease in pH. The brine pH decreased to 3.4 on day 14 and remained stable until day 30. Although a slight increase in pH was observed between day 30 and day 90, the concentrations of lactic acid and acetic acid remained stable at 80 mM and 25 mM, respectively, indicating that the fluctuation in pH was not due to the microbial utilization of these organic acids. Similar chemical profiles were observed at the 8-ft depth in the fermentation tank (data not shown). The conversion of sugars primarily to lactic acid and the decrease in pH indicated that the fermentation was typical for commercial, brined cucumbers (12). A small amount (2 to 4 mM) of malic acid was detected on days 1 and 3, but it disappeared on day 7 and thereafter (data not shown). Propionic and butyric acids were not detected.
Fig 2.
Changes in lactic acid concentration and pH during a commercial cucumber fermentation. Each data point represents the mean (± standard deviation) of three replicates. d, days.
Microbiological analysis.
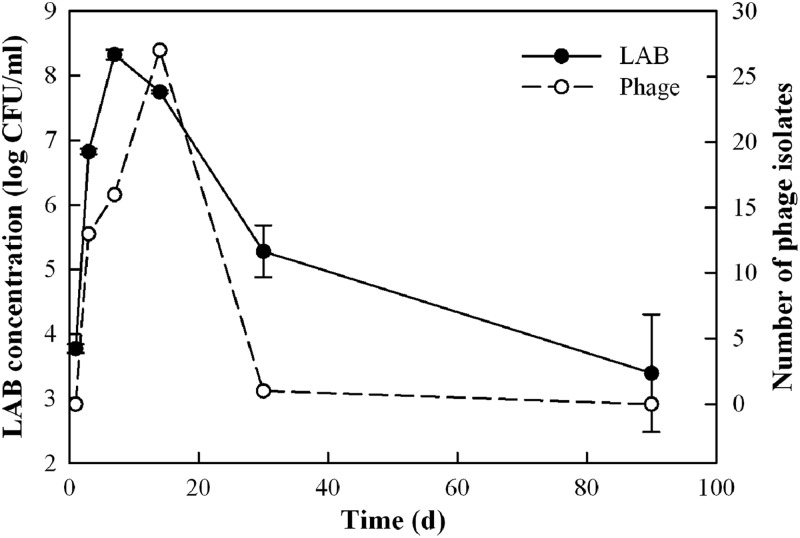
Figure 3 shows the profile of MRS plate counts for LAB in the samples taken at the 2-ft depth in the fermentation tank over a 90-day period. The LAB concentration on day 1 was slightly below 1 × 104 CFU/ml. After day 1, the LAB concentration increased rapidly and reached a maximum (2.4 × 108 CFU/ml) on day 7, which is in agreement with the massive lactic acid production shown in Fig. 2. As the fermentation continued, the LAB concentration decreased to 2 × 105 CFU/ml and 6 × 103 CFU/ml on days 30 and 90, respectively. Similar plate count profiles were obtained for samples taken from the 8-ft depth in the fermentation tank (data not shown).
Fig 3.
Concentrations of lactic acid bacteria and numbers of LAB phage isolates in a commercial cucumber fermentation. Each data point of LAB concentration represents the mean (± standard deviation) of three replicates.
Isolation of LAB and phages.
A total of 576 LAB isolates were obtained by randomly picking colonies from MRS plates over a 90-day period. Using these LAB isolates as potential hosts, 57 phage isolates were obtained through spot tests (Tables 1 and 2). Thirty-two of the phage isolates were from the 2-ft depth, and 25 were from the 8-ft depth in the fermentation tank. These data indicated that about 10% (57 of the 576) of the randomly picked LAB isolates were infected by phages, which could result in significant mortality to LAB, thereby influencing the bacterial ecology, the dynamics of the fermentation, and subsequently the quality of the fermented products.
Table 2.
LAB phages which form turbid pinpoint plaques and their corresponding hosts
| Phage | Host ID | Host namea |
|---|---|---|
| Φ3.2.22 | 3.2.22 | L. plantarum |
| Φ3.8.8 | 3.8.8 | L. plantarum |
| Φ3.8.40 | 3.8.40 | L. plantarum |
| Φ3.8.42 | 3.8.42 | L. plantarum |
| Φ7.2.21 | 7.2.21 | L. plantarum |
| Φ7.2.40 | 7.2.40 | L. brevis |
| Φ7.2.46 | 7.2.46 | L. brevis |
| Φ7.2.48 | 7.2.48 | L. brevis |
| Φ7.2.33 | 7.2.33 | L. brevis |
| Φ7.2.35 | 7.2.35 | L. brevis |
| Φ7.8.6 | 7.8.6 | L. plantarum |
| Φ7.8.15 | 7.8.15 | L. brevis |
| Φ7.8.26 | 7.8.26 | L. brevis |
| Φ14.2.9 | 14.2.9 | L. brevis |
| Φ14.2.41 | 14.2.41 | L. plantarum |
| Φ14.8.15 | 14.8.15 | L. plantarum |
| Φ14.8.17 | 14.8.17 | P. ethanolidurans |
L. represents Lactobacillus; P. represents Pediococcus.
Among the 57 phage isolates, 13, 16, 27, and 1 were isolated on days 3, 7, 14, and 30, respectively, which correlated well with total LAB concentrations (high on days 3, 7, and 14 and low on day 30) (Fig. 3). Fifty (almost 90%) of the phage isolates were found to attack Lactobacillus brevis and L. plantarum (Tables 1 and 2), including 38 L. brevis phages (1 from day 3, 14 from day 7, and 23 from day 14) and 12 L. plantarum phages (6 from day 3, 2 from day 7, 3 from day 14, and 1 from day 30). The finding that L. brevis phages (38 isolates) were more abundant than L. plantarum phages (12 isolates) in the cucumber fermentation was unexpected because L. plantarum (not L. brevis) has been historically shown to be the dominant LAB species in cucumber fermentations (7, 9). However, our data showed that the ratios of L. brevis to L. plantarum isolates were 50:46 on day 7 and 32:58 on day 14, suggesting that the two bacteria codominated the LAB population, which explained why almost 90% of phages were active against L. brevis and L. plantarum. Interestingly, 27 (or 47%) of the phage isolates were obtained on day 14, and the majority of them attacked L. brevis strains, which may be one of the major reasons why the number of L. brevis isolates deceased significantly from 50 on day 7 to 32 on day 14. In contrast to this cucumber fermentation, an ecology study of commercial sauerkraut fermentations showed that the majority of LAB isolated on or after the 7th day of the fermentation were L. plantarum bacteria (31) and that most of the phages isolated during this period were against L. plantarum (20).
One phage which was active against L. plantarum was isolated on day 30 when the brine pH was 3.4, suggesting that this phage and its host were highly persistent in such an acidic environment while other phages and/or their hosts were greatly inhibited. No phages were isolated on day 1 or day 90 in the fermentation, indicating that the phage activity was below our theoretical detection limit (20 PFU/ml). This correlated with the low LAB counts at the beginning and the end of the fermentation (Fig. 3). It is known that LAB on fresh vegetables account for only a very small portion of the total bacterial population (27, 28, 29), and this small LAB population is dominated by Leuconostoc mesenteroides, one of the least salt tolerant of the LAB (16, 22) and also less acid resistant than other LAB (23, 30) involved in vegetable fermentations. The high salt concentration (6%) and low pH (4.4) of the brine used in the cucumber fermentation may be inhibitory to L. mesenteroides. Therefore, very few LAB hosts were present on day 1 for phage replication. Consequently, phage activity was below the detection limit on day 1. The fact that no phage was isolated on day 90 was likely due to the inhibitory effects of low pH and lactic acid on both LAB and phages.
Forty phage isolates (70% of 57) produced clear or slightly turbid plaques on bacterial lawns (Table 1) while the other 17 phage isolates (30%) produced turbid pinpoint plaques or only caused reduced cell growth on bacterial lawns (Table 2). Among the opaque-plaque formers, eight are active against L. plantarum, eight are active against L. brevis, and one is active against Pediococcus ethanolidurans. In contrast, phages isolated from sauerkraut fermentations rarely form such turbid pinpoint plaques (20). Plaque morphology may reflect a type of viral life cycle. Phages forming turbid plaques may be temperate phages. During infection, a portion of the phages may enter the lysogenic cycle instead of the lytic cycle, resulting in turbid plaques. Clark et al. (6) found that rickettsial phages that cause lytic infections in cell culture form clear plaques, while nonlytic strains form opaque plaques in which the cells remain intact. Plaque size may correlate with virulence: the plaques produced by foot-and-mouth disease viruses are generally larger than the plaques from attenuated strains and those causing milder clinical illness (8). Some researchers noticed that not all phages are efficient plaque formers under typical plating conditions. Bacillus megaterium phage G, the largest myovirus known (genome of ca. 500 kb), is unable to form plaques under normal conditions due to its large virion size (13, 14). Bacillus thuringiensis phage 0305ϕ8-36, another large phage, forms plaque only in ultradilute agarose gels (34). Phage 0305ϕ8-36 does not propagate in the traditional gels used for phage plaque formation and also does not produce visible lysis of liquid cultures. Some phages are nonlytic and do not lyse their hosts during infection, thereby not producing clear plaques. Phage M13 is a well-studied nonlytic phage. The progeny phages are released from the infected cell without causing cell death. M13 plaques are seen as areas of reduced growth in the bacterial lawn (32, 33). Such reduced cell growth was also observed on several LAB lawns in our study. It may also be possible that some LAB contain certain phage defense systems (such as abortive mechanisms) that can act at multiple targets within the phage lytic cycle, resulting in the formation of turbid phage plaques. Since very turbid plaques and diminished cell growth (no lysis) could be difficult to recognize, only phages forming clear plaques (Table 1) were further characterized. Characterization of phages forming turbid plaques will be the subject of future research.
Host identification.
The 57 phage host isolates shown in Tables 1 and 2 were identified by 16S rRNA gene sequence analysis. Four are Weissella cibaria (from day 3), 2 are Weissella paramesenteroides (from day 3), 12 are L. plantarum (6 from day 3, 2 from day 7, 3 from day 14, and 1 from day 30), 38 are L. brevis (all except one from day 7 or day 14) bacteria, and 1 is P. ethanolidurans (from day 14). Based on phage typing, there were at least 18 distinct LAB hosts (Table 1). Some hosts were isolated only once (e.g., hosts 3.2.27, 3.8.32, 3.2.31, and 3.8.48) or twice (e.g., host 3.8.43 or 3.8.44 and 7.2.49 or 7.8.33). Several hosts were isolated multiple times at one or more time points, suggesting that they are more abundant and persistent than other hosts. For instance, host isolates 7.2.36, 7.2.50, 7.8.27, 14.2.4, 14.2.12, 14.2.13, 14.2.15, 14.2.18, 14.2.20, 14.2.30, 14.2.39, 14.2.46, 14.2.48, 14.8.7, 14.8.23, and 14.8.24 have the same phage typing and were all identified as L. brevis. Therefore, they are most likely to be the same strain, representing the most frequently isolated phage host (at least 3 times on day 7 and 13 times on day 14). Most of these isolates (12 out of 16) were obtained from a 2-ft depth in the tank. The second most abundant host was host 14.2.31, which was also identified as L. brevis. It had the same phage typing as other three L. brevis isolates (14.8.6, 14.8.36, and 14.8.40). All of the four L. brevis isolates were obtained on day 14. It was noteworthy that host 30.2.8 (identified as L. plantarum) was the only host isolated on day 30 (Table 1) when the pH was 3.4, indicating that phage activities correlated with bacterial growth. There was more than a 2-log reduction in LAB population on day 30 compared to day 14 (Fig. 3). The exposure to a highly acidic environment since day 7 (pH 3.5) and the nutrient depletion (no glucose detected since day 7) may have inhibited most LAB hosts and their phages on day 30. Interestingly, host 30.2.8 had the same phage typing as host 3.2.35 (also identified as L. plantarum) isolated on day 3, suggesting that these two L. plantarum isolates were probably the same strain, and the bacterium and its phage were highly persistent in the fermentation. Table 1 revealed that phage hosts were highly diverse in their sensitivities to different phages. A number of hosts, such as 3.2.31 and 3.8.48 (two different W. paramesenteroides isolates), 14.8.19 (L. brevis), and 14.2.33 (L. plantarum), were sensitive to only a single phage, while other hosts were susceptible to two or more phages. Some hosts were sensitive to up to six distinct phages. For example, host 7.2.36 was sensitive to Φ7.2.36, Φ7.8.27, Φ7.2.50, Φ7.8.17, Φ14.2.46, and Φ14.2.4. One of the phage hosts, P. ethanolidurans, was isolated only on day 14 (Table 2). This homofermentative LAB was not seen as a phage host in sauerkraut fermentations (20).
Phage characterization.
Based on host range, there were at least 17 distinct LAB phages among the 40 phage isolates listed in Table 1. A number of phage isolates appear to be the same according to their host ranges, indicating that the same phage was isolated more than once on the same or different days and that the phage was abundant and/or persistent in the fermentation. For example, Φ7.8.17 shared the same host range as Φ14.2.14, Φ14.2.18, Φ14.2.31, Φ14.8.6, Φ14.8.24, Φ14.8.36, Φ14.8.40, Φ14.8.44, and Φ14.8.23, suggesting that Φ7.8.17 may be the same phage as these nine phage isolates. These data suggest that this phage (as well as its host) was the most abundant phage isolated in this study, especially on day 14. Similarly, Φ7.2.36 was independently isolated eight times (once on day 7 and seven times on day 14), representing the second most abundant phage.
The data in Table 1 show that diverse phages were present on days 3, 7, and 14 (seven types on day 3, six types on day 7, and eight types on day 14 based on host range). Many phages had very broad host ranges, capable of infecting multiple hosts. Surprisingly, Φ3.2.27 (same phage as Φ3.8.30) and Φ3.8.18 were able to infect Weissella cibaria, L. plantarum, and L. brevis. Phages with such a broad host range, crossing three species in two genera, have not been previously reported in vegetable fermentations. In a study of phage ecology in sauerkraut fermentations (20), no phages were found to be able to attack hosts from different genera. Two phages were found to be capable of infecting two species (L. brevis and L. plantarum) in the same genus, but most phages are species specific. For example, Φ1-A4 infects three Leuconostoc mesenteroides strains but not other species (20). ΦJL-1 (isolated from another cucumber fermentation) infects only two L. plantarum strains (19). A variety of molecules have been suggested to act as host receptors for phages infecting LAB. Among these are polysaccharides and (lipo)teichoic acids, as well as a single-membrane protein (26). Broad-host-range phages may be able to use more than one type of receptor present on different hosts, or the same receptors may be present on different hosts, allowing these phages to attack a wider variety of host strains within a genus or between different genera. These phages may play a key role in phage ecology and gene transfer in nature, thereby promoting genetic diversity in microbial communities (15). Further study is needed to explore ecological roles of broad-host-range phages and their impacts on the fermentations. In contrast to phages with a broad host range, several phages (e.g., Φ3.2.31, Φ14.8.19, and Φ14.2.33) listed in Table 1 had a very narrow host range. Each of them attacked only one host strain. Φ30.2.8 (the only phage isolated on day 30) or Φ3.2.35 (same phage as Φ30.2.8 but isolated on a different day) was also strain specific, infecting only one L. plantarum strain.
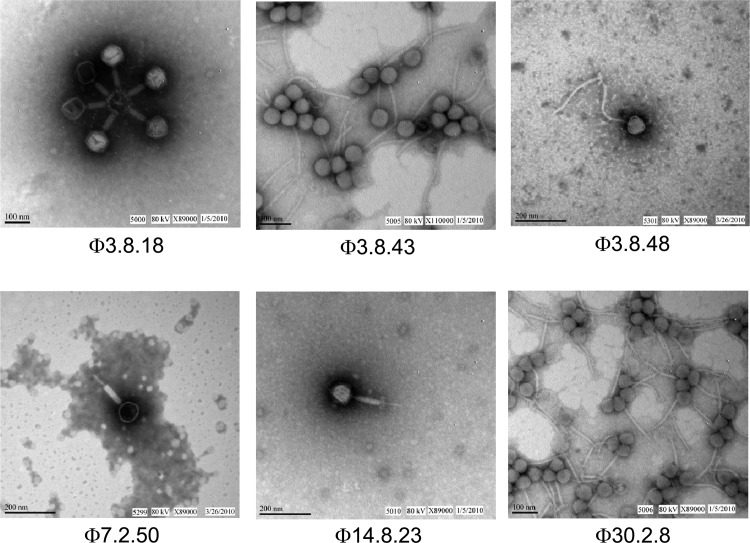
Six representative phages (Φ3.8.18, Φ3.8.43, Φ3.8.48, Φ7.2.50, Φ14.8.23, and Φ30.2.8) with different host ranges (highlighted in bold in Table 1) were selected for further characterization. The principal hosts of these phages included W. cibaria (3.8.43), W. paramesenteroides (3.8.48), L. plantarum (30.2.8), and two L. brevis strains (3.8.18, and 7.2.50 which happened to be the same strain as 14.8.23 based on phage typing). The electron micrographs (Fig. 4) show that all six phages are tailed phages with icosahedral heads. Three phages (Φ3.8.18, Φ7.2.50, and Φ14.8.23) belong to the Myoviridae family, and the other three (Φ3.8.43, Φ3.8.48, and Φ30.2.8) belong to the Siphoviridae family.
Fig 4.
Transmission electron micrographs of six phages isolated from a commercial cucumber fermentation.
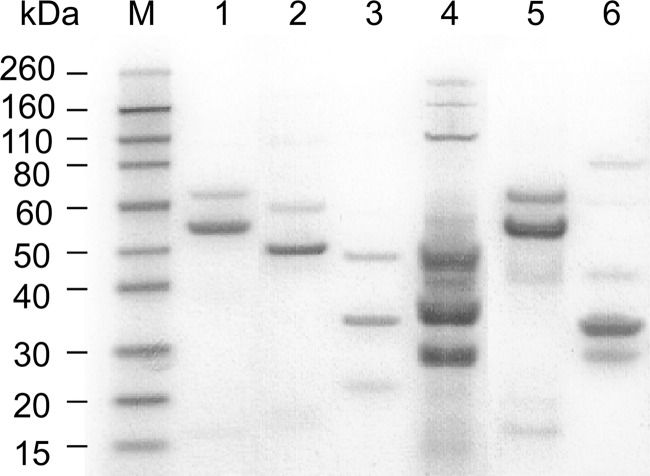
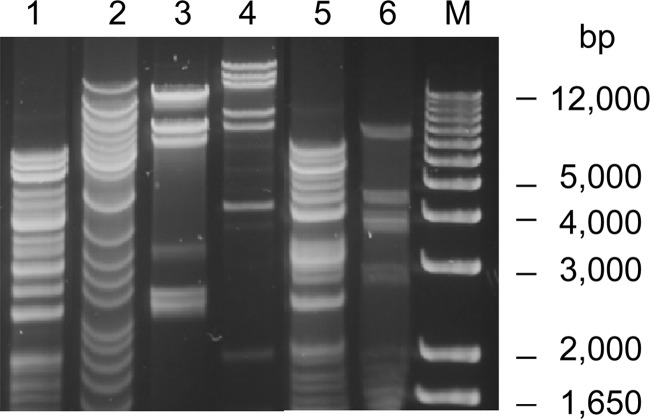
SDS-PAGE analysis showed that the six phages had unique structural protein profiles although the banding patterns from Φ7.2.50 and Φ14.8.23 were only slightly different (Fig. 5), suggesting that they were different phages. HindIII restriction digestion analysis of these phage genomes revealed six different restriction banding patterns (Fig. 6), indicating that these phages were genetically distinct. The data from these selected phages suggest that there is genetic diversity among the phage population in the cucumber fermentation. Further study will include sequence analysis of these phages.
Fig 5.
SDS-PAGE analysis of structural proteins of six phages isolated from a commercial cucumber fermentation. Lane M, molecular mass standard; lane 1, Φ7.2.50; lane 2, Φ3.8.18; lane 3, Φ3.8.43; lane 4, Φ3.8.48; lane 5, Φ14.8.23; lane 6, Φ30.2.8.
Fig 6.
Restriction analysis of DNAs of six phages isolated from a commercial cucumber fermentation. Phage DNAs were digested with HindIII. Lane 1, Φ 7.2.50; lane 2, Φ3.8.18; lane 3, Φ3.8.43; lane 4, Φ3.8.48; lane 5, Φ14.8.23; lane 6, Φ30.2.8; lane M, 1-kb DNA ladder.
In conclusion, the data from this study showed that abundant and diverse LAB phages were present in the commercial cucumber fermentation, which could result in significant mortality in the LAB population, thereby influencing the bacterial ecology and the dynamics of the fermentation. The data suggested that a phage-control strategy may be needed in the proposed low-salt cucumber fermentations that may require the use of LAB starter cultures. Phage-resistant LAB strains isolated in this study may deserve further evaluation as starter cultures for commercial use in the pickle industry. More research is needed to explore phage ecology in other commercial cucumber fermentations in the same and different geographic locations. To our knowledge, this is the first report on phage ecology in commercial cucumber fermentations.
ACKNOWLEDGMENTS
We thank the Mount Olive Pickle Company in Raleigh, NC, and in particular Lisa Moeller and Kelly Beene for help with the manipulation of the commercial cucumber fermentation tank for this study. We also thank Valerie M. Knowlton at the Center for Electron Microscopy, North Carolina State University, Raleigh, NC, for assistance with electron microscopic analysis. In addition, we thank Sandra Parker for excellent secretarial assistance.
Footnotes
Published ahead of print 28 September 2012
REFERENCES
- 1. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 2. Barrangou R, Yoon S-S, Breidt F, Fleming HP, Klaenhammer TR. 2002. Characterization of six Leuconostoc fallax bacteriophages isolated from a commercial sauerkraut fermentation. Appl. Environ. Microbiol. 68:5452–5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benson DA, et al. 2000. GenBank. Nucleic Acid Res. 28:15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breidt F, Hayes JS, Fleming HP. 2000. Reduction of microflora on whole pickling cucumbers by blanching. J. Food Sci. 65:1354–1358 [Google Scholar]
- 5. Breidt F, McFeeters RF, Díaz-Muñiz I. Fermented vegetables. In Doyle MP, Buchanan RL. (ed), Food microbiology: fundamentals and frontiers, 4th ed, in press ASM Press, Washington, DC: http://estore.asm.org/viewItemDetails.asp?ItemID=1069 [Google Scholar]
- 6. Clark TR, Ellison DW, Kleba B, Hackstadt T. 2011. Complementation of Rickettsia rickettsii RelA/SpoT restores a Nonlytic plaque phenotype. Infect. Immun. 79:1631–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costilow RN, Coughlin FM, Robach DL, Ragheb HS. 1956. A study of the acid-forming bacteria from cucumber fermentations in Michigan. Food Res. 21:27–33 [Google Scholar]
- 8. Crandell RA, Gomes I. 1970. Plaque morphology of some South American strains of foot-and-mouth disease virus and the effects of polyionic compounds on plaque formation. Arch. Gesamte Virusforsch. 30:137–146 [DOI] [PubMed] [Google Scholar]
- 9. Etchells JL, Jones ID. 1946. Characteristics of lactic acid bacteria from commercial cucumber fermentations. J. Bacteriol. 52:593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Etchells JL, Bell TA, Fleming HP, Kelling RE, Thompson RL. 1973. Suggested procedure for the controlled fermentation of commercially brined pickling cucumbers–the use of starter cultures and reduction of carbon dioxide accumulation. Pickle Pak Sci. 3:4–14 [Google Scholar]
- 11. Fajans K, Hassel O. 1923. A new method for titration of silver and halogen ions with organic dyestuff indicators. Z. Elektrochem. 29:495–500 [Google Scholar]
- 12. Fleming HP, Kyung KH, Breidt F. 1995. Vegetable fermentations, p 629–661 In Rehm HJ, Reed G. (ed), Biotechnology: enzymes, biomass, food and feed, 2nd ed, vol 9 VCH Publishers, Inc., New York, NY [Google Scholar]
- 13. Gill JJ, Hyman P. 2010. Phage choice, isolation, and preparation for phage therapy. Curr. Pharm. Biotechnol. 11:2–14 [DOI] [PubMed] [Google Scholar]
- 14. Hendrix RW. 2008. Phage evolution, p 177–194 In Abedon ST. (ed), Bacteriophage ecology. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 15. Jensen EC, et al. 1998. Prevalence of broad-host-range lytic bacteriophages of Sphaerotilus natans, Escherichia coli, and Pseudomonas aeruginosa. Appl. Environ. Microbiol. 64:575–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Konisky I. 1989. Colicins and other bacteriocins with established modes of action. Annu. Rev. Microbiol. 36:125–144 [DOI] [PubMed] [Google Scholar]
- 17. Lu Z, Altermann E, Breidt F, Kozyavkin S. 2010. Sequence analysis of Leuconostoc mesenteroides bacteriophage Φ1-A4 isolated from industrial vegetable fermentation. Appl. Environ. Microbiol. 76:1955–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lu Z, et al. 2005. Sequence analysis of the Lactobacillus plantarum bacteriophage ΦJL-1. Gene 348:45–54 [DOI] [PubMed] [Google Scholar]
- 19. Lu Z, Breidt F, Fleming HP, Altermann E, Klaenhammer TR. 2003. Isolation and characterization of a Lactobacillus plantarum bacteriophage ΦJL-1 from a cucumber fermentation. Int. J. Food Microbiol. 84:225–235 [DOI] [PubMed] [Google Scholar]
- 20. Lu Z, Breidt F, Plengvidhya V, Fleming HP. 2003. Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69:3192–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu Z, Fleming HP, McFeeters RF. 2002. Effects of fruit size on composition and sugar utilization of fermented cucumbers. J. Food Sci. 67:2934–2939 [Google Scholar]
- 22. Lund BM, Baird-Parker TC, Gould GW. 2000. The microbiological safety and quality of food. Aspen Publishers, Inc., Gaithersburg, MD [Google Scholar]
- 23. McDonald LC, Fleming HP, Hassan HM. 1990. Acid tolerance of Leuconostoc mesenteroides and Lactobacillus plantarum. Appl. Environ. Microbiol. 56:2120–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McFeeters RF, Barish AO. 2003. Sulfite analysis of fruits and vegetables by high-performance liquid chromatography (HPLC) with ultraviolet spectrophotometric detection. J. Agric. Food Chem. 51:1513–1517 [DOI] [PubMed] [Google Scholar]
- 25. McFeeters RF, Pérez Diaz IM. 2010. Fermentation of cucumbers brined with calcium chloride instead of sodium chloride. J. Food Sci. 75:C291–C296 [DOI] [PubMed] [Google Scholar]
- 26. McGrath S, van Sinderen D. 2007. Bacteriophage: genetics and molecular biology. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 27. Mundt JO. 1970. Lactic acid bacteria associated with raw plant food material. J. Milk Food Technol. 33:550–553 [Google Scholar]
- 28. Mundt JO, Graham WF, McCarty IE. 1967. Spherical lactic acid-producing bacteria of southern-grown raw and processed vegetables. Appl. Microbiol. 15:1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mundt JO, Hammer JL. 1968. Lactobacilli on plants. Appl. Microbiol. 16:1326–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pederson CS, Albury MN. The sauerkraut fermentation. New York State Agricultural Experiment Station Technical Bulletin 824; Geneva, New York: 1969. [Google Scholar]
- 31. Plengvidhya V, Breidt F, Lu Z, Fleming HP. 2007. DNA fingerprinting of lactic acid bacteria in sauerkraut fermentations. Appl. Environ. Microbiol. 73:7697–7702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Salivar WO, Tzagoloff H, Pratt D. 1964. Some physical-chemical and biological properties of the rod-shaped coliphage M13. Virology 24:359–371 [DOI] [PubMed] [Google Scholar]
- 33. Salivar WO, Henry TJ, Pratt DD. 1967. Purification and properties of diploid particles of coliphage M13. Virology 32:41–51 [DOI] [PubMed] [Google Scholar]
- 34. Serwer P, Hayes SJ, Thomas JA, Hardies SC. 2007. Propagating the missing bacteriophages: a large bacteriophage in a new class. Virol. J. 4:21 doi:10.1186/1743-422X-4-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson KH, Blitchington RB, Greene RC. 1990. Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction. J. Clin. Microbiol. 28:1942–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoon S-S, Barrangou-Poueys R, Breidt F, Fleming HP. 2007. Detection and characterization of a lytic Pediococcus bacteriophage from the fermenting cucumber brine. J. Microbiol. Biotechnol. 17:262–270 [PubMed] [Google Scholar]
- 37. Yoon S-S, Barrangou-Poueys R, Breidt F, Klaenhammer TR, Fleming HP. 2002. Isolation and characterization of bacteriophages from fermenting sauerkraut. Appl. Environ. Microbiol. 68:973–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7:203–214 [DOI] [PubMed] [Google Scholar]