Abstract
The physiology of a newly recognized Serratia species, termed South African Caenorhabditis briggsae Isolate (SCBI), which is both a nematode mutualist and an insect pathogen, was investigated and compared to that of Serratia marcescens Db11, a broad-host-range pathogen. The two Serratia strains had comparable levels of virulence for Manduca sexta and similar cytotoxic activity patterns, but motility and lipase and hemolytic activities differed significantly between them.
TEXT
Members of the genus Serratia are found widespread around the globe and are well known for their roles as insect pathogens (14). A newly recognized Serratia species, termed South African Caenorhabditis briggsae Isolate (SCBI), was identified following its isolation from the nematode C. briggsae KT0001 (2). These C. briggsae KT0001 nematodes were recovered from soil samples through Galleria mellonella bait traps in three provinces in South Africa (2). Serratia sp. strain SCBI is lethal to G. mellonella. When directly injected into the hemocoel in numbers of less than 1,000 CFU, larvae die within 72 h. A distinct characteristic of Serratia sp. SCBI is that it forms an apparent association with C. briggsae KT0001 as well as other Caenorhabditis nematodes, including Caenorhabditis elegans, which results in entomopathogenicity of the nematode (1, 2). This microbe-nematode association between Serratia sp. SCBI and C. briggsae may represent a potential emerging entomopathogenic association. Only two other Serratia species are known to use a nematode partner to establish an infection in an invertebrate host (40, 43).
Based on 16S phylogeny, Serratia sp. SCBI is closely related to S. marcescens Db11 (3), a reported pathogen of C. elegans (24, 34, 36). We have sequenced the entire Serratia sp. SCBI genome and have performed an analysis comparing it to the S. marcescens Db11 genome (F. Abebe-Akele, L. S. Tisa, V. Cooper, P. J. Hatcher, E. Abebe, and W. K. Thomas, unpublished data). S. marcescens Db11 is a well-known pathogen of vertebrates and invertebrates, including Caenorhabditis nematodes. Although their 16S RNA genes are 99% identical, genome-wide assessment (23) supports the idea that Serratia sp. SCBI and S. marcescens Db11 represent two distinct species. However, the high (about 80%) similarity at the 16S rRNA gene and within open reading frames indicates that Serratia sp. SCBI and S. marcescens Db11 share a close evolutionary relationship despite their distinct associations with Caenorhabditis nematodes. Since mutualistic associations have the potential to evolve from parasitic relationships (10), it is possible that Serratia sp. SCBI diverged from S. marcescens, making the leap from Caenorhabditis pathogen to a mutualistic relationship.
Although the virulence factors of S. marcescens have been well studied (6, 8, 24, 26, 33), there have been no studies on the physiological properties of Serratia sp. SCBI and their contribution to pathogenesis. Due to their close evolutionary history, a comparative study of Serratia sp. SCBI and S. marcescens Db11 was conducted to determine if they shared putative virulence factors such as exoenzymatic activity and cytotoxicity. In addition, the pathogenicity of these bacteria was assessed on a well-known insect model system, Manduca sexta. Since temperature affects virulence factor activities in S. marcescens Db11 (21, 25, 33), the effect of temperature on the putative virulence traits of both Serratia sp. was investigated at 22, 28, and 37°C, which represent soil, an intermediate, and vertebrate host temperatures, respectively.
Serratia sp. SCBI, S. marcescens Db11, and Escherichia coli EPI300 were grown in an overnight shake culture in Luria-Bertani (LB) broth at 22, 28, or 37°C for all assays. The effect of temperature on growth rate for both Serratia strains was determined, and results indicate that growth rate is not a major factor accounting for the differences in physiological activities investigated in this study (data not shown).
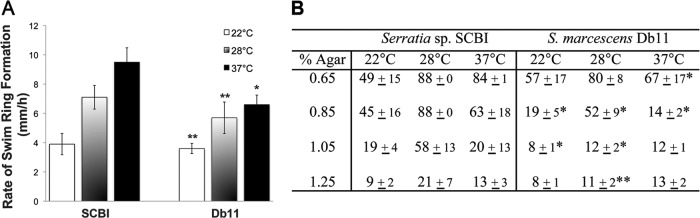
Regulatory networks linking motility and virulence have been elucidated in S. marcescens (26), and therefore motility was investigated in Serratia sp. SCBI and S. marcescens Db11. The plate migration assay (20) was used to investigate the swimming behavior of the Serratia strains. Temperature had a strong effect on swim ring formation (Fig. 1A; see also Fig. S1 in the supplemental material). For Serratia sp. SCBI, swim ring formation was faster (9.5 mm/h) at 37°C than at 28°C and 22°C (P < 0.001). A similar pattern was observed with S. marcescens Db11, with 37°C as the optimum temperature (6.5 mm/h; P < 0.001). In addition to the swimming behavior, we observed that Serratia sp. SCBI and S. marcescens Db11 exhibited swarming, or surface movement, utilizing methods described previously (31). Serratia sp. SCBI exhibited optimal swarming motility on medium with low agar concentrations and at elevated temperatures but was also capable of swarming movement on medium with elevated (1.25%) agar concentrations at 28°C (Fig. 1B; see also Fig. S2 in the supplemental material). S. marcescens Db11 similarly demonstrated optimal swarming on medium with low agar concentrations and at elevated temperatures but did not swarm on medium with agar concentrations greater than 0.85%.
Fig 1.
Temperature influences swimming and swarming behavior. (A) Average rates of swim ring formation for Serratia sp. SCBI (SCBI) and S. marcescens Db11 (Db11) at 22, 28, and 37°C. Results are shown as the averages of 6 measurements from 2 independent experiments, with the standard deviations indicated by error bars. (B) Swarm ring diameter measurements (expressed in millimeters) at 48 h. The maximum measurement was 88 mm (the width of the petri dish). Results are shown as the averages of 9 measurements ± standard deviations from 3 independent experiments (3 measurements each). ** (P < 0.01) and * (P < 0.001) denote significant differences from Serratia sp. SCBI at the same temperature.
Serratia spp. secrete multiple extracellular enzymes such as proteases, lipases, and chitinases that can contribute to their pathogenicity (11, 15, 19, 22, 28). Protease, gelatinase, DNase, chitinase, and siderophore activities were determined using agar plate assays described previously (17, 32, 37). Serratia sp. SCBI secreted a wide variety of exoenzymes and had siderophore activity (see Table S1 in the supplemental material). At 28°C and 37°C, both Serratia sp. SCBI and S. marcescens Db11 exhibited gelatinase, DNase, and chitinase activities on agar test media that were higher than those seen at the lower temperature (P < 0.01). Protease and lipase activities were also determined by quantitative liquid assays using methods described previously (5, 42). For quantitative liquid assays, 28°C was the optimal temperature for protease and lipase production (see Fig. S3 in the supplemental material). At 37°C, both bacterial species had levels of protease and lipase activities significantly lower than those seen at 28°C (P < 0.01). Although Serratia sp. SCBI and S. marcescens Db11 protease activities did not differ significantly at any temperature, Serratia sp. SCBI showed a significantly higher level of lipase activity than S. marcescens Db11 at 22°C and 37°C. Furthermore, the lipase and protease activities of both Serratia spp. were growth phase dependent and found only with post-exponential-growth-phase cells (data not shown).
Hemolytic activity is an important virulence factor for many pathogens (4, 18). For S. marcescens Db11, hemolytic activity is required for C. elegans or Drosophila melanogaster pathogenesis (24) and plays a role in cytotoxic activity against the human bronchial epithelial cell line 16HBE14o- (24). Hemolytic activity was measured using a modified liquid assay (16) which measured the rate of sheep red blood cell (SRBC) lysis by bacterial culture (∼4.0 × 106 cells) over 4 h. Serratia sp. SCBI showed a rate of hemolytic activity at 22°C that was lower than that seen at the other 2 temperatures (P < 0.001) (Fig. 2). Hemolytic activity occurred at similar levels at 28 and 37°C. SRBC lysis initiated after 1 h of incubation and was completed by 3 h. S. marcescens Db11 exhibited an optimal rate of hemolysis at 28°C compared to 22°C and 37°C (P < 0.001). At the 2-h time point, the hemolytic activities of Serratia sp. SCBI and S. marcescens Db11 were similar at 22°C and 28°C. However, at 37°C, Serratia sp. SCBI had significantly greater hemolytic activity (lysing 64% of the SRBC population) at the 2-h time point than S. marcescens Db11 (P < 0.001), which lysed ∼33% of the population. In contrast to protease and lipase activities, hemolytic activity was not influenced by growth phase (data not shown).
Fig 2.
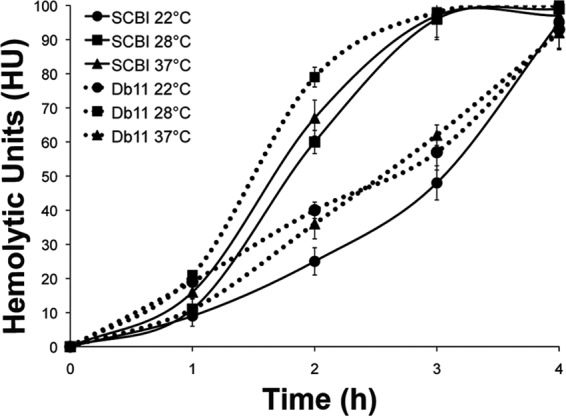
Comparison of the rates of hemolysis by Serratia sp. SCBI and S. marcescens Db11 at different incubation temperatures. Hemolytic activity against SRBCs was measured over 4 h of incubation. Bacterial cultures (≥4.0 × 106 CFU) were assayed for hemolytic activity at their growth temperature. Solid lines represent Serratia sp. SCBI, and dashed lines represent S. marcescens Db11. Values are the averages of 6 measurements from 2 independent experiments (3 measurements each), with the standard deviations indicated by error bars.
Experiments were performed to determine the location of Serratia sp. SCBI hemolytic activity. Untreated culture had the highest rate of hemolysis, lysing 96% of SRBCs at 3 h, whereas bacterial cells washed in phosphate-buffered saline (PBS) lysed an average of 29% of SRBCs at 3 h (data not shown). Reduced activity of the washed cells likely resulted from removal of surface-associated hemolysins during washing. Cell lysates also showed drastically reduced activity in comparison to culture, with an average of only 15% of SRBCs lysed after 3 h. Formalin-killed Serratia sp. SCBI or cell-free supernatant showed no hemolysis. These results indicate the hemolysin was not secreted but might have been surface associated, requiring live cells for functionality.
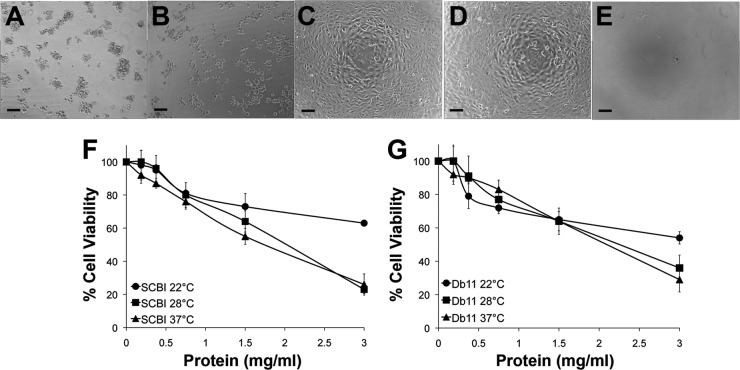
Cytotoxins are well-known virulence factors for bacterial pathogens because they can cause extensive damage to host cell morphology, resulting in cell death (8, 27, 35). S. marcescens culture filtrates are cytotoxic to a number of different mammalian cell lines (6, 8), with a protease, a hemolysin, or a combination of factors being identified as the agents responsible for these activities (17, 29). Therefore, both Serratia sp. were investigated for cytotoxic effects on Buffalo green monkey kidney (BGMK) cells following a 24-h exposure to bacterial supernatants grown at the 3 test temperatures. Various levels (0.18 to 3.0 mg/ml) of protein concentrations of the supernatant were also tested, and cell viability was measured using the MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide] assay (29, 41). BGMK cells exposed to Serratia sp. SCBI and S. marcescens Db11 supernatant fluids from cultures grown at 37°C appeared unhealthy, rounding upward and detaching from the surface of the plates (Fig. 3A and B). There was no significant difference between Serratia sp. SCBI and S. marcescens Db11 in cytotoxic effects at 3 mg/ml protein concentration (Fig. 3F and G). Cultures grown at 28°C and 37°C were significantly more toxic than cultures grown at 22°C. Between 20% and 30% of BGMK cells remained viable after exposure to 28°C and 37°C cultures, while 60% of cells were viable when exposed to 22°C cultures. Cell lysates from both strains were also tested and produced results similar to those seen with the bacterial supernatant (data not shown).
Fig 3.
Cytotoxic effects of filter-sterilized bacterial supernatant fluids from Serratia sp. SCBI, S. marcescens Db11, and E. coli EPI300 on BGMK cells after 24 h. (A to E) Photomicrographs (100× magnification) of BGMK cells treated with supernatant fluids at a concentration of 1.5 mg/ml total protein. Bars, 100 μm. The supernatant was from bacterial cultures grown at 37°C. (A) Serratia sp. SCBI, (B) S. marcescens Db11, (C) E. coli EPI300, (D) no-treatment control, (E) 2% SDS. (F and G) Cell viability measurements by MTT assay after 24 h of exposure at 37°C to bacterial supernatant fluids from (F) Serratia sp. SCBI or (G) S. marcescens Db11. Values are the averages of 6 measurements from 2 independent experiments (3 measurements each), with the standard deviations indicated by error bars.
Serratia spp. are well-known insect pathogens that infect a variety of invertebrates (12, 14, 24, 39). To assess the virulence of Serratia sp. SCBI and S. marcescens Db11, virulence was measured using the M. sexta model system and a modified method described previously (7). Larvae were injected through the larval head with 10 μl of bacterial suspension (500, 4.0 × 104, or 1.0 × 105 CFU) from cultures grown at the 3 test temperatures. Larvae were monitored for mortality and delays in development over 7 days. In addition, larvae were weighed on day 0 and day 7 or on the day of death. Larva injection resulted in mortality, and there were noticeable defects in insect development (Table 1; see also Fig. S4 in the supplemental material). Elevated temperatures and cell dosage increased the virulence of both Serratia strains. There were no significant differences observed between the bacterial strains, as they had similar mortality rates and LT50 values (the latter representing the average time by which 50% of the larval population had died), though S. marcescens Db11 had a slightly higher rate of killing. The controls, E. coli EPI300 or LB broth, showed no insect mortality or developmental effects. Insect larvae that survived Serratia infection for 7 days showed stunted development and often turned pale green and pink around the head and legs. Those larvae injected with Serratia most likely would have succumbed to infection with continued incubation.
Table 1.
Effect of Serratia dosage and temperature on insect mortality and healtha
| Strain and inoculum (CFU 10 μl−1) | 22°C |
28°C |
37°C |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Mortality (%) by day 7 | LT50 (no. of days ± SD) | Change in wt (%) | Mortality (%) by day 7 | LT50 (no. of days ± SD) | Change in wt (%) | Mortality (%) by day 7 | LT50 (no. of days ± SD) | Change in wt (%) | |
| Serratia spp. | |||||||||
| Serratia sp. SCBI | |||||||||
| 500 | 40 | >7.0 | +23.2 | 50 | 4.0 | +51.1 | 70 | 5.6 | +85.9 |
| 4.0 × 104 | 50 | 5.0 ± 1.6 | +32.2 | 60 | 2.4 | +25.8 | 100 | 2.8 | +2.6 |
| 1.0 × 105 | 70 | 2.3 ± 1.9 | +5.6 | 80 | 2.2 | +9.2 | 100 | 1.0 | Negative |
| S. marcescens Db11 | |||||||||
| 500 | 40 | >7.0 | +6.7 | 60 | 4.8 | +14.7 | 80 | 3.6 | +18.7 |
| 4.0 × 104 | 90 | 3.3 ± 1.9 | +5.9 | 60 | 3.0 | +15.1 | 100 | 1.8 | Negative |
| 1.0 × 105 | 90 | 3.3 ± 2.0 | Negative | 100 | 1.0 | Negative | 100 | 1.0 | Negative |
| Controls | |||||||||
| E. coli EPI300 | |||||||||
| 1.0 × 105 | 0 | − | +49.8 | 0 | − | +108.9 | 0 | − | +141.2 |
| LB | 0 | − | 100 | 0 | − | 100 | 0 | − | 100 |
M. sexta larvae were injected with each dosage and incubated at the indicated test temperature. Insect health was monitored daily, and insect mortality was noted. Values represent the results of 2 independent experiments. Mortality values represent the total percentage of the larval population that died during the 7-day incubation period. LT50 values represent the average time for 50% of the larval population to die. Percent change in weight values represent the average change in weight of the population during the 7-day period (or by the day of death) and were normalized to the average weight of larvae injected with LB. Negative values indicate that there was an average decrease in larval weight following injection.
Thermoregulation of virulence genes occurs in numerous bacterial pathogens, including Yersinia, Shigella, and Aeromonas (9, 30, 38), and the optimal temperature for their expression often reflects the host environment. The ability of S. marcescens Db11 to produce virulence-associated phenotypes over a wide range of temperatures is reflective of its ability to infect both vertebrate and invertebrate hosts. Our results indicate that Serratia sp. SCBI and S. marcescens Db11 share similar pathogenic and cytotoxic effects. They also shared a wide variety of virulence-associated properties and, in some cases, had similar temperature-dependent responses. These results suggest that Serratia sp. SCBI may potentially have the same capabilities for infecting a wide host range.
Since Serratia sp. SCBI and S. marcescens Db11 differed in their associations with Caenorhabditis nematodes, we expected that there would be some physiological differences between these two strains. Patterns of motility and lipase activity shown by Serratia sp. SCBI were different. The observed differences in surface swarming were striking and may play a role in insect colonization or nematode interactions. Serratia sp. SCBI bacteria were able to swarm on media with a wide range of agar concentrations (Fig. 1), suggesting that these bacteria are able to move on surfaces with different viscosities or degrees of wetness. Furthermore, we expected Serratia sp. SCBI to have altered hemolysis patterns since this activity is a virulence factor for S. marcescens Db11 against Caenorhabditis nematodes (24). At elevated temperatures, Serratia sp. SCBI showed significantly greater hemolytic activity than S. marcescens Db11 (Fig. 2), but we found several similarities between these two organisms. First, Serratia sp. SCBI and S. marcescens Db11 shared similar rates of hemolysis at lower temperatures. Second, the modes of action for hemolysis of the two bacterial strains may be similar. Cell-to-cell contact is required for full hemolytic activity by S. marcescens (13). Our results show that Serratia sp. SCBI also required contact between live bacteria and blood cells for hemolytic activity. Filter-sterilized supernatant fluids and formalin-killed cells were not hemolytic. Cell lysates had reduced hemolytic activity. However, cell lysates or supernatants from Serratia sp. SCBI were cytotoxic toward the BGMK cell line (Fig. 3). This result indicates that there may be two separate toxicity mechanisms for mammalian blood cells and epithelial cells.
Although only three Serratia species are currently known to form mutualistic relationships with nematodes (2, 40, 43), all three species share close evolutionary histories and appear to be at the nascent stages of mutualistic microbe-nematode relationships. Our study results indicate that Serratia sp. SCBI and S. marcescens Db11 could harbor similar virulence factors required for insect pathogenesis, but their relationship with Caenorhabditis nematodes clearly separates these two strains. Future research could shed light on how Serratia sp. SCBI has made the switch from Caenorhabditis nematode pathogen to mutualist and whether regulation of their virulence factors plays a role in altering their relationship with nematodes.
Supplementary Material
ACKNOWLEDGMENTS
This investigation was supported in part by Hatch grant NH00496 and the College of Life Science and Agriculture, University of New Hampshire—Durham.
This is scientific contribution 2481 from the NH Agricultural Experiment Station.
Buffalo green monkey kidney (BGMK) cells were a kind gift from Aaron Margolin, University of New Hampshire. We thank Robert M. Q. Shanks for S. marcescens Db11, Kaitlyn D. LaCourse for her help with 16S sequencing, and Ben Coffey and Cintia Felix for their initial contributions to early stages of this project.
Footnotes
Published ahead of print 5 October 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Abebe E, Abebe-Akele F, Morrison J, Cooper V, Thomas WK. 2011. An insect pathogenic symbiosis between a Caenorhabditis and Serratia. Virulence 2:158–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abebe E, et al. 2010. An entomopathogenic Caenorhabditis briggsae. J. Exp. Biol. 213:3223–3229 [DOI] [PubMed] [Google Scholar]
- 3. Abebe-Akele F, Tisa LS, Abebe E, Cooper V, Thomas WK. 2011. Characterizing a novel entomopathogenic partnership between Serratia spp. and Rhabditid nematodes. J. Nematol. 43:54 [Google Scholar]
- 4. Arthur M, et al. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caldas C, Cherqui A, Pereira A, Simões N. 2002. Purification and characterization of an extracellular protease from Xenorhabdus nematophila involved in insect immunosuppression. Appl. Environ. Microbiol. 68:1297–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carbonell GV, Falcón R, Yamada AT, da Fonseca BAL, Yano T. 2004. Morphological and intracellular alterations induced by Serratia marcescens cytotoxin. Res. Microbiol. 155:25–30 [DOI] [PubMed] [Google Scholar]
- 7. Daborn PJ, et al. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. U. S. A. 99:10742–10747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Escobar MM, Carbonell GV, Beriam LO, Siqueira WJ, Yano T. 2001. Cytotoxin production in phytopathogenic and entomopathogenic Serratia marcescens. Rev. Latinoam. Microbiol. 43:165–170 [PubMed] [Google Scholar]
- 9. Falconi M, Colonna B, Prosseda G, Micheli G, Gualerzi CO. 1998. Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity; a temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17:7033–7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Genkai-Kato M, Yamamura N. 1999. Evolution of mutualistic symbiosis without vertical transmission. Theor. Popul. Biol. 55:309–323 [DOI] [PubMed] [Google Scholar]
- 11. Givskov M, Eberl L, Molin S. 1997. Control of exoenzyme production, motility and cell differentiation in Serratia liquefaciens. FEMS Microbiol. Lett. 148:115–122 [Google Scholar]
- 12. Glare TR, Corbett GE, Sadler TJ. 1993. Association of a large plasmid with amber disease of the New Zealand grass grub, Costelytra zealandica, caused by Serratia entomophila and Serratia proteamaculans. J. Invertebr. Pathol. 62:165–170 [Google Scholar]
- 13. Goluszko P, Nowacki M. 1989. Extracellular haemolytic activity of Serratia marcescens. FEMS Microbiol. Lett. 52:207–211 [DOI] [PubMed] [Google Scholar]
- 14. Grimont F, Grimont PAD. 2006. The genus Serratia. Prokaryotes 6:219–244 [Google Scholar]
- 15. Hejazi A, et al. 1997. Serratia marcescens. J. Med. Microbiol. 46:903–912 [DOI] [PubMed] [Google Scholar]
- 16. Hertle R, et al. 1997. Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol. Microbiol. 26:853–865 [DOI] [PubMed] [Google Scholar]
- 17. Hertle R, Hilger M, Weingardt-Kocher S, Walev I. 1999. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect. Immun. 67:817–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hertle R. 2005. The family of Serratia type pore forming toxins. Curr. Protein Pept. Sci. 6:313–325 [DOI] [PubMed] [Google Scholar]
- 19. Hines Da Saurugger PN, Ihler GM, Benedik MJ. 1988. Genetic analysis of extracellular proteins of Serratia marcescens. J. Bacteriol. 170:4141–4146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hodgson MM, Day B, White DJ, Tisa LS. 2003. Effect of growth conditions on the motility of Photorhabdus temperata. Arch. Microbiol. 180:17–24 [DOI] [PubMed] [Google Scholar]
- 21. Jepsen PK, Riise E, Biedermann K, Kristensen PC, Emborg C. 1987. Two-level factorial screening for influence of temperature, pH, and aeration on production of Serratia marcescens nuclease. Appl. Environ. Microbiol. 53:2593–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaska M, Lysenko O, Chaloupka J. 1976. Exocellular proteases of Serratia marcescens and their toxicity to larvae of Galleria mellonella. Folia Microbiol. (Praha) 21:465–473 [DOI] [PubMed] [Google Scholar]
- 23. Konstantinidis KT, Ramette A, Tiedje JM. 2006. The bacterial species definition in the genomic era. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361:1929–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kurz CL, et al. 2003. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J. 22:1451–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lai H, et al. 2005. The RssAB two-component signal transduction system in Serratia marcescens regulates swarming motility and cell envelope architecture in response to exogenous saturated fatty acids. J. Bacteriol. 187:3407–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin C, et al. 2010. RssAB-FlhDC-ShlBA as a major pathogenesis pathway in Serratia marcescens. Infect. Immun. 78:4870–4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund T, De Buyser ML, Granum PE. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38:254–261 [DOI] [PubMed] [Google Scholar]
- 28. Lysenko O. 1976. Chitinase of Serratia marcescens and its toxicity to insects. J. Invertebr. Pathol. 27:385–386 [Google Scholar]
- 29. Marty KB, Williams CL, Guynn LJ, Benedik MJ, Blanke SR. 2002. Characterization of a cytotoxic factor in culture filtrates of Serratia marcescens. Infect. Immun. 70:1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mateos D, Anguita J, Naharro G, Paniagua C. 1993. Influence of growth temperature on the production of extracellular virulence factors and pathogenicity of environmental and human strains of Aeromonas hydrophila. J. Appl. Microbiol. 74:111–118 [DOI] [PubMed] [Google Scholar]
- 31. Michaels B, Tisa LS. 2011. Swarming motility by Photorhabdus temperata is influenced by environmental conditions and uses the same flagella as that used in swimming motility. Can. J. Microbiol. 57:196–203 [DOI] [PubMed] [Google Scholar]
- 32. Parani K, Shetty GP, Saha BK. 2011. Isolation of Serratia marcescens SR1 as a source of chitinase having potentiality of using as a biocontrol agent. Indian J. Microbiol. 51:247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poole K, Braun V. 1988. Influence of growth temperature and lipopolysaccharide on hemolytic activity of Serratia marcescens. J. Bacteriol. 170:5146–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pradel E, et al. 2007. Detection and avoidance of a natural product from the pathogenic bacterium Serratia marcescens by Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 104:2295–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sato H, et al. 2003. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. EMBO J. 22:2959–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schulenburg H, Ewbank JJ. 2004. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 4:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwyn B, Neilands JB. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47–56 [DOI] [PubMed] [Google Scholar]
- 38. Straley S, Perry R. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310–317 [DOI] [PubMed] [Google Scholar]
- 39. Tan B, Jackson TA, Hurst MR. 2006. Virulence of Serratia strains against Costelytra zealandica. Appl. Environ. Microbiol. 72:6417–6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torres-Barragan A, Suazo A, Buhler WG, Cardoza YJ. 2011. Studies on the entomopathogenicity and bacterial associates of the nematode Oscheius carolinensis. Biol. Control 59:123–129 [Google Scholar]
- 41. Twentyman PR, Luscombe M. 1987. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br. J. Cancer 56:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Winkler UK, Stuckmann M. 1979. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 138:663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C-X, et al. 2009. Serratia nematodiphila sp. nov., symbiotically associated with entomopathogenic nematode Heterorhabditidoides chongmingensis (Rhabditida: Rhabditidae). Int. J. Syst. Evol. Microbiol. 59:1603–1608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.