Abstract
Viruses have been discovered in numerous fungal species, but unlike most known animal or plant viruses, they are rarely associated with deleterious effects on their hosts. The knowledge about viruses among entomopathogenic fungi is very limited, although their existence is suspected because of the presence of virus-like double-stranded RNA (dsRNA) in isolates of several species. Beauveria bassiana is one of the most-studied species of entomopathogenic fungi; it has a cosmopolitan distribution and is used as a biological control agent against invertebrates in agriculture. We analyzed a collection of 73 isolates obtained at different locations and from different habitats in Spain and Portugal, searching for dsRNA elements indicative of viral infections. The results revealed that the prevalence of viral infections is high; 54.8% of the isolates contained dsRNA elements with viral characteristics. The dsRNA electropherotypes of infected isolates indicated that virus diversity was high in the collection analyzed and that mixed virus infections occurred in fungal isolates. However, a hybridization experiment indicated that dsRNA bands that are similar in size do not always have similar sequences. Particular virus species or dsRNA profiles were not associated with locations or types of habitats, probably because of the ubiquity and efficient dispersion of this fungus as an airborne species. The sequence of one of the most common dsRNA elements corresponded to the 5.2-kbp genome of a previously undescribed member of the Totiviridae family, termed B. bassiana RNA virus 1 (BbRV1).
INTRODUCTION
The entomopathogenic fungus Beauveria bassiana is a natural enemy of numerous species of insects and arachnids and has a cosmopolitan distribution (28, 37). This fungus can live in very different niches. In addition to invertebrates, it can infect plants asymptomatically as an endophyte (34, 44, 52), and it is commonly found in soils (36). B. bassiana is known to produce an array of bioactive metabolites that limit the growth of some fungal plant pathogens, and it has been suggested that its endophytic colonization may induce plant systemic resistance against the pathogenic bacterium Xanthomonas axonopodis pv. malvacearum in cotton (30). Because of its entomopathogenic and endophytic characteristics, this fungus has been considered for potential use as a biocontrol agent against plant pests and pathogens and commercial formulations of B. bassiana for the control of agricultural pests have been developed in several countries (10, 16).
The presence of double-stranded RNA (dsRNA) elements indicative of viral infections has been reported in 2 of 13 Brazilian isolates of B. bassiana obtained from insects, 2 of 12 Canadian soil isolates, 6 of 30 U.S. insect isolates, and 10 of 15 endophytic isolates from Spain. These dsRNA elements ranged in size from 0.7 to 6 kbp, and the number of elements harbored by each fungal isolate varied from one to five. However, none of these dsRNA elements has been sequenced, so the fungal virus to which they might correspond has not been identified (3, 8, 17, 27).
Viruses have been detected in many species, covering all four phyla of the true fungi: Chytridiomycota, Zygomycota, Ascomycota, and Basidiomycota. In general, mycoviruses are very persistent, they are vertically transmitted to spores, they have no known biological vectors for their transmission, and unlike animal or plant viruses, they normally infect their hosts asymptomatically (14). The symptomless phenotype of many mycoviral infections could be explained by the ancient-infection hypothesis, reflecting a long period of coevolution in which reciprocal influences between the fungal host and mycoviruses would have evolved to a nonvirulent state of the virus, resulting in a symptomless virus-fungus association (33, 43). Nevertheless, some fungal viruses affect the virulence of plant-pathogenic fungi like Cryphonectria parasitica and Rosellinia necatrix or affect basidiocarp formation in the commercial production of Agaricus bisporus (4, 20, 40). Other fungal viruses are involved in fascinating and complex interactions among organisms; for instance, a virus infecting a Curvularia root endophyte has been reported to increase the thermal tolerance of the plant host of the endophyte (26). Also, a fungal virus of the yeast Saccharomyces cerevisiae maintains a satellite dsRNA that encodes an allelopathic toxin which inhibits the growth of yeast strains lacking the virus and its satellite. RNA silencing machinery, which inhibits the presence of this virus and its satellite, is absent from fungal taxa harboring them, which suggests that this type of interaction with viruses is beneficial to their fungal hosts (9).
The presence of fungal viruses has been commonly diagnosed by the presence of dsRNA elements, because most known mycoviruses have either dsRNA genomes or single-stranded RNA (ssRNA) genomes that produce dsRNA replicative intermediates (29). dsRNA elements observed in fungal isolates can be quite diverse in terms of the size and number of molecules, and several dsRNAs of different sizes infecting the same fungus might correspond to multipartite viral genomes, to mixed infections, or even to defective products of virus replication (14, 33). Because of characteristics like their persistence in infected hosts or the efficient transmission of mycoviruses to spores, the polymorphic dsRNA profiles detected in fungi have been proposed as markers for distinguishing isolates of different origins within a species (15, 21, 48). These dsRNA profiles have been associated with a geographical structure in some fungal species (31, 54), but often this is not the case (42, 49).
The main objective of this work was to study the prevalence, variability, and patterns of distribution of dsRNA elements in a collection of soil and endophytic isolates of B. bassiana obtained in different locations and habitats of Spain and Portugal. In addition, we sequenced a dsRNA element which provided the first identification of a virus in B. bassiana.
MATERIALS AND METHODS
Fungal isolates.
Seventy-three isolates of B. bassiana were analyzed for the presence of mycoviruses. Fifty-eight isolates collected from soil in different cultivated and natural habitats on the Iberian Peninsula and in the Canary and Balearic Islands came from the collection of the Entomology Laboratory in the School of Agricultural and Forest Sciences and Resources at the University of Córdoba, Córdoba, Spain (Table 1). Although these isolates were isolated from soil, the pathogenicity of several of them was successfully tested on insects (35, 36). Fifteen additional isolates were isolated as endophytes from different grasses in natural habitats in Spain (Table 1).
Table 1.
Soil and endophytic isolates of B. bassiana collected in Spain and Portugal and analyzed for the presence of dsRNA elements
| Isolate | Location | Habitat | Isolate | Location | Habitat or host grass |
|---|---|---|---|---|---|
| Soil | Soil | ||||
| EABb 01/145-Su | Seville | Olive grove | EABb 04/03-Su | Cantabria | Grassland |
| EABb 01/110-Su | Seville | Oak grove | EABb 09/09-Su | Ciudad Real | Olive grove |
| EABb 01/105-Su | Seville | Cotton field | EABb 04/10-Su | Gerona | Olive grove |
| EABb 01/112-Su | Seville | Wheat field | EABb 06/01-Su | Ibiza | Pine forest |
| EABb 01/103-Su | Seville | Woodland | EABb 06/02-Su | Fuerteventura | Fallow land |
| EABb 01/125-Su | Cádiz | Fallow land | EABb 06/03-Su | Fuerteventura | Fallow land |
| EABb 01/33-Su | Cádiz | Olive grove | EABb 07/15-Su | Lugo | Fallow land |
| EABb 01/130-Su | Cádiz | Pine forest | EABb 08/08-Su | Portugal | Olive grove |
| EABb 01/132-Su | Cádiz | Cotton field | EABb 08/09-Su | Portugal | Olive grove |
| EABb 01/15-Su | Almería | Desert | EABb 01/87-Su | Portugal | Pine forest |
| EABb 01/75-Su | Almería | Beach | EABb 01/88-Su | Portugal | Sunflower field |
| EABb 00/16-Su | Almería | Scrubland | EABb 01/89-Su | Portugal | Unknown |
| EABb 01/164-Su | Huelva | Pine forest | Bs20 | Seville | Oak grove |
| EABb 01/168-Su | Huelva | Scrubland | Bs5 | Seville | Oak grove |
| EABb 01/171-Su | Huelva | Cotton field | EABb 04/05-Su | Álava | Leek field |
| EABb 01/19-Su | Granada | Wheat field | EABb 04/09-Su | Madrid | Grassland |
| EABb 01/64-Su | Granada | Woodland | EABb 09/03-Su | Ciudad Real | Eucalyptus grove |
| EABb 01/73-Su | Granada | Scrubland | EABb 09/04-Su | Ciudad Real | Oak grove |
| EABb 07/08-Su | Granada | Olive grove | EABb 09/06-Su | Ciudad Real | Eucalyptus grove |
| EABb 01/34-Su | Málaga | Olive grove | EABb 09/07-Su | Ciudad Real | Oak grove |
| EABb 01/35-Su | Málaga | Scrubland | EABb 09/08-Su | Ciudad Real | Wild olive grove |
| EABb 01/36-Su | Málaga | Meadow | Endophytic | ||
| EABb 00/10-Su | Jaén | Olive grove | E 183 | Salamanca | Dactylis glomerata |
| EABb 00/11-Su | Jaén | Scrubland | E 1764 | Salamanca | Dactylis glomerata |
| EABb 00/13-Su | Jaén | Woodland | E 2720 | La Coruña | Elymus farctus |
| EABb 01/43-Su | Jaén | Olive grove | E 2773 | La Coruña | Ammophila arenaria |
| EABb 01/07-Su | Córdoba | Meadow | E 2854 | La Coruña | Ammophila arenaria |
| Bs7 | Seville | Oak grove | E 2857 | La Coruña | Elymus farctus |
| Bs1 | Seville | Oak grove | E 3079 | La Coruña | Elymus farctus |
| EABb 01/22-Su | Córdoba | Scrubland | E 3080 | La Coruña | Elymus farctus |
| EABb 01/25-Su | Córdoba | Olive grove | E 3111 | La Coruña | Elymus farctus |
| EABb 01/27-Su | Córdoba | Wheat field | E 3154 | Cáceres | Holcus lanatus |
| EABb 01/39-Su | Málaga | Almond grove | E 3155 | Cáceres | Holcus lanatus |
| EABb 04/06-Su | Córdoba | Cork oak grove | E 3158 | Cáceres | Holcus lanatus |
| EABb 04/08-Su | Córdoba | Hazel grove | E 1923 | La Coruña | Ammophila arenaria |
| EABb 00/08-Su | Badajoz | Grassland | E 2175 | Cáceres | Dactylis glomerata |
| EABb 04/02-Su | Cantabria | Meadow | E 2980 | La Coruña | Elymus farctus |
Analysis of the presence of dsRNA.
The presence of dsRNA molecules of sizes ranging from 1 to 12 kbp in fungal isolates was used as an indicator of virus infection. This type of nucleic acid can represent the genomes of dsRNA mycoviruses, as well as replicative forms of viruses with single-stranded RNA (ssRNA) genomes (29). However, DNA viruses, recently discovered in fungi (56), would not be detected by this technique.
To detect the presence of dsRNA, each fungal isolate was cultured for 3 weeks over cellophane disks layered on top of potato dextrose agar (PDA; Scharlau) in petri plates. Approximately 1.5 g of fresh mycelium of each isolate was harvested and ground with liquid nitrogen, and dsRNA was extracted by CF-11 cellulose (Whatman) chromatography (29). To eliminate contaminating DNA, the purified dsRNA was treated with 5 U of DNase I (Promega) for 30 min at 37°C and extracted with 1 volume of phenol-chloroform (1:1; Sigma-Aldrich). Contaminating ssRNA was removed by treatment with 1 U of S1 nuclease (Promega) at 37°C for 15 min and extracted in the same way. dsRNA extracts were subjected to agarose gel electrophoresis and visualized after staining with ethidium bromide. All dsRNA extractions were independently repeated three times. The sizes of the different dsRNA elements were estimated in relation to DNA size standards (1-kb DNA ladder; Promega).
cDNA synthesis.
Isolate EABb 06/02-Su (Fig. 1), harboring a single dsRNA element of about 5.5 kbp, according to the DNA size standard, was cultured for 3 weeks over cellophane disks layered on top of PDA petri plates. The dsRNA was purified as explained before, and approximately 0.6 μg dissolved in water was used for cDNA synthesis. An RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE) procedure (7) was adapted in the present work for the construction of a cDNA library. The cDNA products obtained were cloned in T-A vectors (Invitrogen). Escherichia coli strain DH5α (Invitrogen) was transformed and screened to select transformants containing inserts, which were sequenced. Gaps in the assembled sequences, which were not covered by clones derived from the cDNA library, were filled by reverse transcription and PCR primed by oligonucleotides designed according to sequences flanking the gaps. The ends of the molecule were cloned and confirmed by using the RLM-RACE method (7) again in three independent experiments.
Fig 1.
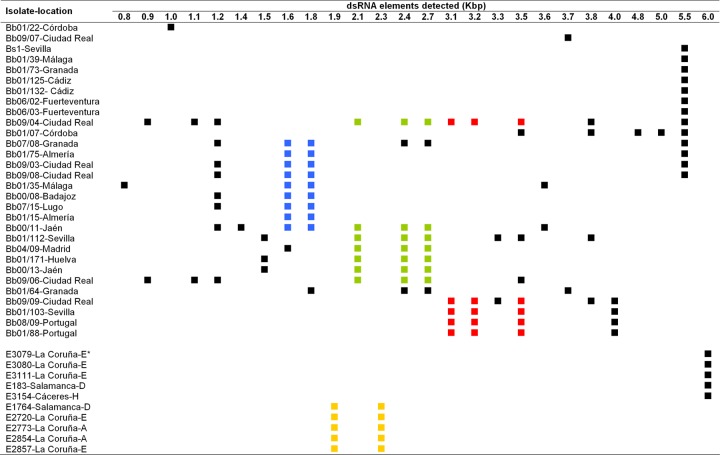
dsRNA electropherotypes observed in soil (Bb and Bs isolates) and endophytic (E isolates, bottom) isolates of B. bassiana. The squares indicate the presence in an isolate of a dsRNA molecule of the size shown at the top of each column. Similar sets of two or three dsRNA elements observed in different isolates are indicated by identical colors.
Sequence and phylogenetic analyses.
Sequence similarity searches in the EMBL virus sequence database were conducted by using the FASTA program (32). For phylogenetic analyses, sequence alignments were performed by using the ClustalX program (47), and genetic distances among amino acid sequences were calculated according to the Poisson correction model using MEGA3 software (23). Phylogenetic trees were made by using the neighbor-joining method, and bootstrap test values were based on 1,000 replications. Prediction of RNA pseudoknots was done with the program DotKnot (45).
Northern blotting experiments.
Thirteen different isolates contained a 5.5-kbp dsRNA element (Fig. 1, isolates EABb 06/2, EABb 06/03, EABb 01/125, EABb 01/132, EABb 01/39, EABb 01/73, Bs1, EABb 09/03, EABb 09/08, EABb 01/75, EABb 07/08, EABb 09/04, and EABb 01/07). To determine if these dsRNA molecules of equal size had homologous nucleotide sequences, a Northern hybridization was done. dsRNA extracts from these isolates were electrophoresed in agarose gels, denatured, and transferred to nylon membranes (57). Hybridization and detection were done by using DIG High Prime DNA Labeling and Detection Starter Kit II (Roche). A clone from the 5′ untranslated region (UTR) of the genome of B. bassiana RNA virus 1 (BbRV1), which is a nonconserved region of the genome of the Totiviridae, was used as a specific probe.
Nucleotide sequence accession number.
The complete genome sequence of BbRV1 has been deposited in the EMBL nucleotide sequence database under accession number HE572591.
RESULTS
Prevalence and patterns of dsRNA elements.
Forty (54.8%) of the 73 soil and endophytic B. bassiana isolates analyzed harbored dsRNA elements. Regarding the substrate where the isolates were obtained, 30 (51.7%) of the 58 soil isolates and 10 (66.7%) of the 15 endophytic isolates contained dsRNA elements.
The diversity of dsRNA profiles observed was high. Twenty-six different dsRNA elements with estimated sizes ranging from 0.8 to 6.0 kbp were detected among the infected isolates. Some infected isolates contained only 1 dsRNA element, while others had as many as 11, and some elements of the same size were common to several isolates (Fig. 1). In addition, several dsRNA elements were always together in different isolates: a set of two dsRNA molecules of 1.8 and 1.6 kbp occurred in seven soil isolates; another set of three dsRNAs of 2.7, 2.4, and 2.1 kbp was present in six soil isolates; and a third set of 3.5-, 3.2-, and 3.1-kbp dsRNAs was found in three soil isolates (Fig. 1). Another set of two dsRNAs of 2.3 and 1.9 kbp occurred in five endophytic isolates (Fig. 1, bottom). Each of these sets of dsRNA elements could represent genomes of mycoviruses belonging to families with multipartite genomes like Chrysoviridae or Partitiviridae (11, 46).
Soil and endophytic isolates were grouped according to the similarity of their dsRNA electropherotypes (Fig. 1). According to this classification, 19 different dsRNA profiles were observed among the soil isolates. A 5.5-kbp dsRNA was the most widespread element, being present in 13 isolates (Fig. 1). This molecule was observed alone in some isolates and together with other dsRNA elements in others, and the same occurred with other dsRNA elements (Fig. 1 and 2). These combinations of dsRNA elements suggested the existence of mixed virus infections.
Fig 2.
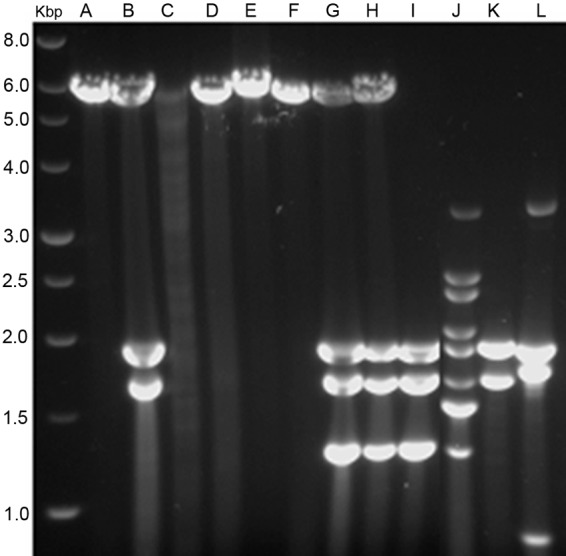
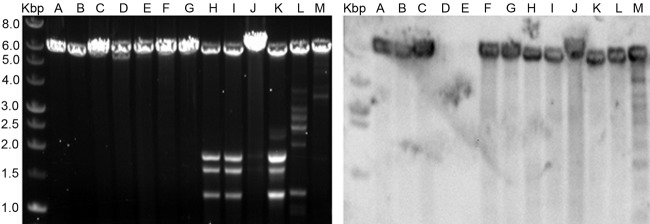
Electrophoretic profiles of dsRNA elements present in several isolates of B. bassiana. Lanes: A, isolate EABb 01/39-Su; B, EABb 01/75-Su; C, EABb 01/33-Su; D, EABb 01/132-Su; E, EABb 06/02-Su; F, EABb 06/03-Su; G, EABb 09/03-Su; H, EABb 09/08-Su; I, EABb 00/08-Su; J, EABb 00/11-Su; K, EABb 01/15-Su; L, EABb 01/35-Su. Lane Kbp contains molecular size markers, and the values on the left are sizes in kilobase pairs.
Surprisingly, the dsRNA patterns found among the endophytic B. bassiana isolates were not as variable as those from soils; only two different dsRNA profiles were found among them (Fig. 1, bottom right). Therefore, as deduced from the diversity of dsRNA electrophoretic profiles, virus diversity was greater in soil isolates (19 electropherotypes in 30 infected isolates) than in endophytic isolates (2 electropherotypes in 10 isolates) (Fig. 1).
In general, no concordance between similar dsRNA profiles and particular locations or habitats was found. For example, the 5.5-kbp dsRNA was found in isolates collected in different habitats in southern and central Spain and in the Canary Islands.
Nucleotide sequence and genome organization of a B. bassiana virus.
A 5.5-kbp dsRNA which was the most abundant element in the survey, occurring in 13 soil isolates, was completely sequenced. Forty-two different clones from a cDNA library were sequenced, and four contigs were obtained from their assembly. The gaps between the four contigs were resolved by using specific primers flanking the gaps. These experiments were repeated three times. Six identical clones of the 5′ end and four identical clones of the 3′ end from two independent RLM-RACE experiments of each terminus were sequenced.
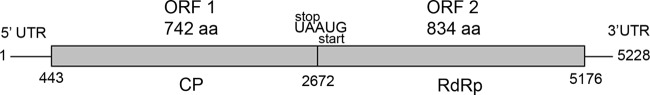
The complete sequence of the dsRNA element harbored by isolate EABb 06/02-Su (Fig. 2, lane E) is 5,228 bp in length and has a GC content of 55%. It contains two open reading frames (ORFs); ORF1 has a length of 2,229 bp and encodes a 742-amino-acid protein (78.41 kDa), and ORF2 is 2,505 bp long and encodes an 834-amino-acid protein (91.15 kDa) (Fig. 3). These two ORFs are separated by a pentanucleotide, UAAUG, that constitutes the stop codon of ORF1 (UAA) and the start codon (AUG) of ORF2, and the two codons overlap by one nucleotide. A sequence predicted to form a pseudoknot (bold and italic lowercase letters; estimated free energy, −14.02 kcal/mol) was detected 11 nucleotides upstream of the UAAUG pentanucleotide (underlined): AAUUGCCggugCUgccccaccCGGAgggcCGAACCCCGAGUAAUG. A similar pseudoknot in this position occurs in totiviruses of the Victorivirus genus, and is involved in the reinitiation of translation for ORF2 (24).
Fig 3.
Genome organization of BbRV1. The 5,228-bp genome contains two ORFs that overlap by one nucleotide. ORF1 encodes a putative CP, and ORF2 encodes a putative RdRp. aa, amino acids.
Other ORFs longer than 528 nucleotides were not found in any strand. The 5′ and 3′ UTRs were 443 and 52 bp long, respectively. The 5′ UTR starts with a GAATA sequence similar to a GAAAA motif that might be involved in RNA transcription in several S. cerevisiae viruses, including the totivirus ScV-L-A (39). The amino acid sequence deduced from ORF1 exhibits a high degree of identity to those of the capsid proteins (CP) of viruses of the family Totiviridae, particularly to that of Tolypocladium cylindrosporum virus 1 (TcV1; 59.7%). The C terminus of this putative CP has an Ala/Gly/Pro-rich region that is shared by viruses in the Victorivirus genus (13). The deduced amino acid sequence of ORF2 also resembled those of RNA-dependent RNA polymerases (RdRps) of viruses of the family Totiviridae, particularly that of Sphaeropsis sapinea RNA virus 1 (57.3% identity). The eight conserved motifs of the sequences of RdRps of dsRNA viruses of simple eukaryotes (2) were found in the amino acid sequence deduced from ORF2.
Phylogenetic analysis of BbRV1.
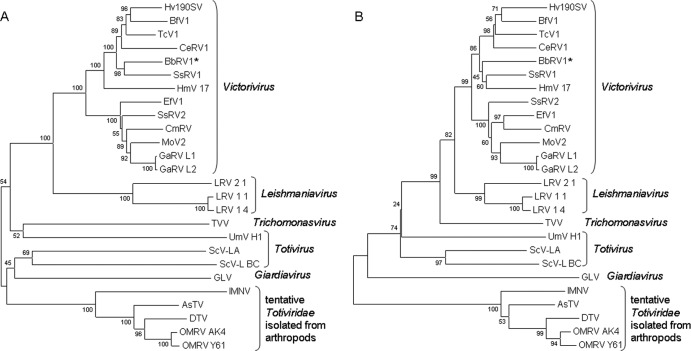
Phylogenetic analyses based on the complete amino acid sequence of the CP and RdRps of selected members of the Totiviridae family and those deduced from ORF1 and ORF2 of the 5.5-kbp dsRNA were done (see Table S1 in the supplemental material; Fig. 4). The two phylogenetic trees constructed revealed that the dsRNA from isolate EABb 06/02-Su most strongly resembles the genomes of viruses included in a clade within the genus Victorivirus. This genus is composed of viruses that infect filamentous fungi (13). Therefore, these phylogenetic analyses and other characteristics of this dsRNA mentioned, like having a nonsegmented dsRNA genome of 4.6 to 6.7 kbp that codes for a CP and an RdRp, having a Pro/Ala/Gly-rich region near the C terminus of the CP, a potential pseudoknot located upstream of the pentanucleotide that overlaps both ORFs, and 5′ and 3′ UTRs with sizes similar to those from the genus Victorivirus (13), indicated that this dsRNA element represents the complete genome of a new member of this genus, BbRV1. This is the first virus identified in B. bassiana.
Fig 4.
Phylogenetic analysis of BbRV1. Multiple alignments of amino acid sequences of the CP (A) and RdRp (B) of viruses of the family Totiviridae were performed. The unrooted neighbor-joining phylogenetic trees shown were made with MEGA software. The values at nodes are bootstrap values as percentages estimated by 1,000 replicates. The accession numbers of the sequences used in the analyses are given in Table S1 in the supplemental material.
Northern blotting experiments.
A Northern blot hybridization was done to check whether B. bassiana isolates sharing the 5.5-kbp dsRNA profile are infected with the same mycovirus. A 543-bp clone complementary to the 5′ UTR of the genome of BbRV1 was used as a probe. This probe represents an area not conserved among the members of the family Totiviridae, and its specificity for BbRV1 is likely to be high. Only 11 of the 13 isolates of B. bassiana harboring the 5.5-kbp dsRNA element hybridized with the BbRV1 probe (Fig. 5). Therefore, although isolates EABb 01/132-Su and EABb 01/39-Su harbor a single 5.5-kbp dsRNA element, they seem to be infected with a mycoviral species different from BbRV1.
Fig 5.
Northern blot hybridization of dsRNA elements of about 5.5 kbp present in several B. bassiana isolates using a probe complementary to the 5′ end of BbRV1. The left panel shows the electrophoretic profiles of 13 isolates, and the right panel shows the resulting hybridization with a chemiluminescent BbRV1 probe. The letters at the top indicate different isolates as follows: A, EABb 06/2-Su; B, EABb 06/03-Su; C, EABb 01/125-Su; D, EABb 01/132-Su; E, EABb 01/39-Su; F, EABb 01/73-Su; G, Bs1; H, EABb 09/03-Su; I, EABb 09/08-Su; J, EABb 01/75-Su; K, EABb 07/08-Su; L, EABb 09/04-Su; M, EABb 01/07-Su. Lanes Kbp contain molecular size markers, and the values on the left are sizes in kilobase pairs. The isolate used to clone and sequence BbRV1 is EABb 06/2-Su (lanes A), which was obtained in Fuerteventura, Canary Islands.
DISCUSSION
The results of the dsRNA analyses of our collection indicate that mycovirus infections are common among B. bassiana isolates from Spain and Portugal; 54.8% of the 73 isolates analyzed harbored dsRNAs with viral characteristics. The prevalence of virus-like dsRNA in soil isolates was lower (51.7%) than that observed in endophytic isolates, which was 66.7% (see below). In both cases, the prevalence values are higher than those reported in other surveys of viruses in soil or insect isolates of B. bassiana (3, 8, 27).
A dsRNA element with an electrophoretic profile of 5.5 kbp was the most common element detected in Beauveria isolates; it was present in 13 of the 73 isolates analyzed. In some isolates, this dsRNA element was found alone, and in others it was accompanied by other dsRNA elements (Fig. 2 and 5). The size of this dsRNA and the nucleotide sequences of the genes that encode it indicate that it is the genome of a new member of the Victorivirus genus (family Totiviridae), BbRV1. The 5,228-bp dsRNA genome of BbRV1 has characteristics of the Totiviridae family (13); it contains two ORFs that overlap by one nucleotide (UAAUG), ORF1 encodes a CP, and ORF2 encodes an RdRp (Fig. 3). Like other members of the Victorivirus genus, BbRV1 has a predicted RNA pseudoknot structure in close proximity to and upstream of the CP stop codon (24). This structure and the overlapping stop and start codons are involved in the coupled termination-reinitiation mechanism of translation that occurs in victoriviruses (24). In this type of translation, both ORFs are translated as independent proteins. In contrast, in totiviruses of other genera, like ScV-L-A, the RdRp is translated as a fusion protein with the CP (12).
Additionally, phylogenetic analysis grouped this virus within the Victorivirus genus (Fig. 4), and like other members of this genus, it has a Pro/Ala/Gly-rich region near the C terminus of the CP and 5′ and 3′ UTRs with sizes similar to those of other victoriviruses (13). Another victorivirus infecting an entomopathogenic fungus has been recently described, TcV1 (19). Totiviruses have hosts in three kingdoms; they infect fungi, insects, and protozoans (12, 22, 55); and it will be interesting to know if they can move from kingdom to kingdom. Insects and fungi are connected through entomopathogenic fungi, and further studies might reveal whether some viruses have jumped from fungal to insect hosts or vice versa. In addition, recent works show evidence of the integration of dsRNA viruses into fungal, insect, and other eukaryotic genomes (5, 25).
BbRV1 seems to be geographically widespread, isolates infected with a dsRNA similar in size and sequence (Fig. 5) were obtained in several provinces in southern and central Spain (Cádiz, Almería, Granada, Córdoba, Sevilla, Ciudad Real), as well as in the Canary Islands. In addition, the isolates infected with BbRV1 came from different habitats, like beaches, olive or eucalyptus groves, oak grasslands, meadows, or fallow land. The presence of BbRV1 in fungal isolates obtained at distant locations may be related to the population dynamics of B. bassiana. This fungus sporulates profusely in dead insect hosts, and its spores are dispersed by wind and rain but also by living infected hosts that may migrate long distances before dying (28). As a result of its abundant sporulation and efficient dispersion, B. bassiana seems to be a common component of the airborne mycobiota at different locations (1, 51). This abundance and heterogeneity of airborne propagules might explain why a clear geographical or habitat distribution of isolates harboring similar viral infections was not found in this analysis.
The existence of mixed virus infections could be deduced from the dsRNA profiles observed; in some isolates, one or several dsRNA elements similar in size occurred, but in others, those elements were accompanied by different dsRNAs. For example, the 5.5-kbp dsRNA which corresponds to the genome of BbRV1 was alone in some isolates and together with other dsRNAs in others (Fig. 2 and 5). Mixed mycovirus infections seem to be a common occurrence in several fungal species, including the entomopathogenic fungus T. cylindrosporum (14, 19).
The large number of different dsRNA profiles observed among all of the B. bassiana isolates analyzed suggests that there is an important diversity of mycoviruses associated with this entomopathogenic species (Fig. 1). The fact that dsRNAs similar in size found in different isolates may represent the genome of the same virus was supported by the hybridization of a DNA probe complementary to the 5′ end of BbRV1 to the dsRNA of 11 other isolates (Fig. 5). However, in the same experiment, 2 of the 13 isolates tested were not sequence homologous to BbRV1, which suggests that not all dsRNA elements with similar electrophoretic profiles correspond to the same viral species. Therefore, the diversity of viruses existing in the collection of isolates that we analyzed is likely to be greater than what can be estimated by using electrophoretic profiles of dsRNA elements.
Although partial or complete sequences of all of the dsRNA elements within a mycovirus family would be necessary for their correct classification, characteristics of the electrophoretic band patterns, like band number and estimated size, could be helpful for a hypothetical classification of Beauveria mycoviruses. Some sets of dsRNA elements found in several isolates could correspond to the multipartite genome of a single virus. For instance, some dsRNA profiles could correspond to members of the family Partitiviridae (bipartite genomes of 1.4 to 2.2 kbp) or Chrysoviridae (genomes composed of four segments of 2.4 to 3.6 kbp) (11, 46). Replication intermediates of members of the Barnaviridae family could also be harbored by some isolates (genomes formed by a linear ssRNA molecule of 4 kbp) (Fig. 1) (38). Other observed dsRNA elements did not show characteristics of known mycovirus families but could constitute members of new families, satellite RNAs, or defective derivatives of replication (14).
In contrast to the relatively high variation of dsRNA patterns found among soil isolates (0.33 dsRNA profile/isolate analyzed), only two different dsRNA profiles were found among the 15 endophytic isolates (0.13 dsRNA profile/isolate). Among the infected endophytic isolates, no relationship between their dsRNA profile and the location or species of the grass host was found. We do not know if the strains isolated from grasses as endophytes might represent a cryptic lineage within B. bassiana (37). This situation could explain the maintenance of a particular set of viruses in a group of grass endophytes. Whether certain strains of entomopathogens might be better endophytes than others or have different rates of survival inside plants has been questioned (53). Some evidence indicates that mycoviruses might affect the endophytic capability of fungal strains; in a study of virus-infected and virus-free strains of the entomopathogen T. cylindrosporum inoculated into tomato and bean leaves, the presence of the mycovirus TcV1 affected the performance of isogenic strains in the different host plants (18). A situation like this in B. bassiana could favor the maintenance of some particular viruses in strains that become endophytes of some particular hosts.
The different combinations of dsRNA elements found among isolates could be generated by different rates of virus transmission. For example, in the entomopathogen T. cylindrosporum, different rates of transmission of the three mycoviruses that infect it were observed (19). In such a situation, dsRNA profiles could hardly be used as markers for distinguishing between different isolates of B. bassiana, since the dsRNA profiles detected in this species could be unstable. Alternatively, equal rates of transmission of dsRNA elements to conidia have been observed in other ascomycetes with mixed virus infections (6, 41, 50). A study of transmission of dsRNAs to asexual spores carried out in our laboratory showed 100% transmission of 10 dsRNAs harbored by a B. bassiana isolate to its conidial progeny (data not shown), but this might not be the case for other dsRNA elements or combinations. Variation among isolates in dsRNA elements could also be generated by transmission among compatible isolates of B. bassiana by hyphal anastomoses, although the large number of vegetative compatibility groups existing in the species may limit this system of transmission (3).
In our laboratory cultures, we did not observe any obvious phenotype associated with virus-infected cultures of B. bassiana. A lack of obvious symptoms is common in virus-infected fungi, and it is interesting that one of the few known examples of a virus causing a disease in fungi occurs under conditions of commercial cultivation of Agaricus bisporus mushrooms (40). There is a certain parallel between this and recent views on plant virus systems suggesting that virulence might appear in plant viruses as a consequence of high host density and other outcomes of agriculture, while many neutral viruses might be occurring in wild plant species (43).
In conclusion, an analysis of B. bassiana isolates representative of different habitats and locations on the Iberian Peninsula and in the Balearic and Canary Islands revealed that the presence of fungal viruses is quite common in this species. Mixed virus infections occurred in several isolates. One of these viruses, the totivirus BbRV1, is the first virus in B. bassiana whose genome has been sequenced, and it was found in 36.6% of the dsRNA-infected soil isolates and at distant locations. Although the dsRNA electrophoretic profiles of infected isolates indicated relatively high virus diversity, hybridizations with a probe complementary to BbRV1 showed that dsRNA elements similar in size do not always have the same nucleotide sequence; therefore, virus species diversity should be higher than that estimated by dsRNA electropherotypes. The effects that fungal viruses produce in their Beauveria hosts were not evaluated in the present work, but given the high prevalence of virus infections observed, few antagonistic associations might be expected, as is the case with most known fungus-virus associations.
Supplementary Material
ACKNOWLEDGMENT
This research was financed by the Spanish Ministry of Science and Innovation, projects AGL2008-01159 and AGL2008-01137.
Footnotes
Published ahead of print 21 September 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Airaudi D, Marchisio VF. 1996. Fungal biodiversity in the air of Turin. Mycopathologia 136:95–102 [DOI] [PubMed] [Google Scholar]
- 2. Bruenn JA. 1993. A closely-related group of RNA-dependent RNA polymerases from double-stranded-RNA viruses. Nucleic Acids Res. 21:5667–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castrillo LA, Griggs MH, Vandenberg JD. 2004. Vegetative compatibility groups in indigenous and mass-released strains of the entomopathogenic fungus Beauveria bassiana: likelihood of recombination in the field. J. Invertebr. Pathol. 86:26–37 [DOI] [PubMed] [Google Scholar]
- 4. Chiba S, et al. 2009. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: molecular and biological characterization, taxonomic considerations, and potential for biological control. J. Virol. 83:12801–12812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiba S, et al. 2011. Widespread endogenization of genome sequences of non-retroviral RNA viruses into plant genomes. PLoS Pathog. 7:e1002146 doi:10.1371/journal.ppat.1002146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu YM, et al. 2004. Complexity of dsRNA mycovirus isolated from Fusarium graminearum. Virus Genes 28:135–143 [DOI] [PubMed] [Google Scholar]
- 7. Coutts RHA, Livieratos IC. 2003. A rapid method for sequencing the 5′ and 3′ termini of double-stranded RNA viral templates using RLM-RACE. J. Phytopathol. 15:525–527 [Google Scholar]
- 8. Dalzoto PR, et al. 2006. Horizontal transfer and hypovirulence associated with double-stranded RNA in Beauveria bassiana. Mycol. Res. 110:1475–1481 [DOI] [PubMed] [Google Scholar]
- 9. Drinnenberg IA, Fink GR, Bartel DP. 2011. Compatibility with killer explains the rise of RNAi-deficient fungi. Science 333:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faria MR, Wraight SP. 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 43:237–256 [Google Scholar]
- 11. Ghabrial SA. 2010. Chrysoviruses, p 500–509 In Mahy BWJ, Van Regenmortel MHV. (ed), Desk encyclopedia of plant and fungal virology Elsevier, Oxford, United Kingdom [Google Scholar]
- 12. Ghabrial SA. 2010. Totiviruses, p 565–576 In Mahy BWJ, Van Regenmortel MHV. (ed), Desk encyclopedia of plant and fungal virology Elsevier, Oxford, United Kingdom [Google Scholar]
- 13. Ghabrial SA, Nibert ML. 2009. Victorivirus, a new genus of fungal viruses in the family Totiviridae. Arch. Virol. 154:373–379 [DOI] [PubMed] [Google Scholar]
- 14. Ghabrial SA, Suzuki N. 2010. Fungal viruses, p 517–524 In Mahy BWJ, Van Regenmortel MHV. (ed), Desk encyclopedia of plant and fungal virology. Elsevier, Oxford, United Kingdom [Google Scholar]
- 15. Gillings MR, Tesoriero LA, Gunn LV. 1993. Detection of double-stranded RNA and virus-like particles in Australian isolates of Pythium irregulare. Plant Pathol. 42:6–15 [Google Scholar]
- 16. Goettel S, Eilenberg J, Glare T. 2005. Entomopathogenic fungi and their role in regulation of insect populations, p 361–406 In Gilbert LB, Latrou K. (ed), Comprehensive molecular insect science. Elsevier Pergamon, Oxford, United Kingdom [Google Scholar]
- 17. Herrero N, Sánchez Márquez S, Zabalgogeazcoa I. 2009. Mycoviruses are common among different species of endophytic fungi of grasses. Arch. Virol. 154:327–330 [DOI] [PubMed] [Google Scholar]
- 18. Herrero N, Sánchez Márquez S, Zabalgogeazcoa I. 12 August 2012. Mycovirus effect on the endophytic establishment of the entomopathogenic fungus Tolypocladium cylindrosporum in tomato and bean plants. BioControl (Epub ahead of print.) doi:10.1007/s10526-012-9476-9 [Google Scholar]
- 19. Herrero N, Zabalgogeazcoa I. 2011. Mycoviruses infecting the endophytic and entomopathogenic fungus Tolypocladium cylindrosporum. Virus Res. 160:409–413 [DOI] [PubMed] [Google Scholar]
- 20. Hillman BI, Suzuki N. 2004. Viruses of the chestnut blight fungus, Cryphonectria parasitica. Adv. Virus Res. 63:423–472 [DOI] [PubMed] [Google Scholar]
- 21. Howitt RLJ, Beever RE, Pearson MN, Forster RLS. 1995. Presence of double-stranded RNA and virus-like particles in Botrytis cinerea. Mycol. Res. 99:1472–1478 [Google Scholar]
- 22. Isawa H, et al. 2011. Identification and molecular characterization of a new nonsegmented double-stranded RNA virus isolated from Culex mosquitoes in Japan. Virus Res. 155:147–155 [DOI] [PubMed] [Google Scholar]
- 23. Kumar S, Tamura K, Nei M. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150–163 [DOI] [PubMed] [Google Scholar]
- 24. Li H, Havens WM, Nibert ML, Ghabrial SA. 2011. RNA sequence determinants of a coupled termination-reinitiation strategy for downstream open reading frame translation in Helminthosporium victoriae virus 190S and other victoriviruses (family Totiviridae). J. Virol. 85:7343–7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu H, et al. 2010. Widespread horizontal gene transfer from double-stranded RNA viruses to eukaryotic nuclear genomes. J. Virol. 84:11876–11887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. 2007. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315:513–515 [DOI] [PubMed] [Google Scholar]
- 27. Melzer MJ, Bidochka MJ. 1998. Diversity of double-stranded RNA viruses within populations of entomopathogenic fungi and potential implications for fungal growth and virulence. Mycologia 90:586–594 [Google Scholar]
- 28. Meyling NV, Eilenberg J. 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biol. Control 43:145–155 [Google Scholar]
- 29. Morris TJ, Dodds JA. 1979. Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology 69:854–858 [Google Scholar]
- 30. Ownley BH, Gwinn KD, Vega FE. 2010. Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. BioControl 55:113–128 [Google Scholar]
- 31. Park YJ, Chen XB, Punja ZK. 2006. Diversity, complexity and transmission of double-stranded RNA elements in Chalara elegans (synanam. Thielaviopsis basicola). Mycol. Res. 110:697–704 [DOI] [PubMed] [Google Scholar]
- 32. Pearson WR. 1990. Rapid and sensitive sequence comparison with FASTP and FASTA. Methods Enzymol. 183:63–98 [DOI] [PubMed] [Google Scholar]
- 33. Pearson MN, Beever RE, Boine B, Arthur K. 2009. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol. Plant Pathol. 10:115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quesada-Moraga E, Landa BB, Muñoz-Ledesma J, Jiménez-Díaz RM, Santiago-Álvarez C. 2006. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 161:323–329 [DOI] [PubMed] [Google Scholar]
- 35. Quesada-Moraga E, Maranhao EAA, Valverde-García P, Santiago-Álvarez C. 2006. Selection of Beauveria bassiana isolates for control of the whiteflies Bemisia tabaci and Trialeurodes vaporariorum on the basis of their virulence, thermal requirements and toxicogenic activity. Biol. Control 36:274–287 [Google Scholar]
- 36. Quesada-Moraga E, Navas-Cortés JA, Maranhao EAA, Ortiz-Urquiza A, Santiago-Álvarez C. 2007. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 111:947–966 [DOI] [PubMed] [Google Scholar]
- 37. Rehner SA, et al. 2011. A phylogeny and systematics of the anamorphic, entomopathogenic genus Beauveria. Mycologia 103:1055–1073 [DOI] [PubMed] [Google Scholar]
- 38. Revill PA. 2010. Barnaviruses, p 498–500 In Mahy BWJ, Van Regenmortel MHV. (ed), Desk encyclopedia of plant and fungal virology. Elsevier, Oxford, United Kingdom [Google Scholar]
- 39. Rodríguez-Cousiño N, et al. 2011. A new wine Saccharomyces cerevisiae killer toxin (Klus), encoded by a double-stranded RNA virus, with broad antifungal activity is evolutionarily related to a chromosomal host gene. Appl. Environ. Microbiol. 77:1822–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Romaine CP, Goodin MM. 2002. Unraveling the viral complex associated with La France disease of the cultivated mushroom, Agaricus bisporus, p 237–257 In Tavantzis SM. (ed), DsRNA genetic elements. Concepts and applications in agriculture, forestry, and medicine. CRC Press, Boca Raton, FL [Google Scholar]
- 41. Romo M, Leuchtmann A, Garcia B, Zabalgogeazcoa I. 2007. A totivirus infecting the mutualistic fungal endophyte Epichloë festucae. Virus Res. 124:38–43 [DOI] [PubMed] [Google Scholar]
- 42. Rong R, Rao SJ, Scott SW, Tainter FH. 2001. Common multiple dsRNAs are present in populations of the fungus Discula destructiva originating from widely separated geographic locations. Curr. Microbiol. 42:144–148 [DOI] [PubMed] [Google Scholar]
- 43. Roossinck MJ. 2010. Lifestyles of plant viruses. Philos. Trans. R. Soc. B Biol Sci. 365:1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sánchez S, Bills GF, Herrero N, Zabalgogeazcoa I. 2012. Nonsystemic fungal endophytes of grasses. Fungal Ecol. 5:289–297 [Google Scholar]
- 45. Sperschneider J, Datta A, Wise MJ. 2011. Heuristic RNA pseudoknot prediction including intramolecular kissing hairpins. RNA 17:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tavantzis S. 2010. Partitiviruses of fungi, p 547–552 In Mahy BWJ, Van Regenmortel MHV. (ed), Desk encyclopedia of plant and fungal virology. Elsevier, Oxford, United Kingdom [Google Scholar]
- 47. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The Clustal_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tooley PW, Hewings AD, Falkenstein KF. 1989. Detection of double-stranded RNA in Phytophthora infestans. Phytopathology 79:470–474 [Google Scholar]
- 49. Tsai PF, Pearson MN, Beever RE. 2004. Mycoviruses in Monilinia fructicola. Mycol. Res. 108:907–912 [DOI] [PubMed] [Google Scholar]
- 50. Tuomivirta TT, Hantula J. 2005. Three unrelated viruses occur in a single isolate of Gremmeniella abietina var. abietina type A. Virus Res. 110:31–39 [DOI] [PubMed] [Google Scholar]
- 51. Ulevičius V, Pečiulytė D, Lugauskas A, Andriejauskienė J. 2004. Field study on changes in viability of airborne fungal propagules exposed to UV radiation. Environ. Toxicol. 19:437–441 [DOI] [PubMed] [Google Scholar]
- 52. Vega FE, et al. 2008. Entomopathogenic fungal endophytes. Biol. Control. 46:72–82 [Google Scholar]
- 53. Vega FE, et al. 2009. Fungal entomopathogens: new insights on their ecology. Fung. Ecol. 2:149–159 [Google Scholar]
- 54. Voth PD, Mairura L, Lockhart BE, May G. 2006. Phylogeography of Ustilago maydis virus H1 in the USA and Mexico. J. Gen. Virol. 87(Pt 11):3433–3441 [DOI] [PubMed] [Google Scholar]
- 55. Wu Q, et al. 2010. Virus discovery by deep sequencing and assembly of virus-derived small silencing RNAs. Proc. Nat. Acad. Sci. U. S. A. 107:1606–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yu X, et al. 2010. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Nat. Acad. Sci. U. S. A. 107:8387–8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zabalgogeazcoa I, Benito EP, García Ciudad A, García Criado B, Eslava AP. 1998. Double-stranded RNA and virus-like particles in the grass endophyte Epichloë festucae. Mycol. Res. 102:914–918 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.