Abstract
Spores of wild-type and mutant Bacillus subtilis strains lacking various structural components were exposed to simulated Martian atmospheric and UV irradiation conditions. Spore survival and mutagenesis were strongly dependent on the functionality of all of the structural components, with small acid-soluble spore proteins, coat layers, and dipicolinic acid as key protectants.
TEXT
One major aspect of space biological research is the investigation of the responses of bacterial, viral, archaeal, and fungal species, as well as biomolecules, to simulated Martian conditions and their evaluation as potential forward contamination risks in the context of planetary protection (2, 4–7, 11, 12, 24, 30, 37–40, 44–46). For this reason, Mars environmental simulation experiments have been conducted to estimate (i) the survival rates of terrestrial microorganisms and (ii) the persistence of organic molecules on Mars (3, 4, 10, 15, 25, 29, 30, 37, 38, 45). Historically, several studies have explored the resistance of bacterial spores to simulated Martian conditions (8, 10, 12, 14, and references therein); these studies have concentrated mainly on the survival of spores of wild-type strains of various spore-forming species (10, 32, 38–40). More recent experiments have attempted to better understand the molecular factors causing spore resistance to environmental extremes, in which mainly spores of the model organism Bacillus subtilis that carry mutations affecting spore protective factors or spore DNA repair systems have been used (9, 18–23; reviewed in references 16, 28, 41, and 42). Spores of B. subtilis possess brownish pigmentation, thick layers of highly cross-linked coat proteins, a modified peptidoglycan spore cortex, a low core water content, and abundant intracellular constituents such as the calcium chelate of dipicolinic acid (Ca-DPA) and α/β-type small, acid-soluble spore proteins (SASP), all factors which have been previously found to contribute to spore resistance (reviewed in references 16, 28, 41, and 42). However, the possible roles of these factors in spore resistance to the extreme environmental conditions prevailing on the surface of Mars have not been explored, which is the subject of the present work.
The B. subtilis strains used in this study are listed in Table 1, and all of the strains used were congenic to their respective wild-type strains. Spores were obtained by cultivation under vigorous aeration in double-strength liquid Schaeffer sporulation medium (36) under identical conditions for each strain, purified, and stored as described previously (31). When appropriate, chloramphenicol (5 μg/ml), erythromycin (1 μg/ml), spectinomycin (100 μg/ml), or tetracycline (10 μg/ml) was added to the medium. Spore preparations consisted of single spores with no detectable clumps and were >99% free of growing cells, germinated spores, and cell debris, as seen in a phase-contrast microscope. Triplicate air-dried spore samples with a thickness of approximately 25 spore layers (for the 5 × 108 spore concentration) on presterilized spacecraft-qualified, chemfilm-treated aluminum 6061 coupons (13 mm in diameter by 1 mm thick) (23) were exposed to simulated Martian conditions in a cylindrical Mars simulation chamber (MSC) as described in detail previously (37). The simulated Mars environmental conditions listed in Table 2 represent a worst-case scenario for high UV flux and thus likely equate to an upper bound for UV effects on B. subtilis spore survival under simulated Martian conditions (38). Three types of spore exposure conditions were tested: (i) ambient laboratory conditions (22°C, 54% relative humidity, on the laboratory bench shielded from light), (ii) exposure in the MSC to simulate Mars conditions but shielded from radiation [designated Mars(−)UV], and (iii) exposure to the full suite of simulated Mars conditions, including 8 h of solar radiation [designated Mars(+)UV]. Further details of the design and geometry of the MSC, radiation dosimetry, and fluence calculations have been described in detail elsewhere by Schuerger et al. (38–40). Spore recovery from coupons was accomplished by stripping off spores with polyvinyl alcohol, and viability assays were performed as described previously (18, 19, 21, 22). The surviving fraction of B. subtilis spores was determined from the N/N0 ratio, with N being the number of CFU of the exposed sample and N0 that of the initial spore burden (directly determined after sample preparation). To determine frequencies of mutation to 4-azaleucine resistance (4-azaleur), aliquots of spores of each strain were taken from Mars(+)UV and Mars(−)UV exposure conditions, as well as laboratory control samples. Aliquots from serial dilutions were spread on Spizizen minimal medium plates supplemented with (final concentrations) tryptophan (50 μg/ml), spore germinants (either l-alanine [10 mM] or an equimolar mixture of DPA and CaCl2 [60 mM each] [1]), and 4-dl-azaleucine (100 μg/ml). Colonies that were 4-azaleur were counted 36 h after incubation at 37°C as described by Rivas-Castillo et al. (35). All data are expressed as averages ± standard deviations (n = 3). The significance of the differences in the survival rates and 4-azaleur mutation induction were determined by analysis of variance (ANOVA) using Analyze-it software (Analyze-it Software, Ltd., Leeds, United Kingdom). Values were evaluated in multigroup pairwise combinations, and differences with P values of ≤0.05 were considered statistically significant (18, 19, 22, 34, 43, 44).
Table 1.
B. subtilis strains used in this study
| Strain | Genotype and/or phenotypea | Missing spore component(s) | Source (reference) |
|---|---|---|---|
| 168 (WN131) | Wild-type parent of WN659 and WN661; trpC2 | None | Laboratory stock (4) |
| PS832 (WN552) | Wild-type parent of PS356, PS1899, FB72, and FB108; prototroph | None | P. Setlow (44) |
| PY79 (WN470) | Wild-type parent of AD17, AD28, and AD142; prototroph | None | A. Driks (34) |
| AD17 (WN496) | gerE36 | Inner coat | A. Driks (34) |
| AD28 (WN495) | cotE::cat Cmr | Outer coat | A. Driks (34) |
| AD142 (WN469) | gerE36 cotE::cat Cmr | Inner and outer coats | A. Driks (34) |
| PS356 (WN1273) | ΔsspA ΔsspB | SASP-α and SASP-β | P. Setlow (17) |
| PS1899 (WN1274) | dacB::cat Cmr | Core dehydration | P. Setlow (33) |
| FB72 (WN552) | ΔgerA::spc ΔgerB::cat ΔgerK::erm Spcr Cmr Ermr (referred to as ΔgerABK DPA+ spores) | Germination receptors | P. Setlow (44) |
| FB108 (WN553) | ΔgerA::spc ΔgerB::cat ΔgerK::erm ΔspoVF::tet Spcr Cmr Ermr Tetr (referred to as ΔgerABK DPA− spores) | Germination receptors, DPA | P. Setlow (44) |
| WN661 | trpC2, presumed mutation in cotAb | Pigmentation | W. L. Nicholson (unpublished data) |
Cmr, resistant to chloramphenicol (5 μg/ml); Ermr, resistant to erythromycin (1 μg/ml); Spcr, resistant to spectinomycin (100 μg/ml); Tetr, resistant to tetracycline (10 μg/ml).
Reference 13.
Table 2.
Environmental conditions used for Mars simulation experiments
| Parameter | Value |
|---|---|
| Avg pressure (kPa) ± SD | 0.69 ± 0.01 |
| Avg temp (°C) ± SD | −10 ± 2 |
| Avg relative humidity (%) ± SD | 8 ± 2 |
| UV-Vis-NIR radiationa fluence rate, kJ m−2 h−1 (total applied fluence)a | |
| Total UV (200–400 nm) | 142.9 (1.14 MJ m−2) |
| UV-C (200–280 nm) | 14.4 kJ (115 kJ m−2) |
| UV-B (280–320 nm) | 24.8 kJ (199 kJ m−2) |
| UV-A (320–400 nm) | 103.7 kJ (829 kJ m−2) |
| Vis (400–700 nm) | 864.0 kJ (6.91 MJ m−2) |
| NIR (700–1,100 nm) | 882.0 kJ (7.05 MJ m−2) |
| Total irradiance (200–1,100 nm) | 1,888.9 (15.1 MJ m−2) |
| Time (h) | 24b |
| % CO2 | 99.99 |
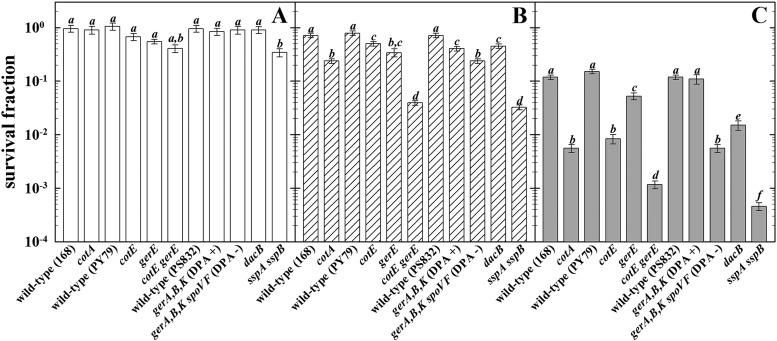
Wild-type and structural-component-deficient spores (Table 1) were exposed to simulated Mars environmental conditions for a 24-h period in the MSC with or without 8 h of exposure to simulated Martian solar radiation, including UV (200 to 400 nm) (Fig. 1). It was noted that a 24-h exposure to ambient lab conditions resulted in a 19% ± 6% average reduction in the survival of wild-type and all mutant spores, with the exception of spores lacking both SASP-α and SASP-β, which exhibited a statistically significant 72% ± 13% loss of spore viability (Fig. 1A). Exposure of spores for 24 h to simulated Mars(−)UV conditions in the MSC did not significantly reduce the viability of wild-type spores but caused a significant reduction in the viability of all of the mutant spores tested, most dramatically, sspA sspB mutant spores lacking the major α/β-type SASP and gerE cotE mutant spores lacking inner and outer spore coats (Fig. 1B). Exposure to the full suite of simulated Mars(+)UV conditions reduced the viability of wild-type spores by ∼1 order of magnitude, whereas spores of all of the mutant strains tested were significantly more sensitive than wild-type spores, again with spores lacking either α/β-type SASP or spore coats being the most sensitive (Fig. 1C).
Fig 1.
Survival of B. subtilis spores in response to simulated Martian environmental conditions, as determined by the ability to form macroscopic visible colonies. The strains used are indicated below the bars; the wild-type strain is listed to the left of the corresponding mutant strain. Spores were exposed as air-dried spore multilayers for 24 h to ambient laboratory conditions (white bars; panel A), Mars(−)UV conditions (hatched bars; panel B), or Mars(+)UV conditions (shaded bars; panel C). Data are expressed as averages and standard deviations (n = 3). The lowercase letters above the bars denote groups significantly different by ANOVA (P < 0.05).
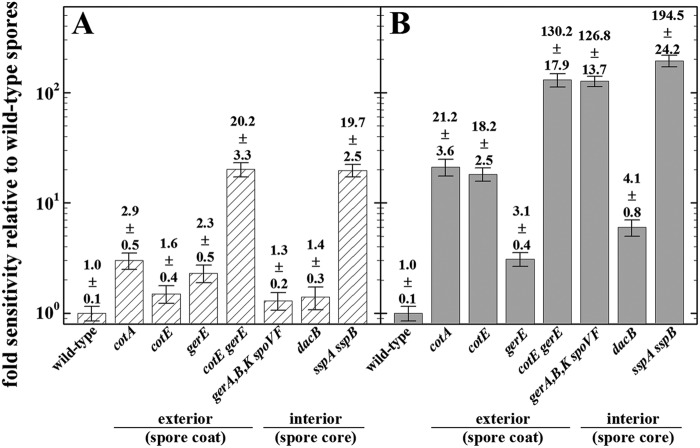
To directly compare the contributions of spore structural components to the resistance of spores to simulated Mars conditions, we calculated the relative increase in the sensitivity of each mutant strain with respect to that of the corresponding wild-type strain (Fig. 2). Ranked in order from most to least important with respect to Mars(−)UV treatment were the inner and outer spore coat (cotE gerE), α/β-type SASP (sspA sspB) >> the coat pigment (cotA), the inner coat (gerE) > the outer coat (cotE), core water content (dacB), DPA (dpaAB), and germination receptors (gerA gerB gerK) (Fig. 2A). Mars(−)UV conditions are dominated by low temperature (−10°C) and low pressure (∼7 mbar), resulting in an extremely desiccating environment. An intact spore coat and α/β-type SASP are the most important factors for dormant-spore survival under Mars(−)UV conditions, whereas DPA, pigmentation, and spore water content play less important roles. Under Mars(+)UV conditions, the spore structural components contributing to resistance, from most to least important, were α/β-type SASP (sspA sspB) > the inner and outer spore coats (cotE gerE), DPA (gerA gerB gerK spoVF) >> pigmentation (cotA), the outer spore coat (cotE) > core water content (dacB) > the inner coat (gerE) ≥ germination receptors (gerA gerB gerK) (Fig. 2B). Under Mars(+)UV conditions, UV radiation is a dominant factor in determining spore survival. In this context, it is not surprising that α/β-type SASP, the spore coat, and DPA make major contributions to spore resistance, as all of these factors have previously been shown to protect air-dried spores from polychromatic UV (21, 34, 43, 44). An intact outer coat, including the outer-coat-associated cotA-encoded pigment, was apparently more important to spore survival under Mars(+)UV conditions than was an intact inner coat (Fig. 2B).
Fig 2.
Impacts of the spore-specific structural attributes (exterior and interior) are displayed as relative sensitivities of spores lacking pigmentation, spore coat assembly, dipicolinic acid (DPA) formation, core dehydration, and α/β-type small, acid-soluble spore protein (SASP) formation to Mars(−)UV conditions (hatched bars; panel A) or Mars(+)UV conditions (shaded bars; panel B). Relative spore sensitivity was expressed as the ratio of the survival of each mutant strain with respect to the survival of the corresponding wild-type strain from the respective exposure to simulated Martian conditions (from Fig. 1B and C) to that of the ambient control (Fig. 1A). Data are averages and standard deviations (n = 3). The actual data values are shown above the corresponding columns (Fig. 1).
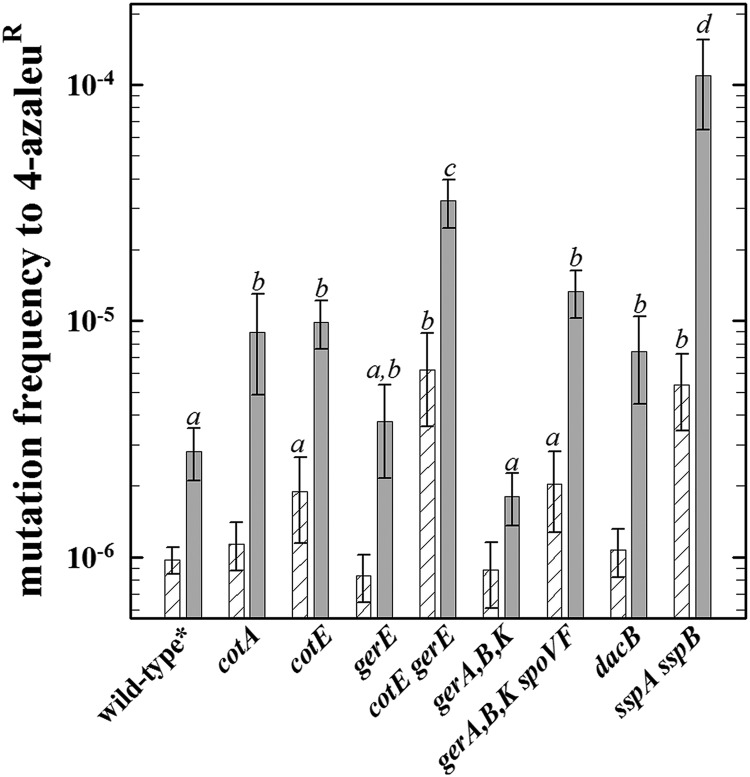
We also measured the frequency of mutation to 4-azaleur in spores exposed to Mars(−)UV or Mars(+)UV conditions (Fig. 3). The relative importance of spore factors for survival of Mars(−)UV exposure was as follows: intact spore coats, α/β-type SASP > an outer coat, DPA >> pigment, reduced core water >>> an inner coat ≥ germination receptors (Fig. 3). Under Mars(+)UV conditions, spore components exhibited importance in mutagenesis to 4-azaleur, in order from highest to lowest frequency, as follows: α/β-type SASP > intact spore coats > pigment, outer coat, DPA, reduced spore core water, and germination receptors (Fig. 3). Mutagenesis to 4-azaleur was strongly UV dependent, which is due mainly to the two ways for UV to exert its lethal effects, i.e., direct interaction with spore DNA and indirectly via the generation of reactive oxygen species, as shown previously (22, 35; reviewed in reference 12).
Fig 3.
Frequencies of mutation to 4-azaleur of spores of different strains exposed to the full suite of simulated Martian conditions shielded (white hatched bars) and including UV radiation (gray bars). Data are averages and standard deviations (n = 3). The lowercase letters above the bars denote groups significantly different by ANOVA (P < 0.05). The spontaneous frequency of mutation to 4-azaleur of the wild-type strain (i.e., the mean spontaneous 4-azaleur mutation frequencies of strains 168, PY79, and PS832 ± the standard deviation) was (9.7 ± 3.6) × 10−7, in good agreement with previous data (35).
On the basis of the above data, it thus appears that α/β-type SASP and intact spore coat layers are the most important factors in determining spore survival and protection from mutagenic damage under simulated Mars conditions either with or without UV exposure. Spore pigmentation, the outer or inner spore coat layers by themselves, reduced spore water content, and DPA play less important roles in spore survival of Mars(−)UV exposure but do play significant roles in spore resistance to Mars(+)UV exposure. Clearly, the data indicate that spore DNA is a major target of both lethal and mutagenic damage, on the basis of the observation that all spore factors contributed significantly to both spore survival (Fig. 2) and the frequency of mutation to 4-azaleur (Fig. 3) in response to the full suite of simulated Mars environmental conditions, including UV. The findings in this communication complement the results of microarray experiments demonstrating the upregulation of DNA repair pathways during the germination of spores exposed to simulated Mars surface conditions (27). Future goals of our work are to (i) compile a complete catalog of all types of cellular damage incurred by spores exposed to simulated Martian conditions; (ii) elucidate the potential additive effects of the sensitive spore phenotypes, such as SASP, core water content, and DPA, with a special focus on the complex interaction of all known spore coat proteins (as reviewed in reference 16 and references therein); and (iii) elucidate the roles of various DNA repair systems in the survival of Mars-exposed spores in order to gain a deeper understanding of the physiological responses used by spore-forming bacteria to cope with the environmental challenges of simulated extraterrestrial environments.
ACKNOWLEDGMENTS
We thank Krystal Kerney and Andrea Rivas-Castillo for their technical assistance during parts of this work and Adam Driks and Peter Setlow for their generous donations of B. subtilis strains. We thank the three anonymous reviewers for insightful comments.
This work was supported in part by grants from the NASA Planetary Protection (NNA06CB58G) and Astrobiology: Exobiology and Evolutionary Biology (NNX08AO15G) programs to W.L.N. and A.C.S. R.M. and G.R. were supported by DLR grant DLR-FuE-Projekt ISS Nutzung in der Biodiagnostik, Programm RF-FuW, Teilprogramm 475.
Footnotes
Published ahead of print 12 October 2012
REFERENCES
- 1. Atluri S, Ragkousi K, Cortezzo DE, Setlow P. 2006. Cooperativity between different nutrient receptors in germination of spores of Bacillus subtilis and reduction of this cooperativity by alterations in the GerB receptor. J. Bacteriol. 188:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berry BJ, Jenkins DG, Schuerger AC. 2010. Effects of simulated Mars conditions on the survival and growth of Escherichia coli and Serratia liquefaciens. Appl. Environ. Microbiol. 76:2377–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bruckner JC, Osman S, Conley C, Venkateswaran K. 2008. Space microbiology: planetary protection, burden, diversity and significance of spacecraft associated microbes, p 52–65 In Schaechter M. (ed), Encyclopedia of microbiology. Elsevier, Oxford, United Kingdom [Google Scholar]
- 4. Fajardo-Cavazos P, Schuerger AC, Nicholson WL. 2008. Persistence of biomarker ATP and ATP-generating capability in bacterial cells and spores contaminating spacecraft materials under Earth conditions and in a simulated Martian environment. Appl. Environ. Microbiol. 74:5159–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fekete A, Kovács G, Hegedüs M, Módos K, Lammer H. 2008. Biological responses to the simulated Martian UV radiation of bacteriophages and isolated DNA. J. Photochem. Photobiol. B 92:110–116 [DOI] [PubMed] [Google Scholar]
- 6. Fendrihan S, et al. 2009. Investigating the effects of simulated Martian ultraviolet radiation on Halococcus dombrowskii and other extremely halophilic archaebacteria. Astrobiology 9:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Foster TL, Winans L, Jr, Casey RC, Kirschner LE. 1978. Response of terrestrial microorganisms to a simulated Martian environment. Appl. Environ. Microbiol. 35:730–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fulton JD. 1958. Survival of terrestrial microorganisms under simulated Martian conditions, p 606–613 In Benson OO, Jr, Strughold H. (ed), Proceedings of Physics and Medicine of the Atmosphere and Space, San Antonio, Texas John Wiley & Sons, New York, NY [Google Scholar]
- 9. Granger AC, Gaidamakova EK, Matrosova VY, Daly MJ, Setlow P. 2011. Effects of Mn and Fe Levels on Bacillus subtilis spore resistance and effects of Mn2+, other divalent cations, orthophosphate, and dipicolinic acid on protein resistance to ionizing radiation. Appl. Environ. Microbiol. 77:32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagen CA, Ehrlich R, Hawrylewicz E. 1964. Survival of microorganisms in simulated Martian environment. I. Bacillus subtilis var. globigii. Appl. Microbiol. 12:215–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hawrylewicz E, Gowdy B, Ehrlich R. 1962. Microorganisms under a simulated Martian environment. Nature 193:497 [Google Scholar]
- 12. Horneck G, Klaus DM, Mancinelli RL. 2010. Space microbiology. Microbiol. Mol. Biol. Rev. 74:121–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hullo MF, Moszer I, Danchin A, Martin-Verstraete I. 2001. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 183:5426–5430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen LL, et al. 2008. A facility for long-term Mars simulation experiments: the Mars environmental simulation chamber (MESCH). Astrobiology 8:537–548 [DOI] [PubMed] [Google Scholar]
- 15. Kerney KR, Schuerger AC. 2011. Survival of Bacillus subtilis endospores on ultraviolet-irradiated rover wheels and Mars regolith under simulated Martian conditions. Astrobiology 11:477–485 [DOI] [PubMed] [Google Scholar]
- 16. Leggett MJ, McDonnell G, Denyer SP, Setlow P, Maillard JY. 2012. Bacterial spore structures and their protective role in biocide resistance. J. Appl. Microbiol. 113:485–498 [DOI] [PubMed] [Google Scholar]
- 17. Mason JM, Setlow P. 1986. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J. Bacteriol. 167:174–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moeller R, et al. 2007. Role of DNA repair by nonhomologous-end joining in Bacillus subtilis spore resistance to extreme dryness, mono- and polychromatic UV, and ionizing radiation. J. Bacteriol. 189:3306–3311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moeller R, et al. 2008. Roles of the major, small, acid-soluble spore proteins and spore-specific and universal DNA repair mechanisms in resistance of Bacillus subtilis spores to ionizing radiation from X rays and high-energy charged-particle bombardment. J. Bacteriol. 190:1134–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moeller R, Rohde M, Reitz G. 2010. Effects of ionizing radiation on the survival of bacterial spores in artificial Martian regolith. Icarus 206:783–786 [Google Scholar]
- 21. Moeller R, Setlow P, Reitz G, Nicholson WL. 2009. Roles of small, acid-soluble spore proteins and core water content in survival of Bacillus subtilis spores exposed to environmental solar UV radiation. Appl. Environ. Microbiol. 75:5202–5208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moeller R, et al. 2011. Role of the Nfo and ExoA apurinic/apyrimidinic endonucleases in radiation resistance and radiation-induced mutagenesis of Bacillus subtilis spores. J. Bacteriol. 193:2875–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moeller R, Schuerger AC, Reitz G, Nicholson WL. 2011. Impact of two DNA repair pathways, homologous recombination and non-homologous end joining, on bacterial spore inactivation under simulated Martian environmental conditions. Icarus 215:204–210 [Google Scholar]
- 24. Morozova D, Möhlmann D, Wagner D. 2007. Survival of methanogenic archaea from Siberian permafrost under simulated Martian thermal conditions. Orig. Life Evol. Biosph. 37:189–200 [DOI] [PubMed] [Google Scholar]
- 25. Newcombe DA, et al. 2005. Survival of spacecraft-associated microorganisms under simulated Martian UV irradiation. Appl. Environ. Microbiol. 71:8147–8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nicholson WL, et al. 2002. Bacterial endospores and their significance in stress resistance. Antonie Van Leeuwenhoek 81:27–32 [DOI] [PubMed] [Google Scholar]
- 27. Nicholson WL, Moeller R, the PROTECT Team, Horneck G. 2012. Transcriptomic responses of germinating Bacillus subtilis spores exposed to 1.5 years of space and simulated Martian conditions on the EXPOSE-E experiment PROTECT. Astrobiology 12:469–486 [DOI] [PubMed] [Google Scholar]
- 28. Nicholson WL, Munakata N, Horneck G, Melosh HJ, Setlow P. 2000. Resistance of bacterial endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nicholson WL, Schuerger AC. 2005. Bacillus subtilis spore survival and expression of germination-induced bioluminescence after prolonged incubation under simulated Mars atmospheric pressure and composition: implications for planetary protection and lithopanspermia. Astrobiology 5:536–544 [DOI] [PubMed] [Google Scholar]
- 30. Nicholson WL, Schuerger AC, Race MS. 2009. Migrating microbes and planetary protection. Trends Microbiol. 17:389–392 [DOI] [PubMed] [Google Scholar]
- 31. Nicholson WL, Setlow P. 1990. Sporulation, germination, and outgrowth, p 391–450 In Harwood CR, Cutting SM. (ed), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England [Google Scholar]
- 32. Osman S, et al. 2008. Effect of shadowing on survival of bacteria under conditions simulating the Martian atmosphere and UV radiation. Appl. Environ. Microbiol. 74:959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Popham DL, Sengupta S, Setlow P. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl. Environ. Microbiol. 61:3633–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riesenman PJ, Nicholson WL. 2000. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 66:620–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivas-Castillo AM, Yasbin RE, Robleto E, Nicholson WL, Pedraza-Reyes M. 2010. Role of the Y-family DNA polymerases YqjH and YqjW in protecting sporulating Bacillus subtilis cells from DNA damage. Curr. Microbiol. 60:263–267 [DOI] [PubMed] [Google Scholar]
- 36. Schaeffer P, Millet J, Aubert J-P. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. U. S. A. 54:704–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuerger AC, et al. 2008. Slow degradation of ATP in simulated Martian environments suggests long residence times for the biosignature molecule on spacecraft surfaces on Mars. Icarus 194:86–100 [Google Scholar]
- 38. Schuerger AC, Mancinelli RL, Kern RG, Rothschild LJ, McKay CP. 2003. Survival of endospores of Bacillus subtilis on spacecraft surfaces under simulated Martian environments: implications for the forward contamination of Mars. Icarus 165:253–276 [DOI] [PubMed] [Google Scholar]
- 39. Schuerger AC, Nicholson WL. 2006. Interactive effects of hypobaria, low temperature, and CO2 atmospheres inhibit the growth of mesophilic Bacillus spp. under simulated Martian conditions. Icarus 185:143–152 [Google Scholar]
- 40. Schuerger AC, Richards JT, Hintze PE, Kern RG. 2005. Surface characteristics of spacecraft components affect the aggregation of microorganisms and may lead to different survival rates of bacteria on Mars landers. Astrobiology 5:545–559 [DOI] [PubMed] [Google Scholar]
- 41. Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525 [DOI] [PubMed] [Google Scholar]
- 42. Setlow P. 2007. I will survive: DNA protection in bacterial spores. Trends Microbiol. 15:172–180 [DOI] [PubMed] [Google Scholar]
- 43. Slieman TA, Nicholson WL. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane dimers in Bacillus subtilis spore DNA. Appl. Environ. Microbiol. 66:199–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slieman TA, Nicholson WL. 2001. Role of dipicolinic acid in survival of Bacillus subtilis spores exposed to artificial and solar UV radiation. Appl. Environ. Microbiol. 67:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stoker CR, Bullock MA. 1997. Organic degradation under simulated Martian conditions. J. Geophys. Res. 102:10881–10888 [DOI] [PubMed] [Google Scholar]
- 46. Tauscher C, Schuerger AC, Nicholson WL. 2006. Survival and germinability of Bacillus subtilis spores exposed to simulated Mars solar radiation: implications for life detection and planetary protection. Astrobiology 6:592–605 [DOI] [PubMed] [Google Scholar]
- 47. ten Kate IL, et al. 2003. Investigating complex organic compounds in a simulated Mars environment. Int. J. Astrobiol. 1:387–399 [Google Scholar]