Abstract
Sortases are a class of enzymes that anchor surface proteins to the cell wall of Gram-positive bacteria. Lactobacillus casei BL23 harbors four sortase genes, two belonging to class A (srtA1 and srtA2) and two belonging to class C (srtC1 and srtC2). Class C sortases were clustered with genes encoding their putative substrates that were homologous to the SpaEFG and SpaCBA proteins that encode mucus adhesive pili in Lactobacillus rhamnosus GG. Twenty-three genes encoding putative sortase substrates were identified in the L. casei BL23 genome with unknown (35%), enzymatic (30%), or adhesion-related (35%) functions. Strains disrupted in srtA1, srtA2, srtC1, and srtC2 and an srtA1 srtA2 double mutant were constructed. The transcription of all four sortase encoding genes was detected, but only the mutation of srtA1 resulted in a decrease in bacterial surface hydrophobicity. The β-N-acetyl-glucosaminidase and cell wall proteinase activities of whole cells diminished in the srtA1 mutant and, to a greater extent, in the srtA1 srtA2 double mutant. Cell wall anchoring of the staphylococcal NucA reporter protein fused to a cell wall sorting sequence was also affected in the srtA mutants, and the percentages of adhesion to Caco-2 and HT-29 intestinal epithelial cells were reduced for the srtA1 srtA2 strain. Mutations in srtC1 or srtC2 result in an undetectable phenotype. Together, these results suggest that SrtA1 is the housekeeping sortase in L. casei BL23 and SrtA2 would carry out redundant or complementary functions that become evident when SrtA1 activity is absent.
INTRODUCTION
In Gram-positive bacteria, many surface proteins are covalently anchored to the cell wall envelope by a membrane-bound thiol-transpeptidase named sortase, which was first identified in Staphylococcus aureus about a decade ago (24). This enzyme, named SrtA for surface protein sorting A, recognizes the conserved carboxylic sorting motif LPXTG of surface proteins (22), cleaves between threonine and glycine and catalyzes the formation of an amide bond between the C-terminal carboxyl group of threonine and an amino group of peptide cross bridges within cell wall peptidoglycan (37). Not all the sortase substrates contain a sequence matching the canonical LPXTG motif, and species-specific variation has been described for this sequence (2).
Sortases can be grouped based on their sequence homology and distinct functions into six (A to F) classes (36). The members of class A, also called housekeeping sortases, are usually encoded in a single copy per genome and are ubiquitously expressed and responsible for the anchoring of the majority of LPXTG proteins. In the other five classes, multiple copies per genome may exist and, in many cases, the genes are positioned in the same transcriptional units as their putative substrates. This is the case for class B sortases, which anchor proteins involved in heme-iron acquisition, class C sortases, which have been shown to participate in the elaboration of pili on the bacterial surface (14), and class D sortases, involved in the spore formation in bacilli. In some high-G+C-content bacteria, class E enzymes (recognizing the LAXTG sorting signal instead of LPXTG) function as the housekeeping sortases instead of class A sortases. Finally, the function of class F sortases present in Actinobacteria is unknown (36).
Bacterial surface proteins play key roles in bacterial-host interactions. The number of LPXTG proteins encoded per genome varies between species, ranging from 1 to more than 40 (2). While the role of sortase enzymes and their substrates is well documented in pathogens (10, 22), only a few reports have examined their functions in other bacteria, such as commensal bacteria in the digestive tract, where secreted and surface-exposed components are important in the establishment of interactions with the host and in probiotic traits (17, 19, 34). The amount of research carried out on sortases and their substrates is still limited in Lactobacillus, one of the most relevant probiotic genera of lactic acid bacteria (LAB). The role of some LPXTG proteins (especially those carrying mucus binding domains) in adhesion to host surfaces has been studied in Lactobacillus acidophilus (5), Lactobacillus plantarum (13, 31), Lactobacillus rhamnosus (42), and Lactobacillus salivarius (39). In lactobacilli, mutant strains in sortase-encoding genes have only been obtained in L. plantarum (4, 13), L. salivarius (39), and Lactobacillus johnsonii (7), and they have been assayed for mucosal adhesion and colonization in several in vitro and in vivo models. Recent results showed that, in addition to intestinal epithelial cell binding (39), the presence of an active sortase gene is required in L. salivarius strain UCC118 to increase the expression of mucin genes in these cells (28), evidencing its role in the cross talk with the host.
Lactobacillus casei is a natural inhabitant of the gastrointestinal tract, and the strain L. casei BL23 has been widely used for genetic, physiological, and biochemical studies (23). We thus decided in this study to search for the presence of sortase gene homologues and sortase-dependent protein-encoding genes into the genome of L. casei BL23. Functional characterization of targeted gene deletion mutants was used to examine the role in adhesion of the sortase proteins.
MATERIALS AND METHODS
Strains and growth conditions.
Lactobacillus casei strains (Table 1) were grown in MRS broth (BD Difco, Le Pont de Claix, France) at 37°C, without agitation. Escherichia coli DH5α was used as a cloning host and was grown in LB medium at 37°C, with vigorous agitation (200 rpm). When needed, antibiotics were used. Ampicillin was used at 100 μg/ml for E. coli, and erythromycin and chloramphenicol were added at 5 μg/ml when used separately and at 2.5 μg/ml each when used together for L. casei. Solid medium was prepared by adding 1.8% agar. Bacterial growth curves were assayed in microtiter plates (200 μl MRS broth per well) at 37°C in a Polarstar Omega plate reader (BMG Labtech, Offenburg, Germany).
Table 1.
Strains and plasmids used in this studya
| Strain or plasmid | Relevant characteristic or description | Source or reference |
|---|---|---|
| Strains | ||
| BL23 | Wild type, genome sequenced | CECT5275 |
| CECT4040 | Cheese isolate | CECT |
| ATCC 25598 | Sour milk isolate | ATCC |
| CECT4043 | Cheese isolate | CECT |
| ATCC 11578 | Oral cavity isolate | ATCC |
| ATCC 334 | Cheese isolate, genome sequenced | ATCC |
| ATCC 4545 | Dental caries isolate | ATCC |
| BL101 | Isolated from commercial probiotic drink | Laboratory stock |
| BL106 | Isolated from commercial probiotic drink | Laboratory stock |
| BL193 | Isolated from commercial probiotic drink | Laboratory stock |
| BL208 | Human intestinal isolate | Laboratory stock |
| BL227 | Commercial probiotic | Laboratory stock |
| BL341 | BL23 srtA1::pRV300 Eryr | This work |
| BL342 | BL23 srtA2::pRV300 Eryr | This work |
| BL343 | BL23 srtC1::pRV300 Eryr | This work |
| BL344 | BL23 srtC2::pRV300 Eryr | This work |
| BL347 | BL23 srtA1::pUCm1 Cmr | This work |
| BL348 | BL23 srtA2::pUCm1 Cmr | This work |
| BL346 | BL23 ΔsrtA2 | This work |
| BL345 | BL23 ΔsrtA2 srtA1::pUCm1 Cmr | This work |
| Plasmids | ||
| pRV300 | SspI-restricted pBluescript SK joined to a 1,130-bp SmaI fragment of pVE6023; Ampr Eryr | (20) |
| pUCm1 | pUC19 derivative carrying the chloramphenicol marker of pC194 at the SmaI site; Ampr Cmr | (25) |
| pNUC-CWA | ColE1 and pAMβ1 origins; P59::spUsp45::nucA::cwaM6D105::t1t2; Ampr Eryr | (9) |
CECT, Colección Española de Cultivos Tipo; ATCC, American Type Culture Collection; Eryr, erythromycin resistance; Cmr, chloramphenicol resistance.
Homology searches.
The genome of L. casei BL23 (23) was screened with pfsearch (pftools package, Swiss Institute for Experimental Cancer Research [ISREC]; http://www.isrec.isb-sib.ch/ftp-server/pftools/pft2.3) using the hidden Markov models from the Pfam database, pf04203 (sortase) and pf00746 (Gram-positive anchor). Homology searches in Lactobacillus genomes were carried out with BLAST at the NCBI genome database. The following strains of the Lactobacillus casei/Lactobacillus paracasei and Lactobacillus rhamnosus group were screened: BL23, ATCC 334, ATCC 25302, Zhang, 8700:2, LC2W, BD-II, GG (ATCC 53103), ATCC 8530, ATCC 21052, HN001, CASL, LMS2-1, Lc705, R001, and MTCC5462.
Construction of L. casei BL23 mutants in srt genes.
Internal DNA fragments ranging from 352 to 385 bp were PCR amplified from the sortase genes srtA1, srtA2, srtC1, and srtC2 (LCABL_23200, LCABL_06160, LCABL_25390, and LCABL_05230, respectively) using primers listed in Table 2. PCRs were performed using L. casei BL23 genomic DNA as a template and Platinum Pfx DNA polymerase (Invitrogen). The fragments were cloned into the integrative vector pRV300 (20) and treated with SmaI, allowing blunt-end cloning. The resulting plasmids, pRVsrtA1, pRVsrtA2, pRVsrtC1, and pRVsrtC2, were transformed by electroporation into the BL23 strain by using a Gene-Pulser (Bio-Rad) as described previously (30) and transformants were selected in solid media by erythromycin. Integration at the correct loci and disruption were checked by Southern blotting on HindIII-digested genomic DNA for srtA1 and srtC2, AccI-digested genomic DNA for srtA2, and KpnI-digested genomic DNA for srtC1. Southern blotting was also performed on genomic DNA from different L. casei strains (Table 1) digested with HindIII. The DNA inserts from pRVsrtA1, pRVsrtA2, pRVsrtC1, and pRVsrtC2, labeled with digoxigenin (DIG) with the PCR DIG-labeling mix (Roche), were used as probes. Hybridization and detection were performed in Hybond-N membranes (GE Healthcare) by using alkaline phosphatase-conjugated anti-DIG and the CDP-Star chemiluminescent reagent as recommended by the manufacturer (Roche). The srtA2 gene was deleted by a double-crossover strategy. The 5′- and 3′-end-flanking regions of the srtA2 gene (LCABL_06160) were amplified using the primer pairs A2_PRE_FOR and A2_PRE_REV and A2_POST_FOR and A2_POST_REV. The amplicons were joined by splicing by overlap extension (SOE)-PCR using the primer pair A2_PRE_FOR and A2_POST_REV. The newly generated 1.9-kb amplicon was digested using SacI and HindIII and cloned into pRV300, digested with the same enzymes. The integrity of the transformants was verified by PCR using pRV300 forward and reverse primers, and after sequencing, plasmid integrants in L. casei BL23 were constructed as described above. Erythromycin-resistant colonies were tested to confirm upstream or downstream integration to srtA2 by PCR using primer pairs SRTA2_REV and pRV300_REV and SRTA2_FOR and pRV300_FOR, respectively. Plasmid integrants where single recombination occurred upstream or downstream were selected and cultured at 37°C without antibiotic selection for at least 200 generations. Colonies were grown on MRS plates and screened for an Erys phenotype by replica plating on MRS plates with 5 μg/ml of erythromycin. The occurrence of a double-crossover event in Erys strains was confirmed by PCR using the primer pair A2_PRE_FOR and A2_POST_REV. The absence of srtA2 (LCABL_06160) was also verified by a negative amplification using the primer pair SRTA2_FOR and SRTA2_REV. Mutants in srtA1 and srtA2 were constructed carrying a chloramphenicol resistance marker in order to allow transformation with the pNUC-CWA plasmid (see below for anchoring of staphylococcal nuclease in srt mutants of L. casei BL23). For this purpose, blunt-ended amplicons of the internal fragments of the genes srtA1 and srtA2 were cloned into pUCm1 (25), digested with HincII. The obtained plasmids pUCsrtA1 and pUCsrtA2 were used to obtain the disruption mutants srtA1::pUCm1 and srtA2::pUCm1 and the ΔsrtA2 srtA1::pUCm1 double mutant. Integration at the correct loci and disruption were checked by Southern blotting on HindIII-digested genomic DNA for srtA1 and AccI-digested genomic DNA for srtA2.
Table 2.
Oligonucleotide primers used in this study
| Primer | Sequencea | Use |
|---|---|---|
| SRTA1_FOR | 5′-GGGTCAAAGCGCTTGATATC-3′ | Amplification of a 352-bp internal fragment of the srtA1 gene |
| SRTA1_REV | 5′-TCATCAATCCACTGAACTTG-3′ | |
| SRTA2_FOR | 5′-CTAAGGCTATGACTAATGATTC-3′ | Amplification of a 355-bp internal fragment of the srtA2 gene |
| SRTA2_REV | 5′-CAGGTAACTAAGGTTAACAAC-3′ | |
| SRTC1_FOR | 5′-GCAGGCTTATGTTAAAAAGC-3′ | Amplification of a 385-bp internal fragment of the srtC1 gene |
| SRTC1_REV | 5′-ATGCGAGTTGATCATGTATG-3′ | |
| SRTC2_FOR | 5′-CAAACGCAATCAGCAAATCTT-3′ | Amplification of a 385-bp internal fragment of the srtC2 gene |
| SRTC2_REV | 5′-AATCATATAAGGCGTGCAGGT-3′ | |
| A2_PRE_FOR | 5′-TTTTGAGCTCGGCTTGGATGATCTAGG G-3′ | Amplification of a 959-bp 5′-end-flanking region of the srtA2 gene/SOE-PCR with the A2_POST_REV primer |
| A2_PRE_REV | 5′-CCTTATCTAAGGGAACGAGCGTTCTACTGCTATGACG-3′ | |
| A2_POST_FOR | 5′-CGTCATAGCAGTAGAACGCTCGTTCCC TTAGATAAGG-3′ | Amplification of a 991-bp 3′-end-flanking region of the srtA2 gene/SOE-PCR with the A2_PRE_FOR primer |
| A2_POST_REV | 5′-TTTTAAGCTTCAAGATTGACACCGATCAAC-3′ |
Restriction sites introduced for cloning are underlined.
Transcriptional analysis.
Total RNA was isolated from 10 ml of exponentially growing L. casei BL23, BL341, and BL342 cells (optical density at 550 nm [OD550] of 0.9) using the TRIzol reagent as recommended by the supplier (Invitrogen). The RNA was treated with RNase-free DNase I (Turbo DNA-free kit; Ambion), and 2 μg was used to synthesize cDNA with the Superscript VILO cDNA synthesis kit as recommended by the manufacturer (Invitrogen). RNA (60 ng), cDNA (5 ng), and chromosomal DNA (100 ng) samples were used to amplify fragments from srtA1, srtA2, srtC1, and srtC2 with the oligonucleotide pairs SRTA1_FOR/SRTA1_REV, SRTA2_FOR/SRTA2_REV, SRTC1_FOR/SRTC1_REV, and SRTC2_FOR/SRTC2_REV, respectively, and the reaction products were separated in 1.5% agarose gels stained with ethidium bromide. The cDNA samples were also used for quantitative PCR (qPCR) analysis using the same oligonucleotide pairs and a LightCycler 480 (Roche) with the LC Fast Start DNA Master SYBR green I mix (Roche). The reaction mixture (10 μl) contained 5 μl of 2× master mix, 0.5 μl of each primer (10 μM), and 5 ng of cDNA. Reaction mixtures without a template or containing RNA samples were run as controls. The cycling conditions were as follows: 95°C for 10 min, followed by 45 cycles of three steps consisting of denaturing at 95°C for 10 s, primer annealing at 60°C for 20 s, and primer extension at 72°C for 30 s. For each set of primers, the cycle threshold values (crossing point [CP]) were determined by the automated method implemented in the LightCycler software 4.0 (Roche). The pyrG gene was selected as a reference gene based on previous studies (18). The relative expression was calculated using REST (relative expression software tool) (29). Linearity and amplification efficiency were determined for each primer pair. Every quantitative reverse transcription-PCR (qRT-PCR) was performed in triplicate with at least three biological independent samples.
MATS test.
A microbial adhesion to solvents (MATS) test was performed essentially as described previously (1). Five ml of overnight cultures of each strain was washed with phosphate-buffered saline (PBS; pH 7.2) and resuspended in PBS to a final OD600 of 0.4 (A0). Three milliliters of this suspension was mixed with 1 ml of different solvents (chloroform, ethyl acetate, or hexadecane), and the mixture was vortexed for 1 min at full speed. The mixture was left to stand for 20 min to allow phase separation, and the absorbance of the aqueous phase was measured at 600 nm (A1). The percentage of adhesion was calculated as follows: % adhesion = 100 × [1 − (A1/A0)]. Each experiment was done in triplicate with cells coming from independent cultures.
Adhesion to Caco-2 and HT-29 epithelial cell lines.
Epithelial cells were seeded at 4 × 104 cells/cm2 (Caco-2) or 2 × 105 cells/cm2 (HT-29) in 24-well plates in Dulbecco modified Eagle medium (DMEM) (with Glutamax, 25 mM glucose; Gibco) supplemented with 1% (vol/vol) nonessential amino acids solution (Gibco), 1% (vol/vol) sodium pyruvate solution (Gibco), 1% (vol/vol) sodium bicarbonate solution (only for HT-29 cells; Gibco), 1% (vol/vol) of antibiotics (100 U per ml penicillin, 100 μg/ml streptomycin; Gibco), and 10% (vol/vol) fetal calf serum and incubated at 37°C in a CO2 incubator. After the cells reached confluence (incubation for 6 and 3 days for Caco-2 and HT-29, respectively), plates were incubated for an additional 15 (Caco-2) or 21 (HT-29) days to allow cell differentiation and the medium was changed every 2 days. L. casei cells were grown overnight, washed two times with PBS, and stained with 75 μM carboxyfluorescein diacetate (CFDA) in the absence of light for 30 min. Subsequently, they were washed two times with PBS, and stained bacterial cells were added to each well in 0.5 ml of PBS adjusted to an OD550 of 0.2 (108 CFU/ml), equivalent to a bacterial cell/epithelial cell ratio of 100 (Caco-2) and 50 (HT-29). The plates were incubated for 1 h at 37°C in the absence of light. Aliquots of the stained cells were maintained in tubes under the same conditions and further treated in the same conditions. Nonadhered bacteria were removed by washing 3 times with 1 ml of PBS, and the bacteria were detached by covering the monolayer with 200 μl of a 15% (vol/vol) solution of trypsin-EDTA (Gibco) in PBS, followed by the addition of 300 μl of culture medium to stop the trypsin reaction. The different fractions (input and output) were transferred to black microtiter plates, and the fluorescence was measured in a Fluoroskan Ascent plate reader (Thermo Scientific) set at a 485-nm excitation wavelength and a 538-nm emission wavelength. The experiments were performed in triplicate with bacteria derived from independent cultures. Adhesion was expressed as a percentage of fluorescence recovered relative to that of the input.
Determination of enzymatic activities.
The N-acetylglucosaminidase assay was carried out in a 250-μl volume containing 10 mM potassium phosphate (pH 6.8), 1 mM MgCl2, 5 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide (Sigma), and cells (OD550 of 6) at 37°C. The reaction was stopped with 250 μl of 5% Na2CO3, and the OD420 was measured. The cell envelope proteinase (PrtP) activity was determined using β-casein as a substrate. L. casei BL23 and the sortase mutants were grown overnight in MRS plates, collected from the surface with PBS, and washed in the same buffer. Preliminary studies showed that L. casei BL23 cells grown on plates displayed a higher PrtP activity than cells grown in liquid medium. The cells were adjusted to an OD550 of 3 in 100 mM MES (morpholineethanesulfonic acid) buffer (pH 7) supplemented with 5 mM CaCl2. The cell suspension was mixed with the substrate β-casein (4 mg/ml) (Sigma), dissolved in the same buffer at a 3:1 (bacterial cells/substrate) volume ratio. After overnight incubation at 37°C, the cells were pelleted by centrifugation and 10-μl aliquots were analyzed by SDS-PAGE.
Anchoring of staphylococcal nuclease in srt mutants of L. casei BL23.
L. casei BL23 and the srtA1 (BL347), srtA2 (BL348), and srtA1 srtA2 (BL345) mutants were transformed with pNUC-CWA, a plasmid which carries a cassette containing the strong constitutive lactococcal promoter P59, the signal peptide (SP) of the lactococcal Usp45 (SPUsp45) protein, and the staphylococcal nuclease A gene (nucA) fused to the sequence encoding the cell wall anchor motif of the Streptococcus pyogenes M6 protein, followed by two transcriptional terminators from the E. coli rrnB operon (9). Strains harboring pNUC-CWA were grown in 10 ml of MRS to mid-exponential phase (OD550 of 0.8 to 0.9; 5.8 × 108 to 6.6 × 108 CFU/ml), and the media were retained as the secreted fraction. The cells were washed two times with PBS and resuspended in 50 mM Tris-HCl (pH 8). The cell pellets were then disrupted with glass beads (0.1 mm) in a Mini-Beadbeater (BioSpec Products, Bartlesville, OK) with four cycles of 30 s at maximal speed, and unbroken cells were discarded by centrifuging the supernatant at 6,000 × g for 5 min. The supernatant was recovered and centrifuged under the same conditions for two additional times. Subsequently, it was centrifuged at 22,000 × g for 20 min at 4°C. The pellet was washed three times at 22,000 × g for 15 min with 50 mM Tris-HCl (pH 8) plus 0.5 M NaCl and retained as the cell envelope fraction (cell wall/membrane fragments). Samples of the different fractions were separated on 15% SDS-PAGE gels (0.5 μg proteins from cell envelope fractions, 0.5 μl of conditioned media), and the proteins were electrotransferred to Hybond-ECL membranes (GE Healthcare). NucA was detected with a rabbit anti-NucA serum (1:5,000) and the ECL Advance Western blotting detection kit (GE Healthcare).
Statistical analysis.
A Student's t test was employed to investigate statistical differences with the PRISM 4.0 software (GraphPad Software, San Diego, CA). Samples with P values of <0.05 were considered statistically different.
RESULTS
The L. casei BL23 genome encodes four sortase genes.
Four sortase-encoding genes were detected in the L. casei BL23 genome: two genes encoding sortases belonging to class A (LCABL_23200 and LCABL_06160, named hereinafter srtA1 and srtA2, respectively) and two encoding sortases belonging to class C (LCABL_25390 and LCABL_05230, named srtC1 and srtC2, respectively). Orthologues to SrtA1 and SrtC1 were identified in 16 sequenced genomes of the Lactobacillus casei/Lactobacillus paracasei and Lactobacillus rhamnosus group by BLAST search (7 and 9 strains, respectively) (Table 3). On the contrary, SrtC2 was present in all L. casei/L. paracasei strains and in only two L. rhamnosus strains (GG and LMS2-1). srtA2 homologues were only found in L. casei BD-II (LCBD_0623) and L. casei LC2W (LC2W_0620). Also, in L. casei ATCC 334, a srtA2 homologue was a pseudogene (LSEI_0555) and, in L. rhamnosus Lc705, a SrtA2 homologue was plasmid encoded (pLC705_00020). Identities in proteins were 99 to 100% for L. casei/L. paracasei, whereas identities between L. casei/L. paracasei and L. rhamnosus were from 78 to 99%. Southern blot analysis of a collection of nonsequenced L. casei/L. paracasei strains from different origins confirmed that copies of srtA1, srtC1, and srtC2 were present in all strains, whereas srtA2 was detected in only two strains (Table 3). srtA1 is probably the housekeeping sortase. On the other hand, the fact that srtA2 is only present in few strains (BL23, ATCC 334, BD-II, LC2W, BL101, and BL106) and that it is plasmid encoded in Lc705, makes its role intriguing.
Table 3.
Presence of srt and spa genes in L. casei/L. paracasei and L. rhamnosus strains
| Strain | Presence (+) or absence (−) ofb: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| srtA1 | srtA2 | srtC1 | spaF | spaE | spaD | srtC2 | spaC | spaB | spaA | |
| L. casei/L. paracasei | ||||||||||
| BL23 | + | + | + | + | + | + | + | + | + | + |
| ATCC 334 | + | +p | + | + | +p | + | + | + | + | + |
| Zhang | + | − | + | + | +p | + | + | + | + | + |
| 8700:2 | + | − | + | + | + | + | + | + | + | + |
| ATCC 25302 | + | − | + | + | + | + | + | + | + | + |
| LC2W | + | + | + | + | + | + | + | +p | + | + |
| BD-II | + | + | + | +p | + | + | + | +p | + | + |
| CECT4040a | + | − | + | + | ||||||
| ATCC 25598a | + | − | + | + | ||||||
| CECT4043a | + | − | + | + | ||||||
| ATCC 11578a | + | − | + | + | ||||||
| ATCC 4545a | + | − | + | + | ||||||
| BL101a | + | + | + | + | ||||||
| BL106a | + | + | + | + | ||||||
| BL193a | + | − | + | + | ||||||
| BL208a | + | − | + | + | ||||||
| BL227a | + | − | + | + | ||||||
| L. rhamnosus | ||||||||||
| GG (ATCC 53103) | + | − | + | + | + | + | + | + | + | + |
| HN001 | + | − | + | + | + | + | − | − | − | − |
| CASL | + | − | + | + | + | + | − | − | − | − |
| LMS2-1 | + | − | + | + | + | + | + | + | + | + |
| ATCC 21052 | + | − | + | + | + | + | − | − | − | − |
| Lc705 | + | + | + | + | + | + | − | − | − | − |
| R001 | + | − | + | + | + | + | − | − | − | − |
| ATCC 8530 | + | − | + | + | + | + | − | − | − | − |
| MTCC5462 | + | − | +p | +p | +p | +p | − | − | − | − |
The genomes of these strains have not been sequenced and the presence of srtA1, srtA2, srtC1, and srtC2 was assessed by Southern blotting on genomic DNA using specific probes derived from the BL23 strain.
+p, presence of a pseudogene. BLAST E values for the predicted peptides were in all cases lower than 1e−164.
Putative sortase substrates in L. casei BL23.
Twenty-three putative sortase substrates carrying the Gram-positive anchor domain (pf00746) were encoded in the L. casei BL23 genome (Table 4). From these 23 proteins, four did not present an N-terminal signal peptide for secretion as predicted by SignalP (27). However, these last proteins were probably encoded by pseudogenes with deletions or frameshifts at the 5′ end. As an example, the protein encoded by LCABL_28490 could be fused to the product of the upstream LCABL_28500 gene, which contains a signal peptide. The resulting peptide was homologous to the product of the L. casei ATCC 334 LSEI_2660 gene (Table 4). Eleven of these putative substrates contained the canonical LPXTG sequence, whereas the rest contained target sequences that deviated from it with one or more mismatches (Table 4). From the substrates identified, 35% did not have any predicted function, 30% were predicted to have an enzymatic function, and 35% were predicted to have a surface adhesin/binding function.
Table 4.
Putative sortase substrates identified in the L. casei BL23 genome
| Gene locus | Anchoring domain sequencea | SPb | Putative function/characteristic | Presence in L. casei ATCC 334 |
|---|---|---|---|---|
| LCABL_02860 | LPKTAEASGWELMLAGLATIFGVISIVFFWRQHRMAV | + | β-N-acetylglucosaminidase; glycosyl hydrolase family 20 | LSEI_0291 |
| LCABL_03600 | LPRTGEREGIEASLWGGLIVAISTLLGILGIDRKRKQN | − | β-fructosidase; five Big-3 domains, Ig-like domain present in extracellular proteins | |
| LCABL_05200 | LPHTGGQGYQRLLGIALGLISAAFLLLLVVLIKRRVVKQHD | + | L. rhamnosus GG SpaC homologue; von Willebrand type A and CnaB domains | LSEI_0455 |
| LCABL_05210 | LPQTGDTVAAWLSTLGLIIFATVLAFNMKKQKIDN | + | L. rhamnosus GG SpaB homologue | LSEI_0456 |
| LCABL_05220 | LPHTGGTGTVIFAILGVALIAFGAVAYRKRRNGF | + | L. rhamnosus GG SpaA homologue; CnaB domain | LSEI_0456 |
| LCABL_05320 | LPPMGMQNSHWLQALGIALLGMVFALSIGLTSKKKHEKN | + | Cell envelope-associated proteinase PrtR | Truncated LSEI_0465 |
| LCABL_05330 | LPQMANSHRNALQILGVIIISLMTTLGIVVTDKKKREKNKVNS | + | Cell envelope-associated proteinase PrtR | Truncated LSEI_0468 |
| LCABL_06690 | APQTPWLWIIIAIILALVILILLWLIWRQRRKRRETDETKQA | + | DUF 916, bacterial protein of unknown function | LSEI_0606 |
| LCABL_06700 | LPQTGNAVQLWYVVIGVELLIIVILGIVLLRGRSRQGGKK | + | Hypothetical protein | LSEI_0607 |
| LCABL_06740 | LPQTSEGGQNWYPLIGFALLMMSSLRLQLRLKRKDRGDDDENRD | + | Hypothetical protein | LSEI_615 |
| LCABL_12630 | KPSSHWWYWLIGIAILLLLALVAWLFYLLGKRRREQDENEEDDR | − | Homologue to LCABL_06690; DUF 916, bacterial protein of unknown function | |
| LCABL_12640 | LPATSEGVTLTAFMLGLMLTFFSLGGLYASRRISK | + | Hypothetical protein | |
| LCABL_21260 | LPETGNRVFEKGGIVGFLMLLATLGLGMVQKMRSKRF | + | SCP-like extracellular protein domain | LSEI_1905 |
| LCABL_24520 | LPKTAETTERPAFGFLGVIVVSLMGVLGLKRKQREE | + | Cell envelope-associated proteinase PrtP | LSEI_2270 |
| LCABL_25040 | LPNTGDNQRTSLIAIGVALLLALISFGSFGLRRREK | + | Homologue to L. rhamnosus GG mucus binding factor (MBF); 3 MucBP domains | LSEI_2320 |
| LCABL_25400 | LPMTGGMGLLAFLLIGIVLMSGGYYVKKQTGKKA | + | L. rhamnosus GG SpaD homologue; CnaB domain | LSEI_2363 |
| LCABL_25410 | LPAMSDWQNLSLVLIGVGLLTLATYFLIKHKKARNHP | + | L. rhamnosus GG SpaE homologue | LSEI_2364 |
| LCABL_25420 | LPKTGGNGIVLFLLMALVAGTSGLLLAIVLKRKEAR | + | L. rhamnosus GG SpaF homologue; CnaB domain | Truncated LSEI_2365/66 |
| LCABL_26030 | LPDTGERVLGWLAIAIGSLLSITGVLLLIKDYQ | + | CnaA and CnaB domains | LSEI_2431 |
| LCABL_26070 | YPATGESQAGTILAEAGAVVIAVLGLAGVRKYRHAK | + | Surface protein with SSASSAA and SSASSAG repetitions; only present in the L. casei/L. rhamnosus group | Truncated LSEI_2436/37 |
| LCABL_28490 | FPKTGEMMMNSLPIIGLVALIVCGFGVIGWRKYVANK | − | Zinc metalloproteinase C | LSEI_2660 |
| LCABL_28750 | MPQTGDKVIQWLSLAGVGMLLLIGGLMIWRQRREQ | + | GAG domain, family 31 of glycosyl hydrolases; secreted bacterial lyase enzymes capable of acting on glycosaminoglycans | Truncated LSEI_2686/87 |
| LCABL_31150 | LPQTGDTSANDLSIVGLILTSIASLFGLAGARNKKRSE | − | Adhesion exoprotein | LSEI_2896 |
The C-terminal regions of the proteins are shown. LPXTG consensus motifs are in bold, transmembrane domains predicted using the TMHMM server (www.cbs.dtu.dk/services/TMHMM/) are underlined, and cationic residues following the transmembrane domain are in bold.
Signal peptides were predicted using SignalP 4.0 (www.cbs.dtu.dk/services/SignalP/) (27). +, present; −, absent.
L. casei BL23 encodes surface-anchored glycosidases that may be involved in the adaptation of this microorganism to exploit the carbohydrate resources present in the gastrointestinal niche. LCABL_02860 is predicted to encode a β-N-acetylglucosaminidase enzyme of the glycosyl hydrolase family 20 (GH20). These enzymes are involved in the removal of terminal β-glycosidically linked N-acetylhexoamine residues and participate in many important physiological and pathological processes (15). They hydrolyze β-1,6 linkages in poly-β-(1,6)-N-acetylglucosamine, a major component of extracellular matrix polysaccharides, and may act on the liberation of N-acetylhexoamine residues from the highly glycosylated mucins from intestinal mucus or from human milk oligosaccharides (12). LCABL_28750 encodes a glycosyl hydrolase of the GH31 family, putatively acting in the hydrolysis of glycosaminoglycans, such as hyaluronan and chondroitin, from the extracellular matrix. A third glycosidase, encoded by LCABL_03600 (fosE), comprises a truncated (493 amino acids) β-fructosidase. Other genes encoding enzymatic functions are predicted to code for three cell envelope-associated proteinases (CEP). In BL23, two are paralogous genes in tandem encoding PrtR proteases (LCABL_05320 and LCABL_05330) that share a 43% sequence identity and one is a PrtP encoding gene (LCABL_24520). The composition of domains varied, but all contain the catalytic serine protease domain showing sequence homology to the active site of subtilases.
At least five proteins among the identified putative sortase substrates contain domains (CnaA and CnaB) present in the S. aureus CNA collagen adhesin (LCABL_05200 and LCABL_05220 in the srtC2 cluster, LCABL_25400 and LCABL_25420 in the srtC1 cluster, and LCABL_26030).
In contrast to other lactobacilli from intestinal origin (3), the presence of proteins with tandem multiple mucin binding domains (MucBP) involved in mucus attachment is not a characteristic of L. casei. The product of LCABL_25040, another sortase substrate, represents the only exception. Although it does not show homology to mucus binding proteins such as Msa or Mub from L. plantarum and Lactobacillus reuteri (21, 31), it harbors three MucBP domains. The product of LCABL_25040 shares a 38% identity with the mucus binding factor (MBF) protein encoded by the L. rhamnosus GG LGG_02337 gene (42).
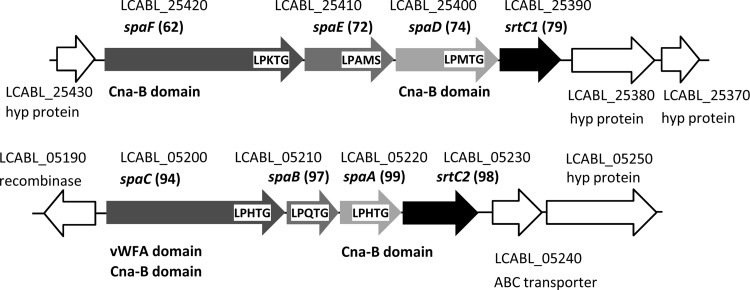
In L. casei BL23, srtC1 and srtC2 appear to cluster with the genes encoding their putative substrates, as typically seen for class C sortases (Fig. 1) (36). This idea was reinforced by the fact that, in these substrates, a glycine residue follows the LPXTG motif (Table 4), which is characteristic for substrates being processed by this class of sortases. Interestingly, srtC1 and srtC2 clusters contained genes homologous to spaCBA and spaFED from L. rhamnosus GG, respectively, which encode pilin subunits responsible for adhesive pilus synthesis (16). The pilin subunits of L. rhamnosus GG SpaB, SpaC, and SpaF, which are 97%, 94%, and 62% identical to the proteins encoded by LCABL_05210, LCABL_05200, and LCABL_25420, respectively, bind to intestinal mucus, and SpaC has been directly linked to the mucus and epithelial cell binding ability of L. rhamnosus GG cells (16, 41, 43). In all sequenced L. casei/L. paracasei and L. rhamnosus strains carrying srtC1 and srtC2, these genes were always linked to spaFED and spaCBA clusters, respectively (Table 3).
Fig 1.
Genomic context of the L. casei BL23 class C sortases srtC1 and srtC2. The locus tags are indicated along with some relevant characteristics, such as known homologues, conserved domains, and sortase cleavage motif sequences, if present. Numbers in parentheses correspond to the percentage of amino acid identities to the L. rhamnosus GG homologue products. hyp, hypothetical; vWFA, von Willebrand factor A.
Transcriptional analysis and construction of L. casei BL23 sortase mutants.
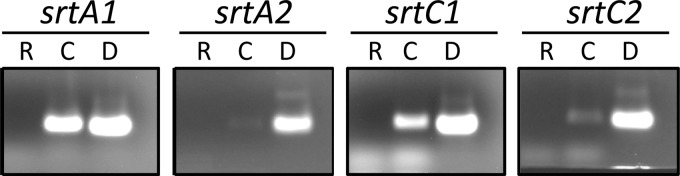
Expression of all four sortase genes was detected in L. casei BL23 by RT-PCR (Fig. 2), but qPCR analysis showed that, compared to srtA1, BL23 expressed 2,633 ± 500-fold lower levels of srtA2 mRNA. Expression of srtC1 and srtC2 was also 59.8 ± 6.1- and 53.5 ± 5.6-fold lower than expression of srtA1, respectively. Subsequently, with the aim of analyzing the contribution of the sortases to protein anchoring and bacterial adhesion, single mutants in all sortase encoding genes were constructed by inserting nonreplicative plasmids carrying internal fragments of the genes (Table 1). A double mutant in srtA1 and srtA2 (strain BL345) was also constructed by deleting the srtA2 gene, followed by insertional inactivation of srtA1. The mutant strains were assessed for their growth in MRS medium, showing no changes in growth rates compared to the parental BL23 strain (data not shown). A qPCR analysis also showed that the deletion of either srtA1 or srtA2 did not result in altered expression of the remaining sortase genes (relative fold changes ranging from 1.2- to 1.6-fold; data not shown).
Fig 2.
Analysis of the expression of srtA1, srtA2, srtC1, and srtC2 genes in L. casei. PCR was performed with RNA samples (lanes R) or cDNA samples (lanes C) or with chromosomal DNA from L. casei BL23 as a control (lanes D).
Cell surface characteristics of srt mutants.
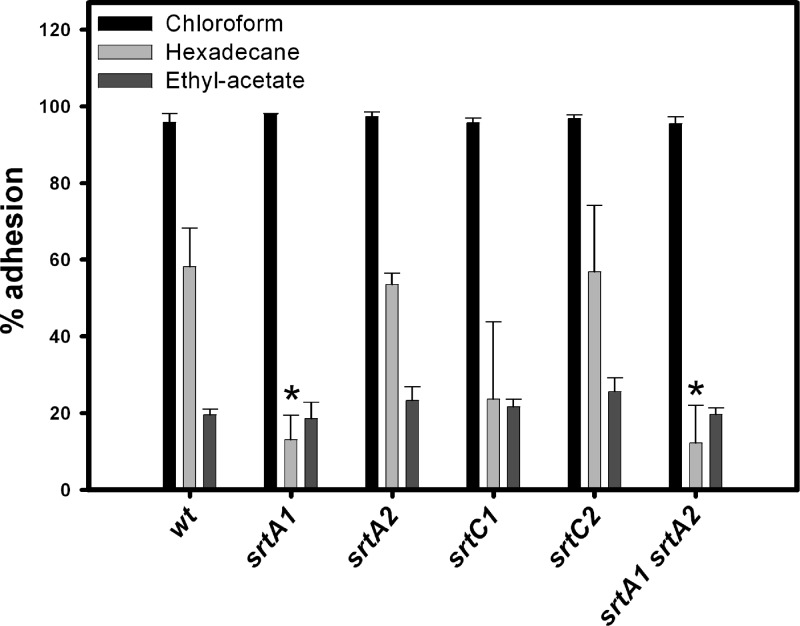
The net hydrophobicity and the surface charge are among the most commonly studied physicochemical properties of the bacterial surface. They determine, to a large extent, the nonspecific interactions with the environment. We used the microbial adhesion to solvents (MATS) test to measure surface characteristics in L. casei strains by measuring affinity to chloroform (acidic solvent and electron acceptor), ethyl acetate (basic solvent and electron donor), and hexadecane (hydrophobic solvent) (Fig. 3) (1). The high adhesion to the acidic solvent chloroform (above 95%) and the low adhesion to the basic solvent ethyl acetate (around 21%) observed with all strains confirmed the nonacidic character of L. casei BL23 (26). The percentages of adherence to the apolar solvent hexadecane varied among strains. The L. casei BL23 wild type was highly hydrophobic (58% adhesion to hexadecane), and three mutant strains, srtA1, srtC1, and the double mutant srtA1 srtA2, experienced reductions in hexadecane adhesion of 77%, 59%, and 79%, respectively, although only changes in srtA1 and the double mutant strains were significant (P < 0.05). This suggests that the surface hydrophobicity of L. casei BL23 is influenced by proteins anchored by SrtA1-dependent mechanisms.
Fig 3.
Cell surface characteristics of the L. casei wild type (wt; BL23 strain) and the sortase mutants (BL341, BL342, BL343, BL344, and BL345 strains) measured by the MATS test. Results are the means from three experiments, and the bars represent standard deviations. An asterisk indicates a statistically significant difference compared to the wild type (P < 0.05).
Adhesion properties of srt mutants to intestinal epithelial cells.
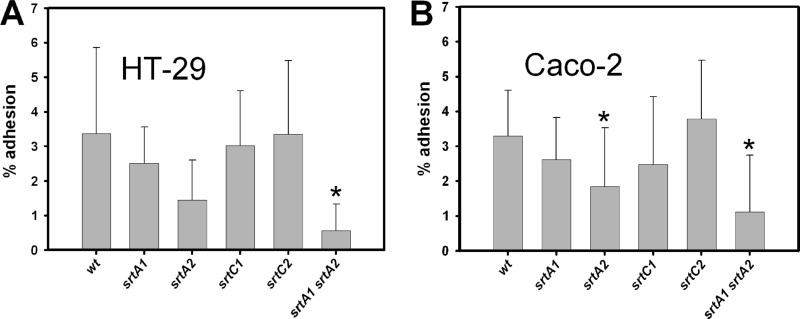
We measured the adhesion of the different strains to cultured intestinal epithelial cell lines. The resulting adhesion profiles were quite similar for the two cell lines employed (Fig. 4). A significant decrease of 83% in adhesion to the HT-29 cell line was observed in the srtA1 srtA2 double mutant strain compared to the parental strain BL23 (P < 0.05) (Fig. 4A). Significant changes were also detected in the binding ability to Caco-2, with decreases of 44% for the srtA2 mutant (P < 0.05) and 66% for the srtA1 srtA2 double mutant (P < 0.001) compared to the wild-type strain (Fig. 4B).
Fig 4.
Binding of wild-type L. casei and sortase mutants to epithelial HT-29 (A) and Caco-2 (B) cell lines. wt, wild type (BL23 strain). Results are the means from at least three independent experiments made in triplicate, and the bars represent standard deviations. An asterisk indicates a statistically significant difference compared to the wild type (P < 0.05).
Quantification of surface-anchored protein reporters.
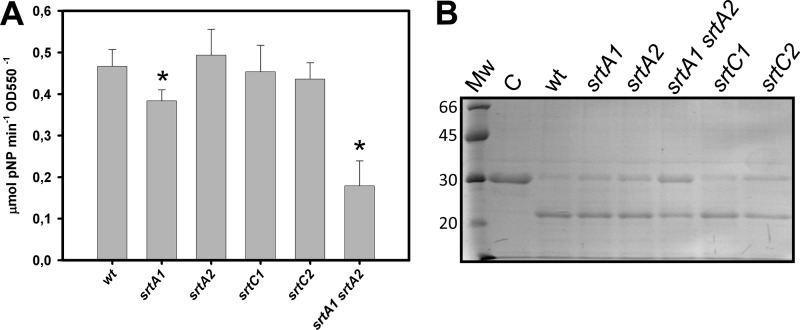
We determined the activity in whole cells of two enzymes that are putative substrates for sortase enzymes (N-acetylglucosaminidase and the cell envelope proteinase PrtP encoded by LCABL_02860 and LCABL_24520, respectively) (Table 4) in the wild type and the different srt mutants. A statistically significant decrease in N-acetylglucosaminidase activity by 18% (P < 0.001) was found in the srtA1 strain. This activity was additionally lowered by 62% (P < 0.0001) in the srtA1 srtA2 double mutant (Fig. 5A). The surface proteinase activity was determined by monitoring its proteolytic activity on β-casein by electrophoresis of the resulting peptides. The hydrolytic activity of L. casei BL23 on this substrate was low and only detectable after long incubation periods. The single srt mutants showed hydrolytic activity comparable to that of the wild type, whereas a reduced β-casein hydrolysis was observed for the srtA1 srtA2 strain (Fig. 5B).
Fig 5.
Activity of cell wall-anchored enzymes in wild-type L. casei and different sortase mutants. (A) Measurement of N-acetyl-β-d-glucosaminidase activity in whole cells. Results are the means from three experiments with cells from independent cultures, and the bars represent standard deviations. An asterisk indicates a statistically significant difference compared to the wild type (P < 0.05). (B) Proteolytic activity on β-casein determined by SDS-PAGE analysis. Mw is a molecular weight protein standard. Numbers on the left are molecular sizes in kDa. C is a β-casein sample incubated without cells. wt, wild type (BL23 strain).
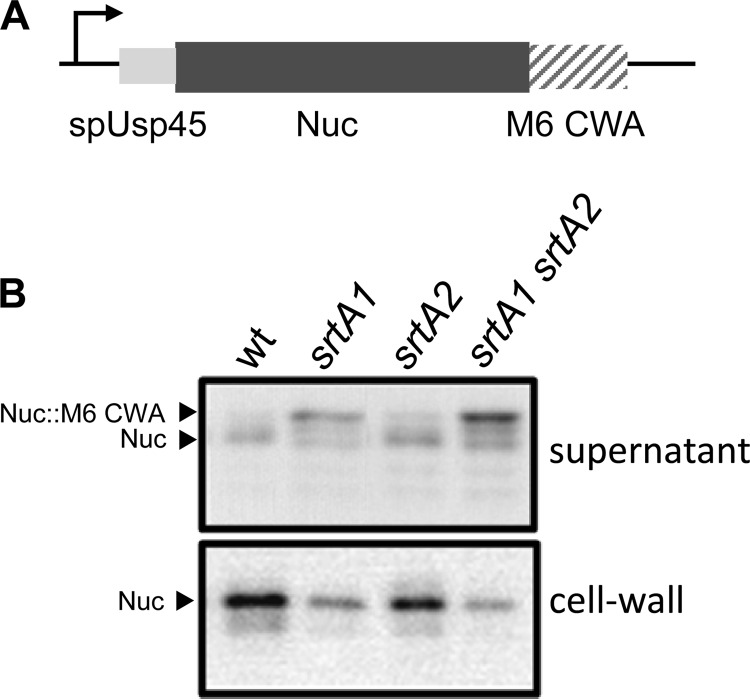
We used a reporter protein to assess the surface-anchoring activity present in srtA1 and srtA2 mutants. For this purpose, the wild type and the srtA1, srtA2, and srtA1 srtA2 mutants were transformed with the pNucA-CWA plasmid (9), which encoded the staphylococcal nuclease fused to the cell wall-anchoring (CWA) domain of the S. pyogenes M6 protein. The presence of NucA in cell culture supernatants and surface fractions was assayed by Western blot analysis (Fig. 6). NucA bands of the same size were detected in surface fractions of all strains, but their intensity decreased in the sortase mutants as follows: wild type > srtA2 > srtA1 > srtA1 srtA2. When the culture supernatants were analyzed, the srtA1 mutation resulted in the occurrence of NucA forms of a greater size which could correspond to nonprocessed LPXTG fusions. These results are in agreement with the lack of a transpeptidase reaction at the LPXTG sequence, which resulted in lack of cleavage of the C-terminal part of the anchoring sequence (3.17 kDa in the M6-anchoring domain fused to NucA). The intensity of the NucA bands in supernatants followed the gradation srtA1 srtA2 > srtA1 > srtA2 > wild type, additionally suggesting that in the absence of sortase activity more NucA protein is released to the supernatant.
Fig 6.
Localization of a Nuc-CWA reporter. (A) Schematic representation of the construct used to express a Nuc-CWA reporter in L. casei. spUsp45 is the signal peptide from lactococcal Usp45. M6 CWA is the cell wall-anchoring sequence of the S. pyogenes M6 protein. (B) Western blot detection with anti-Nuc serum in the supernatant and cell wall fractions of wild-type L. casei (wt; BL23 strain) and different sortase mutants (BL347, BL348, and BL345 strains) transformed with pNUC-CWA.
DISCUSSION
The adaptation of specific probiotics to particular gastrointestinal niche areas relies on the production of a variety of factors that enable their persistence and cross talk with host cells. Among them, the sortase-dependent surface proteins play a key role in processes related to mucosal adhesion (34, 40) but also in some aspects of the maintenance of intestinal homeostasis, as revealed by recent studies on the LPXTG protein PrtP in L. casei/L. paracasei (43). Thus, the study of the role of sortases and their substrates is crucial for deciphering different probiotic modes of action.
The 23 putative sortase substrates identified in the L. casei BL23 genome represent 0.77% of its proteome, which is quite similar to the 0.78% in L. casei ATCC 334. These strains are among the LAB with a higher percentage of sortase substrates in a genome-scale comparative analysis carried out with 26 strains (44), and they are only exceeded by L. plantarum WCFS1, whose genome encodes 27 sortase substrates (1.11%) (2, 44). Variations in sortase-dependent proteins occur between L. casei strains. As an example, compared to BL23, the cheese isolate ATCC 334 strain carries gene deletions or frameshifts in genes related to adhesion (spaF) or scavenging functions (prtR, LCABL_28750 glycosyl hydrolase), which may reflect the absence of selective pressure due to the adaptation to a distinct niche. Similarly, in L. salivarius UCC118 six out of the 10 sortase substrates identified are pseudogenes or gene fragments that would code for proteins related to adhesion in the oral cavity, suggesting an adaptation that was concomitant with the loss of functions (39).
We have shown that the activity of the class A sortases is necessary for the anchoring of at least N-acetylglucosaminidase and the cell wall proteinase PrtP in L. casei BL23 and that they can anchor NucA fused to an heterologous sorting sequence. Although a decrease in the surface display of these reporters was found in srtA1 or srtA1 srtA2 strains, there was never a complete lack of anchoring. This is in contrast to results obtained in Lactococcus lactis IL1403, where a srtA mutation completely abolished the anchoring to the cell wall of all sortase substrates tested, identifying srtA as the single gene responsible for general sortase activity (8). However, results obtained for Streptococcus thermophilus showed that mutation of the single srtA gene did not affect the levels of the cell wall-anchored proteinase PrtP (6). The authors attributed this to a surface retention of PrtP by a sortase-independent mechanism involving its C-terminal hydrophobic helix. In addition, proteins could remain attached to the negatively charged cell wall by electrostatic interactions depending on their pI. This could explain the persistence of the surface-anchored reporters in the L. casei srtA1 srtA2 mutant. Alternatively, compensation in the lack of housekeeping sortases by SrtC1 or SrtC2 which, although recognizing the same LPXTG motifs would catalyze cross-linking between two proteins instead of a protein and peptidoglycan (38), cannot be ruled out.
The existence of two class A sortases in the BL23 strain is remarkable, as the presence of a single sortase A gene per genome is the general rule. In the L. casei/L. paracasei and L. rhamnosus group, this is a characteristic of only few strains, and the plasmid location of srtA2 in L. rhamnosus Lc705 leaves open the possibility of the srtA2 locus being an integrated plasmid remnant in strains like BL23, where it is chromosomally located. In L. rhamnosus Lc705, both SrtA proteins (chromosome and plasmid encoded) are detected by proteome analysis (35). The exact role of L. casei SrtA2 is still unknown. The fact that srtA1 is highly expressed compared to srtA2, the effect of srtA1 on surface hydrophobicity, and the lack of processing of a Nuc::CWA fusion in a srtA1 strain suggest that SrtA1 is responsible for the main sortase activity in L. casei BL23. Nevertheless, we demonstrated that it was necessary to make gene deletions in both srtA genes to detect considerable or significant decreases in surface enzyme activities or bacterial adhesion, evidencing the contribution of SrtA2 to protein cell wall anchoring. Additionally, a trend toward reduction of binding to epithelial cells was shown in the srtA2 mutant. Whether SrtA2 is specific for a particular set of LPXTG proteins remains to be determined.
Studies on the implication of sortase activity in adhesion in LAB have rendered distinct results. An msa mutant of L. plantarum 299v, which lacks a sortase-dependent mannose specific adhesin, showed reduced fitness in a model of pig intestinal colonization (13), but intestinal survival and persistence in pig or mouse models were not affected by a srtA mutation in 299v (13) or WCFS1 (4) strains, respectively. van Pijkeren et al. (39) identified the lspA gene, encoding a protein with MucBP and LPXTG domains, and srtA as factors affecting the binding to human epithelial cells (Caco-2 and HT-29) in L. salivarius UCC118. Finally, deletion of srtA in L. johnsonii NCC533 had no effect on the persistence of this bacterium in the mouse gut (7). Our experiments indicate that one or more sortase-dependent proteins could be involved in the adhesion to Caco-2 and HT-29 cells in L. casei BL23. This is in agreement with the presence of putative sortase substrates with likely adhesion functions such as the proteins encoded by the spaCBA and spaFED clusters, LCABL_25040 (MBF homologue), or LCABL_26030 (CnaA and CnaB domains). However, although L. casei BL23 showed good binding capacities to human colonic resected fragments among several L. casei strains, it had very low binding to pig mucin (26) and, compared to L. rhamnosus GG, it displays a 10-fold lower attachment to human intestinal mucus (our unpublished observations). The ability of the BL23 srtA1 srtA2 mutant to persist in the intestinal environment remains to be investigated.
Within the LAB, adhesive pilus structures that are assembled by sortase-dependent mechanisms have only been reported in L. rhamnosus GG, where the spaCBA cluster codes for pili involved in mucus binding (16, 33, 41). Additionally, genetic evidences for pilus and fimbria structures exist for L. ruminis (11) and L. johnsonii (32). The presence of pilin clusters is widespread in the L. casei/L. paracasei and L. rhamnosus group. All analyzed strains carry a spaFED-srtC1 cluster, and the spaCBA-srtC2 cluster was found in all L. casei/L. paracasei strains, whereas it was only present in L. rhamnosus GG and LMS2-1 strains. Besides a nonsignificant decrease in surface hydrophobicity in the srtC1 mutant, no clear phenotype could be evidenced for L. casei BL23 srtC1 or srtC2 mutants with regard to cell surface characteristics or adhesion. In L. ruminis, expression of the putative pilus locus LRC_00600-00630 was strain specific (11). Therefore, the striking difference in mucus adhesion between L. casei BL23 and L. rhamnosus GG may reside in variations in the expression of the spaCBA genes.
In summary, in L. casei BL23, around 20 proteins are likely anchored to the cell surface by sortases. Although the specific function of most of them is unknown, sequence analyses suggest that many are related to the lifestyle of L. casei at the host mucosal surfaces and most likely participate in adhesion or substrate release from host glycans, which would account for an adaptation to persist in the gastrointestinal niche. SrtA1 is the housekeeping sortase in this strain, while SrtA2 can compensate for its absence to a certain extent. The genes for two other sortases, srtC1 and srtC2, are clustered with genes putatively encoding adhesive pili assembled by sortase-catalyzed reactions. The presence of pilus structures has only been reported for L. rhamnosus GG, but it may constitute a characteristic for the L. casei/L. paracasei and L. rhamnosus group.
ACKNOWLEDGMENTS
This work was supported by projects AGL2004-00176/ALI and Consolider Fun-c-Food CSD2007-00063 from the Spanish Ministry of Economy and Competitiveness. Diego Muñoz-Provencio was the recipient of a research fellowship from the Instituto Danone. The JAE-Post contract to Jesús Rodríguez-Díaz from the Consejo Superior de Investigaciones Científicas and the Ramón y Cajal contract to María Carmen Collado from the Ministry of Economy and Competitiveness are greatly acknowledged.
Amalia Blasco is acknowledged for her excellent technical support. We are very grateful to Jonathan Ulmer (Micalis Institute) for a critical reading of the manuscript.
Footnotes
Published ahead of print 5 October 2012
REFERENCES
- 1. Bellon-Fontaine MN, Rault J, van Oss CJ. 1996. Microbial adhesion to solvents: a novel method to determine the electron-donor/electron-acceptor or Lewis acid-base properties of microbial cells. Colloids Surf. B Biointerfaces 7:47–53 [Google Scholar]
- 2. Boekhorst J, de Been MW, Kleerebezem M, Siezen RJ. 2005. Genome-wide detection and analysis of cell wall-bound proteins with LPxTG-like sorting motifs. J. Bacteriol. 187:4928–4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boekhorst J, Helmer Q, Kleerebezem M, Siezen RJ. 2006. Comparative analysis of proteins with a mucus-binding domain found exclusively in lactic acid bacteria. Microbiology 152:273–280 [DOI] [PubMed] [Google Scholar]
- 4. Bron PA. 2004. The molecular response of Lactobacillus plantarum to intestinal passage and conditions. Ph.D. thesis Wageningen University, Wageningen, The Netherlands [Google Scholar]
- 5. Buck BL, Altermann E, Svingerud T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71:8344–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dandoy D, et al. 2011. The fast milk acidifying phenotype of Streptococcus thermophilus can be acquired by natural transformation of the genomic island encoding the cell-envelope proteinase PrtS. Microb. Cell Fact. 10(Suppl 1):S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denou E, et al. 2008. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC533 using a combination of genomics and transcriptome analysis. J. Bacteriol. 190:3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dieye Y, et al. 2010. Functionality of sortase A in Lactococcus lactis. Appl. Environ. Microbiol. 76:7332–7337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dieye Y, Usai S, Clier F, Gruss A, Piard JC. 2001. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 183:4157–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dramsi S, Magnet S, Davison S, Arthur M. 2008. Covalent attachment of proteins to peptidoglycan. FEMS Microbiol. Rev. 32:307–320 [DOI] [PubMed] [Google Scholar]
- 11. Forde BM, et al. 2011. Genome sequences and comparative genomics of two Lactobacillus ruminis strains from the bovine and human intestinal tracts. Microb. Cell Fact. 10(Suppl 1):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garrido D, Ruiz-Moyano S, Mills DA. 2012. Release and utilization of N-acetyl-d-glucosamine from human milk oligosaccharides by Bifidobacterium longum subsp. infantis. Anaerobe 18:430–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gross G, et al. 2008. Mannose-specific interaction of Lactobacillus plantarum with porcine jejunal epithelium. FEMS Immunol. Med. Microbiol. 54:215–223 [DOI] [PubMed] [Google Scholar]
- 14. Hendrickx AP, Budzik JM, Oh SY, Schneewind O. 2011. Architects at the bacterial surface—sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 9:166–176 [DOI] [PubMed] [Google Scholar]
- 15. Intra J, Pavesi G, Horner DS. 2008. Phylogenetic analyses suggest multiple changes of substrate specificity within the glycosyl hydrolase 20 family. BMC Evol. Biol. 8:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kankainen M, et al. 2009. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human mucus binding protein. Proc. Natl. Acad. Sci. U. S. A. 106:17193–17198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleerebezem M, et al. 2010. The extracellular biology of the lactobacilli. FEMS Microbiol. Rev. 34:199–230 [DOI] [PubMed] [Google Scholar]
- 18. Landete JM, et al. 2010. Requirement of the Lactobacillus casei MaeKR two-component system for L-malic acid utilization via a malic enzyme pathway. Appl. Environ. Microbiol. 76:84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184 [DOI] [PubMed] [Google Scholar]
- 20. Leloup L, Ehrlich SD, Zagorec M, Morel-Deville F. 1997. Single-crossover integration in the Lactobacillus sake chromosome and insertional inactivation of the ptsI and lacL genes. Appl. Environ. Microbiol. 63:2117–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mackenzie DA, et al. 2010. Strain-specific diversity of mucus-binding proteins in the adhesion and aggregation properties of Lactobacillus reuteri. Microbiology 156:3368–3378 [DOI] [PubMed] [Google Scholar]
- 22. Marraffini LA, Dedent AC, Schneewind O. 2006. Sortases and the art of anchoring proteins to the envelopes of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70:192–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maze A, et al. 2010. Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J. Bacteriol. 192:2647–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mazmanian SK, Liu G, Ton-That H, Schneewind O. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285:760–763 [DOI] [PubMed] [Google Scholar]
- 25. Monedero V, Gosalbes MJ, Perez-Martinez G. 1997. Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J. Bacteriol. 179:6657–6664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Munoz-Provencio D, et al. 2009. Adhesion properties of Lactobacillus casei strains to resected intestinal fragments and components of the extracellular matrix. Arch. Microbiol. 191:153–161 [DOI] [PubMed] [Google Scholar]
- 27. Nielsen H, Engelbrecht J, Brunak S, von Heijne G. 1997. A neural network method for identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Int. J. Neural Syst. 8:581–599 [DOI] [PubMed] [Google Scholar]
- 28. O'Callaghan J, Butto LF, Macsharry J, Nally K, O'Toole PW. 2012. Influence of adhesion and bacteriocin production by Lactobacillus salivarius on the intestinal epithelial cell transcriptional response. Appl. Environ. Microbiol. 78:5196–5203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36 doi:10.1093/nar/30.9.e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Posno M, et al. 1991. Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl. Environ. Microbiol. 57:1822–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pretzer G, et al. 2005. Biodiversity-based identification and functional characterization of the mannose-specific adhesin of Lactobacillus plantarum. J. Bacteriol. 187:6128–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pridmore RD, et al. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. U. S. A. 101:2512–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reunanen J, von Ossowski I, Hendrickx AP, Palva A, de Vos WM. 2012. Characterization of the SpaCBA pilus fibers in the probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 78:2337–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanchez B, Bressollier P, Urdaci MC. 2008. Exported proteins in probiotic bacteria: adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host. FEMS Immunol. Med. Microbiol. 54:1–17 [DOI] [PubMed] [Google Scholar]
- 35. Savijoki K, et al. 2011. Comparative proteome cataloging of Lactobacillus rhamnosus strains GG and Lc705. J. Proteome Res. 10:3460–3473 [DOI] [PubMed] [Google Scholar]
- 36. Spirig T, Weiner EM, Clubb RT. 2011. Sortase enzymes in Gram-positive bacteria. Mol. Microbiol. 82:1044–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ton-That H, Liu G, Mazmanian SK, Faull KF, Schneewind O. 1999. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. U. S. A. 96:12424–12429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ton-That H, Marraffini LA, Schneewind O. 2004. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim. Biophys. Acta 1694:269–278 [DOI] [PubMed] [Google Scholar]
- 39. van Pijkeren JP, et al. 2006. Comparative and functional analysis of sortase-dependent proteins in the predicted secretome of Lactobacillus salivarius UCC118. Appl. Environ. Microbiol. 72:4143–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Velez MP, De Keersmaecker SC, Vanderleyden J. 2007. Adherence factors of Lactobacillus in the human gastrointestinal tract. FEMS Microbiol. Lett. 276:140–148 [DOI] [PubMed] [Google Scholar]
- 41. von Ossowski I, et al. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76:2049–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. von Ossowski I, et al. 2011. Functional characterization of a mucus-specific LPXTG surface adhesin from probiotic Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 77:4465–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. von Schillde MA, et al. 2012. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 11:387–396 [DOI] [PubMed] [Google Scholar]
- 44. Zhou M, Theunissen D, Wels M, Siezen RJ. 2010. LAB-secretome: a genome-scale comparative analysis of the predicted extracellular and surface-associated proteins of lactic acid bacteria. BMC Genomics 11:651. [DOI] [PMC free article] [PubMed] [Google Scholar]