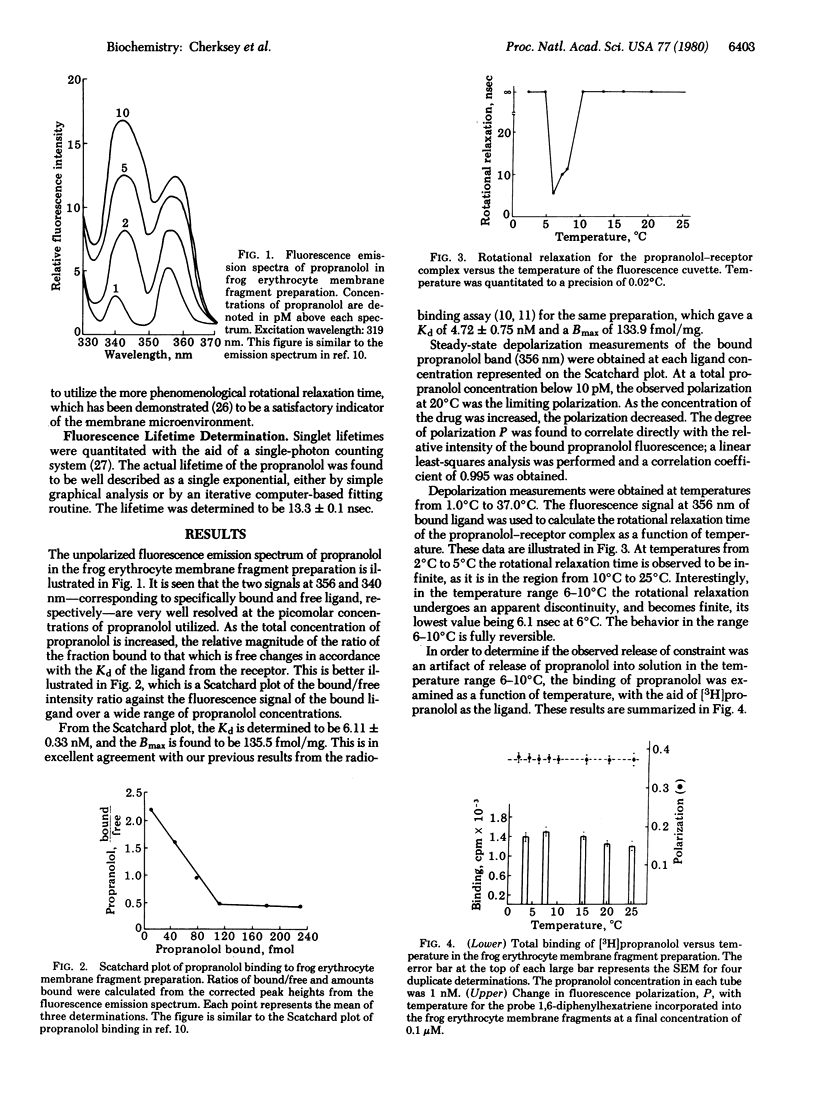
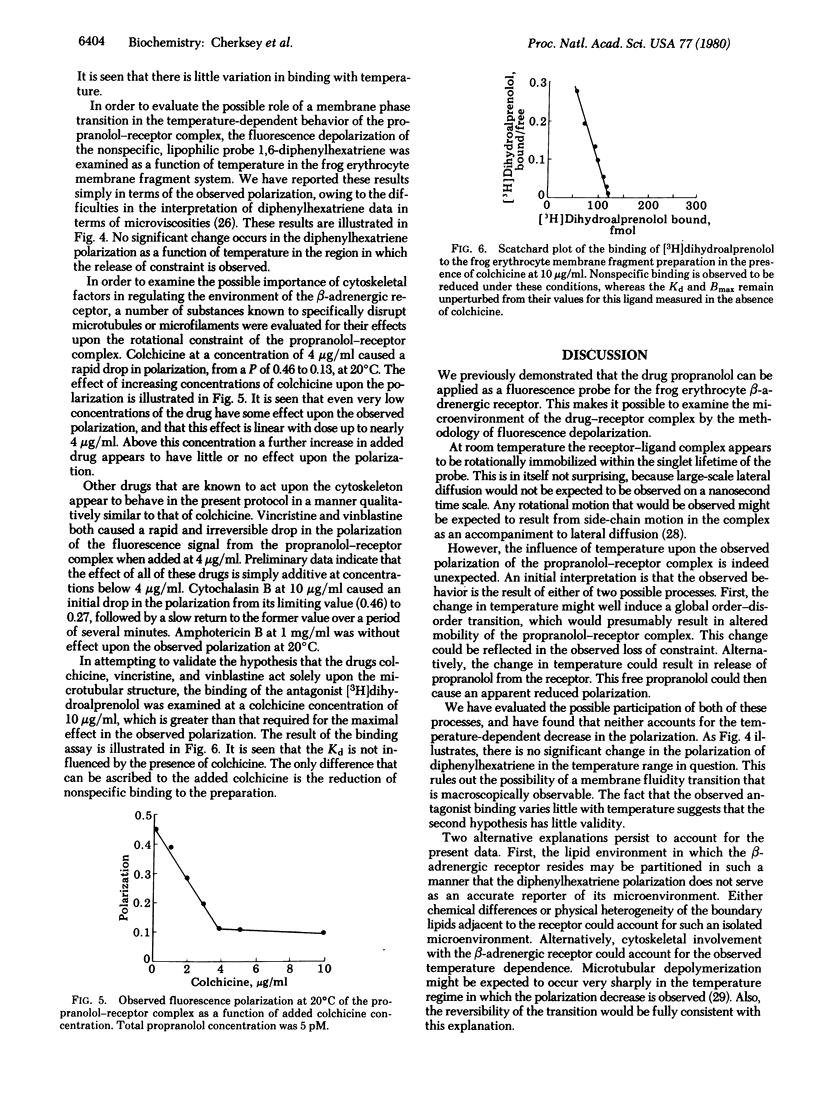
Abstract
A fluorescence receptor binding assay, based upon the high-affinity beta-adrenergic receptor antagonist propranolol, is utilized to probe the microenvironment of the antagonist-receptor complex in the frog (Rana catesbeiana) erythrocyte membrane. The technique of steady-state fluorescence depolarization is applied to the propranolol-receptor complex, allowing quantitation of the rotational relaxation time of the complex. It is found that the complex is dynamically constrained at 20 degrees C. However, in the temperature range 6-10 degrees C a sharp reversible release of constraint is observed. It is further demonstrated that the addition of drugs that are known to specifically disrupt the cytoskeleton (colchicine, vincristine, and vinblastine) causes a similar but irreversible release of constraint at 20 degrees C. Cytochalasin B has a much smaller influence on the rotational mobility of the propranolol-receptor complex than do the other drugs that disrupt the cytoskeleton. Amphotericin B is without effect on the rotational constraint of the complex. Binding of the antagonist [3H]dihydroalprenolol is not influenced by colchicine. A model is proposed which postulates that cytoskeletal elements are linked to the antagonist-receptor complex. Antagonist binding does not result in cytoskeletal release, whereas agonist binding is postulated to lead to dissociation of the agonist-receptor complex from the cytoskeleton, thereby activating adenylate cyclase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas D., Steer M. L., Levitzki A. Stereospecific binding of propranolol and catecholamines to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4246–4248. doi: 10.1073/pnas.71.10.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakardjieva A., Galla H. J., Helmreich E. J. Modulation of the beta-receptor adenylate cyclase interactions in cultured Chang liver cells by phospholipid enrichment. Biochemistry. 1979 Jul 10;18(14):3016–3023. doi: 10.1021/bi00581a017. [DOI] [PubMed] [Google Scholar]
- Belford G. G., Belford R. L., Weber G. Dynamics of fluorescence polarization in macromolecules. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1392–1393. doi: 10.1073/pnas.69.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry R. J. Rotational and lateral diffusion of membrane proteins. Biochim Biophys Acta. 1979 Dec 20;559(4):289–327. doi: 10.1016/0304-4157(79)90009-1. [DOI] [PubMed] [Google Scholar]
- Dale R. E., Chen L. A., Brand L. Rotational relaxation of the "microviscosity" probe diphenylhexatriene in paraffin oil and egg lecithin vesicles. J Biol Chem. 1977 Nov 10;252(21):7500–7510. [PubMed] [Google Scholar]
- Geacintov N., Prusik T., Khosrofian J. M. Properties of benzopyrene-DNA complexes investigated by fluorescence and triplet flash phytolysis techniques. J Am Chem Soc. 1976 Oct 13;98(21):6444–6452. doi: 10.1021/ja00437a002. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Cohn N., Glaser M. Fluidity of LM cell membranes with modified lipid compositions as determined with 1,6-diphenyl-1,3,5-hexatriene. Biochemistry. 1979 Mar 20;18(6):1042–1049. doi: 10.1021/bi00573a017. [DOI] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAINER L. M., CHI Y. M., FREIDBERG S. L., RALL T. W., SUTHERLAND E. W. Adenyl cyclase. IV. The effects of neurohormones on the formation of adenosine 3',5'-phosphate by preparations from brain and other tissues. J Biol Chem. 1962 Apr;237:1239–1243. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lakowicz J. R., Prendergast F. G., Hogen D. Differential polarized phase fluorometric investigations of diphenylhexatriene in lipid bilayers. Quantitation of hindered depolarizing rotations. Biochemistry. 1979 Feb 6;18(3):508–519. doi: 10.1021/bi00570a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Limbird L. E., Mukherjee C., Caron M. G. The beta-adrenergic receptor and adenylate cyclase. Biochim Biophys Acta. 1976 Apr 13;457(1):1–39. doi: 10.1016/0304-4157(76)90012-5. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mullikin D., Caron M. G. Regulation of beta-adrenergic receptors by guanyl-5'-yl imidodiphosphate and other purine nucleotides. J Biol Chem. 1976 Aug 10;251(15):4686–4692. [PubMed] [Google Scholar]
- Potter L. T. Uptake of propranolol by isolated guinea-pig atria. J Pharmacol Exp Ther. 1967 Jan;155(1):91–100. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P., Tedesco J. L., Lee T., Chau-Wong M., Muller P., Bowles J., Whitaker P. M., McManus C., Tittler M., Weinreich P. Dopamine receptors in the central nervous system. Fed Proc. 1978 Feb;37(2):131–136. [PubMed] [Google Scholar]
- Shand D. G., Nuckolls E. M., Oates J. A. Plasma propranolol levels in adults with observations in four children. Clin Pharmacol Ther. 1970 Jan-Feb;11(1):112–120. doi: 10.1002/cpt1970111112. [DOI] [PubMed] [Google Scholar]
- Sinensky M., Minneman K. P., Molinoff P. B. Increased membrane acyl chain ordering activates adenylate cyclase. J Biol Chem. 1979 Sep 25;254(18):9135–9141. [PubMed] [Google Scholar]
- Sinensky M., Pinkerton F., Sutherland E., Simon F. R. Rate limitation of (Na+ + K+)-stimulated adenosinetriphosphatase by membrane acyl chain ordering. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4893–4897. doi: 10.1073/pnas.76.10.4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Mode of coupling between the beta-adrenergic receptor and adenylate cyclase in turkey erythrocytes. Biochemistry. 1978 Sep 5;17(18):3795–3795. doi: 10.1021/bi00611a020. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Fischkoff S., Chance B., Cooper R. A. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes. Biochemistry. 1974 Apr 9;13(8):1589–1595. doi: 10.1021/bi00705a006. [DOI] [PubMed] [Google Scholar]
- Vatner D. E., Lefkowitz R. J. (3H)-Propranolol binding sites in myocardial membranes: nonidentity with beta adrenergic receptors. Mol Pharmacol. 1974 May;10(3):450–456. [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Farris F. J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry. 1979 Jul 10;18(14):3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- Weber G. Uses of fluorescence in biophysics: some recent developments. Annu Rev Biophys Bioeng. 1972;1:553–570. doi: 10.1146/annurev.bb.01.060172.003005. [DOI] [PubMed] [Google Scholar]
- Yguerabide J. Nanosecond fluorescence spectroscopy of macromolecules. Methods Enzymol. 1972;26:498–578. doi: 10.1016/s0076-6879(72)26026-8. [DOI] [PubMed] [Google Scholar]
- Zadunaisky J. A., Schaeffer B. E., Cherksey B. Chloride active transport, membrane lipids and receptors in the corneal epithelium. Ann N Y Acad Sci. 1980;341:233–245. doi: 10.1111/j.1749-6632.1980.tb47175.x. [DOI] [PubMed] [Google Scholar]


