Abstract
We previously reported that the Corynebacterium glutamicum RNase E/G encoded by the rneG gene (NCgl2281) is required for the 5′ maturation of 5S rRNA. In the search for the intracellular target RNAs of RNase E/G other than the 5S rRNA precursor, we detected that the amount of isocitrate lyase, an enzyme of the glyoxylate cycle, increased in rneG knockout mutant cells grown on sodium acetate as the sole carbon source. Rifampin chase experiments showed that the half-life of the aceA mRNA was about 4 times longer in the rneG knockout mutant than in the wild type. Quantitative real-time PCR analysis also confirmed that the level of aceA mRNA was approximately 3-fold higher in the rneG knockout mutant strain than in the wild type. Such differences were not observed in other mRNAs encoding enzymes involved in acetate metabolism. Analysis by 3′ rapid amplification of cDNA ends suggested that RNase E/G cleaves the aceA mRNA at a single-stranded AU-rich region in the 3′ untranslated region (3′-UTR). The lacZ fusion assay showed that the 3′-UTR rendered lacZ mRNA RNase E/G dependent. These findings indicate that RNase E/G is a novel regulator of the glyoxylate cycle in C. glutamicum.
INTRODUCTION
In most bacteria, the rate of mRNA decay depends on the initial cleavage, performed mainly by endoribonucleases of the RNase E/G family (2, 13, 31). Escherichia coli has two RNase E/G homologs, RNase E and RNase G. RNase E is an essential multifunctional RNase, which was initially discovered as a 5S rRNA-processing enzyme (18). RNase E also has a global role in the degradation of mRNA (2). The E. coli RNase G was originally identified as an endoribonuclease involved in the maturation of 16S rRNA (33, 48). RNase G is dispensable for viability (33, 48); RNase G was suggested to be involved in the regulation of central metabolism (26, 32, 42). In RNase G mutant cells, glycolysis is accelerated and pyruvic acid is consequently overproduced (42).
Both RNase E and RNase G cleave their target RNAs within single-stranded AU-rich regions (34, 38, 46). RNase E and G are known to prefer substrates with 5′-monophosphate ends to those with triphosphate ends (24, 35, 46). However, RNase E also cleaves certain mRNA substrates in a 5′-end-independent pathway referred to as “direct entry” (3, 22, 29). A recent study strongly suggested that the noncatalytic C-terminal half of RNase E has an important role in direct entry (1).
Corynebacterium glutamicum is a Gram-positive, nonpathogenic soil bacterium that has been widely applied in the industrial production of numerous metabolites, including amino acids and organic acids (5, 23, 30). C. glutamicum is particularly useful for large-scale production of glutamic acid and lysine. More than 2 million tons of glutamate as well as 1.5 million tons of lysine per year are produced using this bacterium (5). Since these two major metabolites produced from C. glutamicum are derived from tricarboxylic acid (TCA) cycle intermediates, the regulation of the TCA cycle and related pathways such as the glyoxylate cycle has been the subject of intensive study (6).
When acetate or a carbon source entering the central metabolism via acetyl coenzyme A (acetyl-CoA) is the only carbon source for an organism, the operation of the glyoxylate cycle is required to provide oxaloacetate. The glyoxylate cycle consists of 5 of the 8 reactions of the TCA cycle, and it bypasses the two decarboxylation steps via additional reactions involving isocitrate lyase (ICL) and malate synthase (MS). ICL catalyzes the cleavage of isocitrate to succinate and glyoxylate, and MS condenses glyoxylate with acetyl-CoA to form malate (17). It is known that E. coli and Bacillus subtilis do not utilize glucose and acetate simultaneously but preferentially use glucose (9, 19). On the other hand, C. glutamicum can coutilize glucose and acetate (17).
The expression of the aceA gene of C. glutamicum encoding ICL and that of the aceB gene encoding MS are positively regulated by the Lux R-type transcriptional regulator RamA (45). The aceA and aceB genes are also negatively regulated by the transcriptional regulator RamB in the presence of glucose (45). In addition, GlxR, a homolog of cyclic AMP (cAMP) receptor protein (CRP), negatively regulates the expression of aceA and aceB in a cAMP-dependent manner (45). In contrast to the situation in E. coli and other bacteria, the intracellular cAMP level in C. glutamicum is elevated during growth on glucose and lowered during growth on acetate (28). Thus, GlxR acts as a repressor of aceA and aceB during growth on glucose, when the cAMP level is high, but not during growth on acetate, when the cAMP level is low (28).
In contrast to E. coli, B. subtilis, a low-G+C Gram-positive bacterium, does not contain an RNase E/G ortholog. Instead, RNase J1/J2 and RNase Y play a central role in mRNA metabolism (4). C. glutamicum has one RNase E/G ortholog (NCgl2281) and one RNase J ortholog (NCgl1895), but no RNase Y. A previous study suggested that C. glutamicum RNase E/G was more closely related to E. coli RNase G than to RNase E (36). We then showed that RNase E/G is involved in the processing of 5S rRNA, although it is not essential for cell viability (37). Here we show that RNase E/G is a novel posttranscriptional regulator of the glyoxylate cycle in C. glutamicum.
MATERIALS AND METHODS
Bacterial strains and media.
E. coli JM109 [recA1 endA1 gyrA96 thi hsdR17 supE44 relA1 Δ(lac-proAB)/F′ (traD36 proAB+ lacIq lacZΔM15)] and JM110 [dam dcm supE44 hsdR17 thi leu rpsL1 lacY galK galT ara tonA thr tsx Δ(lac-proAB)/F′(traD36 proAB+ lac Iq lacZΔM15)] were used for plasmid construction. The C. glutamicum strains and plasmids used in this study are listed in Table 1. L broth containing 1% polypeptone, 0.5% yeast extract, 0.5% NaCl, and 0.1% glucose (pH 7.0) was used as the complex medium. CGC medium (14) containing an appropriate carbon source, 5.0 g/liter (NH4)2SO4, 5.0 g/liter urea, 21 g/liter morpholinepropanesulfonic acid (MOPS), 1.0 g/liter K2HPO4, 1.0 g/liter KH2PO4, 0.25 g/liter MgSO4 · 7H2O, 0.01 g/liter CaCl2, 16.4 mg/liter FeSO4 · 7H2O, 10 mg/liter MnSO4 · H2O, 0.2 mg/liter CuSO4 · 5H2O, 1.0 mg/liter ZnSO4 · 7H2O, 0.2 mg/liter NiCl2 · 6H2O, 0.2 mg/liter biotin, and 1.0 mg/liter thiamine (pH 6.8) was used as the minimal medium. Kanamycin was added when culturing cells carrying plasmid. d-Glucose, d-fructose, d-sucrose, d-ribose, l-arabinose, sodium acetate, or sodium gluconate (1% each) was added as a carbon source. The cells were grown at 30°C, and cell growth in the liquid medium was monitored by measuring the optical density at 660 nm (OD660). Overnight cultures grown on CGC minimal medium containing 1% glucose were washed and then inoculated into fresh CGC minimal medium containing either 1% glucose or 1% sodium acetate.
Table 1.
C. glutamicum strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| C. glutamicum strains | ||
| ATCC 31831 | Wild type | Laboratory stock |
| D2281 | ΔrneG | 37 |
| Plasmids | ||
| pECt | E. coli-C. glutamicum shuttle vector; Kmr; multiple cloning site; ColE1 ori; trc promoter; lacIq | 43 |
| pECtS | Same as pECt but with ΔlacIq-trc promoter | This study |
| pCrneFL | pECt with rneG | 36 |
| pNaceA | pECt with aceA | This study |
| pA5UZ | pECtS with aceA 5′-UTR-lacZ fusion gene | This study |
| pZA3U | pECt with lacZ-aceA 3′-UTR fusion gene | This study |
Plasmid construction.
A 2.25-kb DNA fragment containing the aceA gene fragment and covering 640 bp upstream of the translational start site and 282 bp downstream of the end of the coding sequence was amplified by performing PCR using the primers 5′-GGTGCTGTCAGTTCCATGGTTGTC-3′ and 5′-GAGGATCCGAACACGCAACGAGAG-3′ (the artificially generated NcoI and BamHI sites are underlined). The amplified fragment was digested with NcoI and BamHI. The digested DNA fragment was cloned into E. coli-C. glutamicum shuttle vector plasmid pECt (43) digested with the same enzymes. The newly constructed plasmid was named pNaceA.
A PCR protocol was used to delete the DNA fragment containing lacIq and the trc promoter from the vector plasmid pECt. A 5.7-kb DNA fragment was amplified by PCR using the primers 5′-CGGATAACCGCGGCACACAGGAAAC-3′ and 5′-GGCATACTCCGCGGCATCGTATAAC-3′ (the artificially generated SacII sites are underlined). The amplified fragment was digested with SacII and subjected to self-ligation. The newly constructed vector plasmid was named pECtS.
A 0.67-kb upstream region of the aceA gene containing the promoter sequence, the 5′ untranslated region (5′-UTR), and the first 14 bp of the coding region was amplified by performing PCR using the primers 5′-GGTGCTGTCAGTTCCATGGTTGTC-3′ and 5′-GCGGTACGTGGATCCCCAACGTTT-3′ (the artificially generated NcoI and BamHI sites are underlined). A 3.09-kb DNA fragment containing the E. coli lacZ gene fragment and covering 4 bp upstream of the translational start site and 3 bp downstream of the end of the coding sequence was amplified by performing PCR using the primers 5′-CACAGGATCCAGCTATGACCATGATT-3′ and 5′-CATGGCCTGCCCGGTACCTATTATT-3′ (the artificially generated BamHI and KpnI sites are underlined). The PCR products were digested with BamHI and ligated. The ligated DNA fragment was digested with NcoI and KpnI. The digested DNA fragment was cloned into plasmid pECtS digested with the same enzymes. The newly constructed plasmid was named pA5UZ.
A two-step PCR protocol was used to generate the gene fusion product consisting of lacZ and the aceA 3′-UTR. A 3.1-kb DNA fragment was amplified by PCR using the primers 5′-CGCCATGGAAAGGAATAATTACTCTAATGACCATGATTACGGATTCAC-3′ and 5′-CTGTAGGTCCTAGTTTTTTTGACACCAGACCAACT-3′ (the artificially generated NcoI site is underlined). The 5′ primer contains the Shine-Dalgarno (SD) sequence of the C. glutamicum pyc gene and the first 22 bp of the coding region of the E. coli lacZ gene. The 3′ primer contains the E. coli lacZ gene fragment lacking the last 3 bp of the coding region and a 15-bp downstream region of the aceA gene containing the last 6 bp of the coding region in frame. A 153-bp DNA fragment was amplified by PCR using the primers 5′-GTCTGGTGTCAAAAAAACTAGGACCTACAGGTTCT-3′ and 5′-TCTTTCGGAAGCTTTGCAGTCAACA-3′ (the artificially generated HindIII site is underlined). The 5′ primer contains the lacZ gene fragment lacking the last 3 bp of the coding region and a 20-bp downstream region of the aceA gene containing the last 6 bp of the coding region in frame. The two amplicons were annealed to each other and used in a second round of PCR to generate the lacZ-aceA 3′-UTR fusion gene using the primers 5′-CGCCATGGAAAGGAATAATTACTCTAATGACCATGATTACGGATTCAC-3′ and 5′-TCTTTCGGAAGCTTTGCAGTCAACA-3′ (the artificially generated NcoI and HindIII sites are underlined). The amplified fragment was digested with NcoI and HindIII. The digested DNA fragment was cloned into the pECt plasmid (43) digested with the same enzymes. The newly constructed plasmid was named pZA3U.
Analysis of cellular proteins.
Cells suspended in sodium phosphate buffer (50 mM, pH 7.0) were disrupted by sonication. After the removal of unbroken cells, cell lysates were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), and the gel was stained with Coomassie brilliant blue. The 45-kDa protein band was cut out and analyzed by mass spectrometry (MALDI-TOF/TOF ultrafleXtreme; Bruker Daltonics, Inc., Billerica, MA).
Total RNA extraction.
Total cellular RNA from C. glutamicum ATCC 31831 and D2281 was isolated as described previously (37). Briefly, overnight cultures grown on CGC minimal medium containing 1% glucose at 30°C were washed and then inoculated into fresh CGC minimal medium containing 1% sodium acetate. Two volumes of RNA Protect bacterial reagent (Qiagen, Valencia, CA) were added directly to one volume of exponentially growing cultures at an OD660 of ∼1 (in logarithmic phase) to stabilize cellular RNAs. The cells were harvested by centrifugation at 5,000 × g for 10 min at 25°C, and total cellular RNAs were isolated using an RNeasy minikit (Qiagen). Total RNA was treated with DNase I at 37°C for 1 h.
Northern hybridization.
For Northern analysis of aceA mRNA and 16S rRNA, total RNAs were separated using the formaldehyde-free RNA gel kit (1% agarose) (Amresco, Solon, OH). A hybridization probe labeled with digoxigenin-dUTP (DIG-dUTP) was generated using the PCR DIG probe synthesis kit (Roche Diagnostics, Basel, Switzerland). For the aceA mRNA, the hybridization probe was amplified by PCR using the primers 5′-CTACACCGCAGACCAGGTAG-3′ and 5′-CTGGCCTTCTTCAGTGGAAC-3′. For the 16S rRNA, the hybridization probe was amplified by PCR using the primers 5′-GTGGAGAGTTTGATCCTGGCTCAGGAC-3′ and 5′-GAAAGGAGGTGATCCAGCCGCACCTTC-3′. Northern hybridizations were performed according to the procedures described in the DIG Northern starter kit (Roche Diagnostics). The hybridization temperature was 50°C. Positive hybridization bands were detected using the CDP-Star reagent (Roche Diagnostics) with exposure times between 30 s and 15 min. The amount of RNA was measured on the basis of the band intensity by using the Just TLC software (Sweday, Lund, Sweden). For the rifampin chase experiment, exponentially growing cells were treated with 150 μg/ml rifampin to inhibit the de novo synthesis of RNA, and after the specified times, total RNA was extracted and analyzed as described above.
qRT-PCR.
The mRNA was quantified using the Eco Real-Time PCR System (Illumine, Inc., San Diego, CA). Primers used in this quantitative real-time PCR (qRT-PCR) analysis are listed in Table 2. A 50-ng total RNA sample was used for each RT-PCR with each primer pair using the QuantiFast SYBR green RT-PCR kit (Qiagen) according to the manufacturer's instructions. Negative controls with no reverse transcriptase were included with each RNA sample to rule out DNA contamination. Amplicons were run on a 2% (wt/vol) agarose gel to verify that only a single band was produced. The target gene transcripts were normalized to the reference gene transcript (16S rRNA) from the same RNA sample. Each gene was analyzed using RNA isolated from three independent samples. The cycle threshold (CT) for each sample was generated according to the procedures described in the Eco real-time PCR system user guide.
Table 2.
Primers used in qRT-PCR analysis
| Target gene | 5′ primer sequence | 3′ primer sequence | Reference or source |
|---|---|---|---|
| 16S rRNA-1 | AGAGTTTGATCCTGGCTCA | ACGTGTTACTCACCCGTTCG | 21 |
| 16S rRNA-2 | ACGTTCCCGGGCCTTGTACA | CGGCTACCTTGTTACGAC | 21 |
| aceA | ATGTCAAACGTTGGAAAGCC | TGTGCTCCTCGATGACGGAA | 21 |
| aceB | TGACTGAACAGGAACTGTTGTC | AGGGAGTACCGCTTCGGTTA | 21 |
| ack | CTCCGGTTCATCTTCCATCA | GAGATCGAACGCCAGGTTTA | This study |
| pta | CTCTGATCACCACGGTCAAC | TCCTACACCGATGATGAGAG | This study |
| ramA | GGATACCCAGCGGATTAAAG | CGATTGTCCTGCAACACAGT | This study |
| gltA | TGTTTGAAAGGGATATCGTG | AGTCTCAGACAGCATCTTGC | 21 |
| acn | GCACCCTTGAAGTTGGCGAC | CGGTACGAAGAAGGTTCTCT | 21 |
| icd | AAGCACCGCTGCTCGCGACCTA | CGTCCAGCGAGTGAAATGTC | 21 |
Primer extension analysis.
Primer extension analysis was performed as described previously (37). Briefly, the 5′ end of aceA mRNA was determined by nonradioactive primer extension analysis. Aliquots of 16 μg of total RNA extracted from ATCC 31831 and D2281 cells were used for primer extension with Superscript II RNase H reverse transcriptase (Invitrogen, Carlsbad, CA) and the biotinylated oligonucleotide 5′-GTGATGCCGTTCCAACGAG-3′, which was complementary to a region within the aceA mRNA coding region. The primer extension products were separated on a denaturing polyacrylamide gel (8 M urea, 6% gel) together with sequencing ladders obtained using the same primer. The template for the sequencing ladders was PCR amplified using the primers 5′-GGTGCTGTCAGTTCCATGGTTGTC-3′ and 5′-GAGGATCCGAACACGCAACGAGAG-3′. The separated products were detected by chemiluminescence using a Phototope-Star detection kit (New England BioLabs Inc., Beverly, MA).
3′ RACE.
3′ rapid amplification of cDNA ends (3′-RACE) analysis was performed as described previously (37). Total RNA was treated with DNase I and dephosphorylated with calf intestinal phosphatase. Dephosphorylated RNA was ligated with the 3′ RNA adapter 5′-P-UUCACUGUUCUUACAGGUUCGCCGGCG-idT-3′ (Gene Design Inc., Ibaraki, Japan) containing 3′-inverted deoxythymidine (3′idT) at 10°C overnight. Further treatment of the RNA with the ligated 3′ RNA adapter and reverse transcription using the primer 5′-CGAACCTGTAAGAACAGTGAA-3′ complementary to the 3′ adapter and Ready-To-Go You-Prime first-strand beads (GE Healthcare, Buckinghamshire, United Kingdom) were performed. The products of the reverse transcriptions were amplified by two consecutive PCRs, using the adapter-specific primer used for RT-PCR and the aceA mRNA-specific primer 5′-GCTTCACCGCTGTTAAGCAC-3′. The 3′ RACE products were separated on a 2% agarose gel. The band of the stable 3′ end was cut out and sequenced using the Applied Biosystems 3730xl DNA analyzer (Applied Biosystems, Foster City, CA).
β-Galactosidase activity.
β-Galactosidase activity was determined by the method of Miller (40). Overnight cultures grown on CGC minimal medium containing 1% glucose at 30°C were washed and then inoculated into fresh CGC minimal medium containing either 1% glucose or 1% sodium acetate. To synthesize the LacZ protein from the lacZ-aceA 3′ UTR fusion gene, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM when cell growth reached the early exponential phase (OD660 of ∼0.3). After 5 h of cultivation, the culture was subjected to the β-galactosidase activity assay.
Determination of glucose and sodium acetate concentrations.
Glucose concentrations in the culture supernatant were measured using the Biotech-analyzer AS-210 (SakuraSeiki, Tokyo, Japan) with a glucose oxidase sensor. Sodium acetate concentrations in the culture supernatant were measured using the Agilent 7100 capillary electrophoresis (CE) system with the Agilent organic acid solution kit (Agilent Technologies, Santa Clara, CA).
RESULTS
Overproduction of the ICL protein in the ΔrneG mutant.
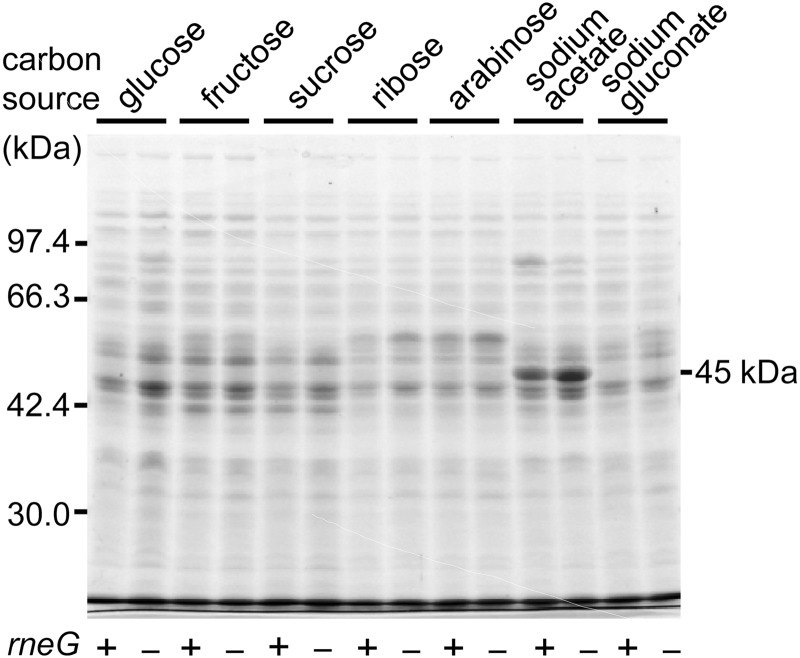
In order to examine whether RNase E/G participates in regulation of carbon metabolism at the mRNA level, we first compared protein expression patterns between the wild type and the ΔrneG mutant when cells were grown on various kinds of sugars and organic acids as the sole carbon source. The total cellular proteins obtained from the cultures were subjected to SDS-PAGE. The carbon sources examined were d-glucose, d-fructose, d-sucrose, d-ribose, l-arabinose, sodium acetate, and sodium gluconate. Although most C. glutamicum strains do not have the ability to utilize l-arabinose, C. glutamicum ATCC 31831, which we used as the wild-type strain in this study, is able to utilize l-arabinose as the sole carbon source (27). As shown in Fig. 1, a ΔrneG mutant strain, D2281, grown on sodium acetate as the sole carbon source synthesized a large amount of protein with a molecular mass of ∼45 kDa, compared with the wild type; the ΔrneG mutant produced 2.3-fold ± 0.4-fold (n = 3) more 45-kDa protein than the wild type. The overproduction of the 45-kDa protein reverted to wild-type levels when the plasmid pCrneFL, carrying the rneG gene (36), was introduced into the D2281 strain (data not shown). The 45-kDa protein band was identified as ICL with a molecular mass of 47.3 kDa by mass spectrometry. Overproduction of ICL in the D2281 strain was also observed when cells were grown on sodium acetate plus glucose (data not shown).
Fig 1.
Overproduction of the ICL protein in the ΔrneG mutant. ATCC 31831 (wild type [+]) and D2281 (ΔrneG [−]) cells were grown on various kinds of sugars and organic acids as the sole carbon source. The total cellular proteins obtained from the cultures were analyzed by 10% SDS-PAGE. A Coomassie brilliant blue-stained gel is shown. The carbon sources are shown at the top of the gel. The position of the 45-kDa protein is shown to the right of the gel.
Increased stability of aceA mRNA in the ΔrneG mutant.
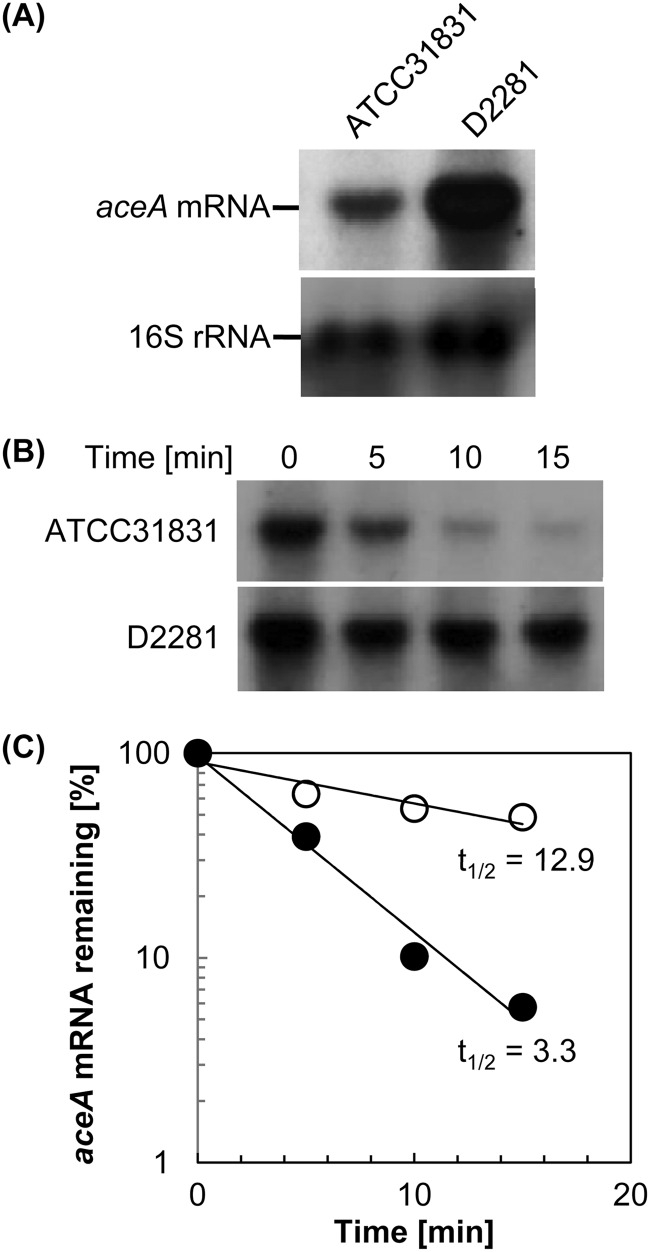
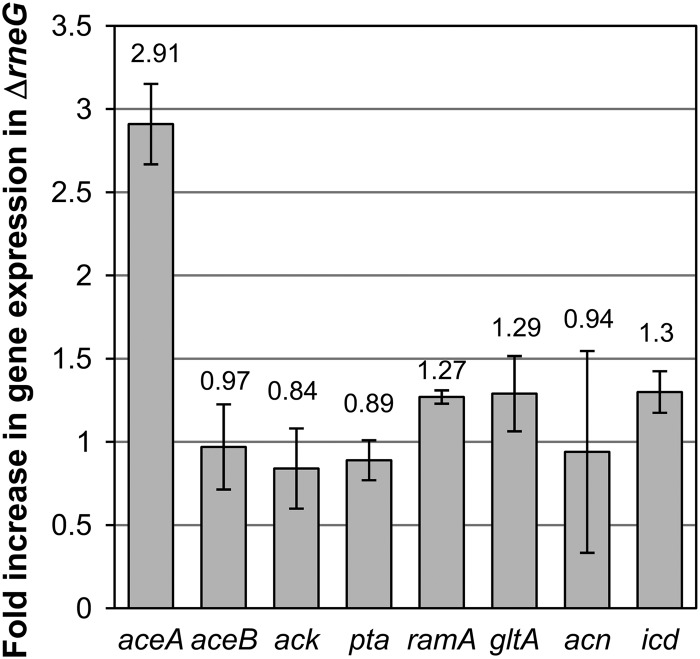
It was likely that overproduction of ICL by the ΔrneG mutant was due to the increased stability of the aceA mRNA encoding ICL. To examine the effect of the ΔrneG mutation on the stability of the aceA mRNA, we first carried out Northern hybridization. The amount of aceA mRNA in the ΔrneG mutant grown on sodium acetate as the sole carbon source was significantly higher than that in the wild type (Fig. 2A). We expected that RNase E/G is involved in degradation of not only aceA mRNA but also other mRNAs encoding proteins involved in acetate metabolism. Therefore, we performed qRT-PCR analysis. Total RNA was prepared from the cultures growing exponentially on sodium acetate as the sole carbon source. The RNA was subjected to qRT-PCR analyses using specific primer sets for genes involved in acetate metabolism. The selected genes were aceA (encoding ICL), aceB (encoding MS), ack (encoding acetate kinase), pta (encoding phosphotransacetylase), ramA (encoding RamA, which positively regulates aceA), gltA (encoding citrate synthase), acn (encoding aconitase), and icd (encoding isocitrate dehydrogenase [ICD]). As shown in Fig. 3, the rneG deletion resulted in an increase in aceA mRNA level of approximately 3-fold. However, the rneG deletion did not affect the amounts of the other mRNAs. These results suggest that RNase E/G specifically degrades the aceA mRNA among these mRNAs, although this does not exclude the possibility that the RNase E/G degrades mRNAs other than the aceA mRNA.
Fig 2.
Increased stability of aceA mRNA in the ΔrneG mutant. (A) Intracellular levels of aceA mRNA and 16S rRNA in ATCC 31831 (wild type) and D2281 (ΔrneG) strains. Total RNAs from ATCC 31831 and D2281 were analyzed by Northern hybridization, as described in Materials and Methods, using specific probes for aceA mRNA and 16S rRNA. (B) Measurement of the half-lives of aceA mRNA in the ATCC 31831 and D2281 strains. Rifampin was added to exponentially growing cultures of ATCC 31831 and D2281 at time zero, and at the indicated times, total RNAs were isolated and analyzed by Northern hybridization using a specific probe for aceA mRNA. (C) The intensities of the hybridized bands in Fig. 2B were quantified with Just TLC software and the half-life (t1/2) (min) of aceA mRNA was calculated. Filled circles, ATCC 31831; open circles, D2281. The half-lives shown are representative of those from three independent rifampin chase experiments with comparable results.
Fig 3.
Relative expression levels of genes involved in acetate metabolism. qRT-PCR was used for analysis of total RNA isolated from ATCC 31831 (wild type) and D2281 (ΔrneG) cells grown on CGC minimal medium containing 1% sodium acetate. Each column represents the gene of interest shown on the x axis. The fold increase in gene expression in the ΔrneG mutant corresponds to the ratio of the transcript level of each gene in the wild-type strain to that of the corresponding gene in the ΔrneG mutant. The levels of gene transcripts were measured in triplicate and normalized using 16S rRNA transcript levels.
We then performed a rifampin chase experiment. Exponentially growing cells on sodium acetate as the sole carbon source were treated with 150 μg/ml rifampin to prevent further initiation of transcription. Total RNA was isolated at various times after the addition of rifampin, and the rates of decay of the aceA mRNA in the wild type and the ΔrneG mutant were determined. The aceA mRNA degraded with a half-life of 3.3 min in the wild type. In contrast, it degraded with a half-life of 12.9 min in the ΔrneG mutant (Fig. 2B and C). The prolonged half-life of the aceA mRNA in the ΔrneG mutant was also confirmed by qRT-PCR (data not shown). These results indicate that the overproduction of ICL in the ΔrneG mutant is due to the increased stability of the aceA mRNA.
Involvement of the 3′-UTR of aceA mRNA in RNase E/G-dependent degradation.
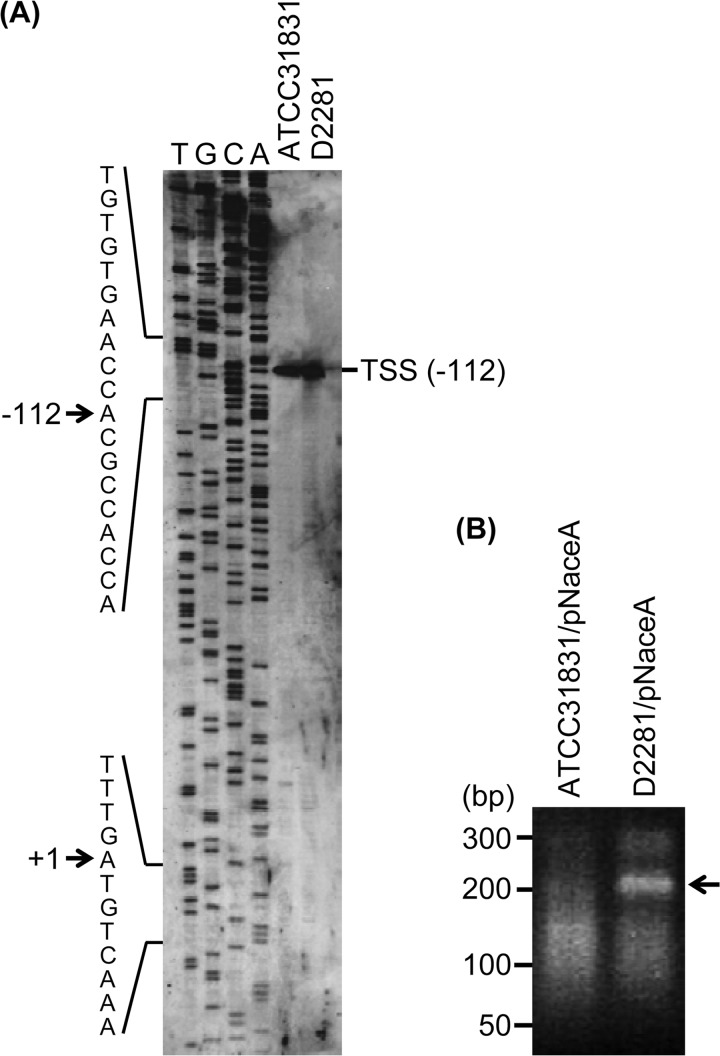
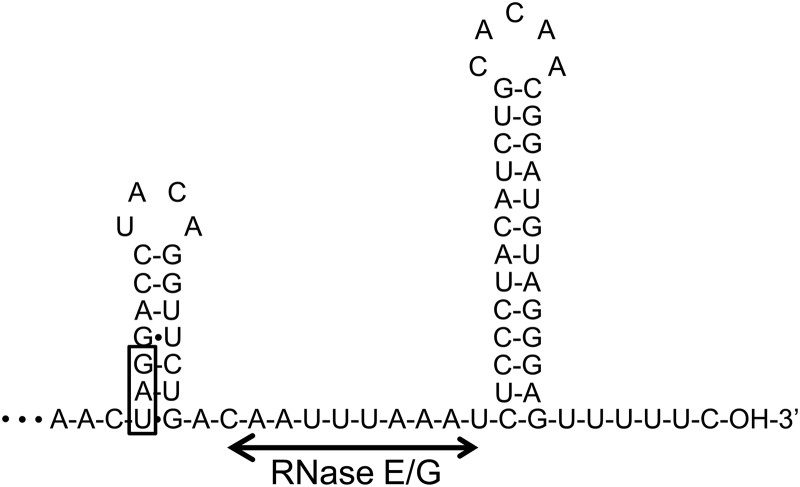
To determine the cleavage site of the aceA mRNA by RNase E/G, we first carried out primer extension analysis using total RNA isolated from wild-type and ΔrneG mutant cells grown on sodium acetate as the sole carbon source. In the case of E. coli, the degradation of many mRNAs is often initiated by RNase E cleavage in the 5′ untranslated region (5′-UTR) of the mRNA (25). However, as shown in Fig. 4A, only one 5′ end, which corresponded to the previously identified transcriptional start site of the aceA gene (17), was detected both in the wild type and in the ΔrneG mutant (Fig. 4A). This primer extension analysis suggested that RNase E/G-mediated degradation of aceA mRNA is 5′-UTR independent. It is also reported that several E. coli mRNAs, such as those of the rpsO and cspA genes, are subjected to RNase E cleavage in their 3′-UTR (20, 22). Therefore, we next performed 3′ RACE analysis to estimate the sites of RNase E/G cleavage in the aceA mRNA using total RNAs isolated from the wild type and the ΔrneG mutant harboring the pNaceA plasmid. The pNaceA plasmid expresses the aceA mRNA from the native promoter. As shown in Fig. 4B, a stable 3′ end of the aceA mRNA was detected in the ΔrneG mutant harboring pNaceA, while such a stable 3′ end was not seen in the wild type harboring pNaceA. This might be because the RNase E/G cleavage product is degraded rapidly by 3′-to-5′ exoribonucleases. The major PCR product obtained from the ΔrneG mutant harboring pNaceA was isolated from the 2% agarose gel and subjected to sequencing analysis. The 3′ end was located at 63 nucleotides (nt) downstream of the end of the aceA coding sequence. Using the RNA fold software (mfold [http://mfold.rna.albany.edu/?q=mfold]), the secondary structure of the aceA 3′-UTR was predicted. As shown in Fig. 5, a GC-rich hairpin loop followed by a run of U residues was found at the 3′ end of the transcript, which resembles typical bacterial rho-independent transcription terminators. It was also predicted that the UAG stop codon can be occluded by base pairing with sequences further downstream in the 3′-UTR (Fig. 5). In addition, a single-stranded AU-rich region was found between the two stem-loops (Fig. 5). Considering the substrate preference of E. coli RNase E and G, RNase E/G cleavage might occur within this AU-rich region, which is somewhat reminiscent of the E. coli rpsO and cspA mRNA (20, 22).
Fig 4.
Estimation of the sites of RNase E/G cleavage in aceA mRNA. (A) Primer extension analysis of aceA transcripts from ATCC 31831 and D2281 cells. Primer extension products derived from the total RNAs of ATCC 31831 (wild type) and D2281 (ΔrneG) grown on CGC minimal medium containing 1% sodium acetate were separated on a denaturing polyacrylamide gel along with sequencing ladders (T, G, C, and A) obtained using the same primer. Parts of the aceA transcript sequence are indicated on the left side. The positions of the transcriptional start site (TSS) and the translational start site are indicated by arrows. The translational start site of the aceA transcript is defined as +1. The detected 5′ end of the aceA transcript corresponded to the TSS (−112). (B) 3′ RACE products derived from total RNA isolated from ATCC 31831 and D2281 cells harboring pNaceA. 3′ RACE products were obtained as described in Materials and Methods. The pNaceA plasmid expresses the aceA mRNA from the native promoter. A 50-bp ladder marker is shown on the left side. The position of the stable 3′ end detected in the ΔrneG mutant is shown on the right side. The band of the stable 3′ end was cut out and sequenced.
Fig 5.
Predicted secondary structure of the aceA 3′-UTR. RNA fold software (mfold [http://mfold.rna.albany.edu/?q=mfold]) was used to predict the secondary structure of the aceA 3′-UTR. The UAG stop codon is boxed. The horizontal line represents the possible cleavage site of RNase E/G (see the text for details).
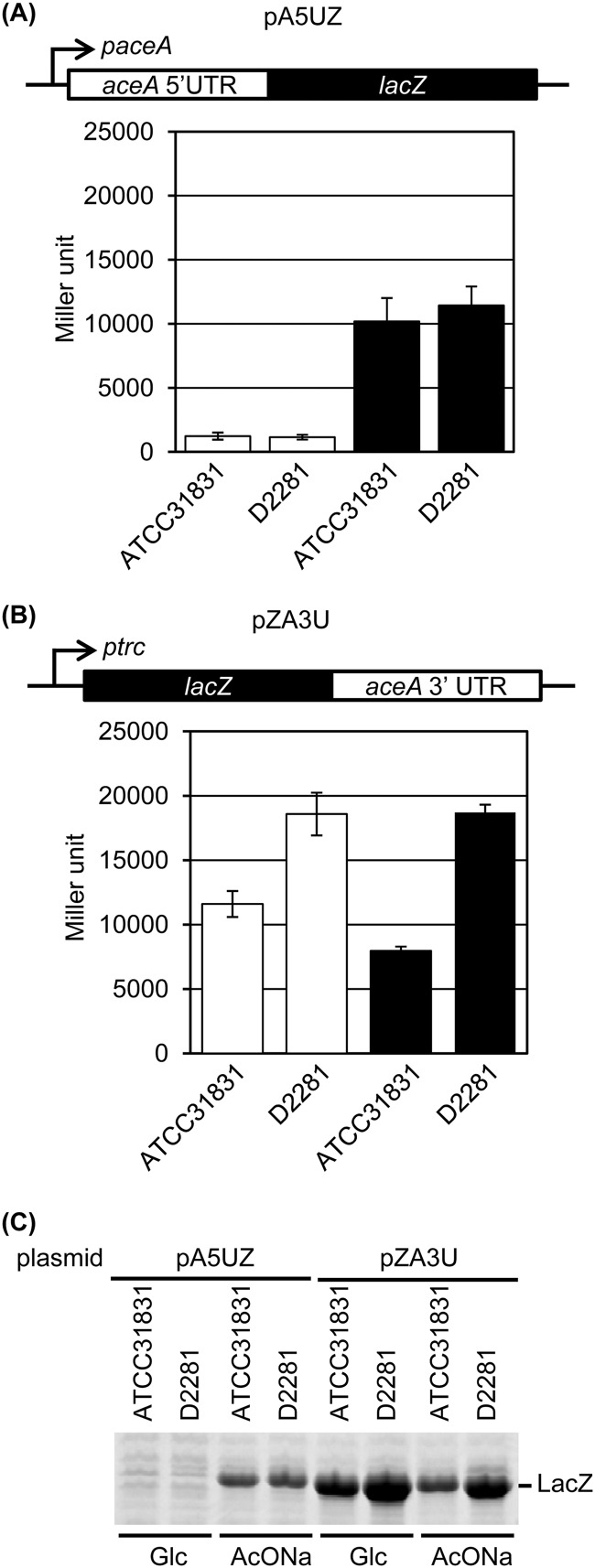
3′-UTR-dependent overproduction of the LacZ protein from a lacZ-aceA 3′-UTR fusion gene in the ΔrneG mutant.
In order to verify the possibility that the 3′-UTR is involved in stability control by RNase E/G, a lacZ-aceA 3′-UTR fusion gene was constructed as described in Materials and Methods (Fig. 6B). An aceA-5′-UTR-lacZ gene was also constructed as a control (Fig. 6A). The plasmid pA5UZ, carrying a 0.67-kb upstream region of the aceA gene containing the promoter sequence, the 5′-UTR, and the first 14 bp of the coding region, joined in frame with the E. coli lacZ gene, was introduced into the wild type and the ΔrneG mutant. As shown in Fig. 6C, in both the wild-type and ΔrneG mutant cells, the expression of the ICL-LacZ fusion protein was repressed in glucose minimal medium. On the other hand, in both the wild-type and ΔrneG mutant cells, the expression of the ICL-LacZ fusion protein significantly increased in sodium acetate minimal medium (Fig. 6C). As expected, there was no significant difference in the expression of the ICL-LacZ fusion protein between the wild type and the ΔrneG mutant (Fig. 6C). β-Galactosidase activities were 8.2- and 9.9-fold higher in sodium acetate minimal medium than in glucose minimal medium in the wild type and in the ΔrneG mutant, respectively (Fig. 6A). In agreement with the results of SDS-PAGE, there was no significant difference in β-galactosidase activity between the wild type and the ΔrneG mutant (Fig. 6A). These results confirmed that the RNase E/G-mediated degradation of the aceA mRNA is 5′-UTR independent.
Fig 6.
Effect of the ΔrneG mutation on the expression of the aceA-lacZ fusion genes. (A) β-Galactosidase activity in ATCC 31831 (wild type) and D2281 (ΔrneG) cells harboring the pA5UZ plasmid. Plasmid pA5UZ carries the aceA 5′-UTR-lacZ fusion gene. A schematic diagram of the aceA 5′-UTR-lacZ fusion gene and the aceA promoter paceA is shown at the top. The wild type and the ΔrneG mutant were grown on CGC minimal medium containing either 1% sodium acetate (filled bars) or 1% glucose (blank bars). (B) β-Galactosidase activity in ATCC 31831 and D2281 cells harboring the pZA3U plasmid. Plasmid pZA3U carries the lacZ-aceA 3′-UTR fusion gene. A schematic diagram of the lacZ-aceA 3′-UTR fusion gene and the trc promoter ptrc is shown at the top. The wild type and the ΔrneG mutant were grown on CGC minimal medium containing either 1% sodium acetate (filled bars) or 1% glucose (blank bars). To synthesize the LacZ protein from the lacZ-aceA 3′-UTR fusion gene, IPTG was added to a final concentration of 0.1 mM. Enzyme activity is expressed in Miller units. All the values are derived from at least three independent cultivations, and the error bars represent the standard deviations. (C) Expression of the LacZ protein expressed from the pA5UZ and pZA3U plasmids was examined by 7.5% SDS-PAGE. The position of the LacZ protein is shown to the right of the gel.
Plasmid pZA3U, carrying the E. coli lacZ gene lacking the last 3 bp of the coding region, joined with a 149-bp downstream region of the aceA gene containing the last 9 bp of the coding region and the 3′-UTR in frame, was introduced into the wild type and the ΔrneG mutant. In the absence of IPTG, the expression of the LacZ protein from the lacZ-aceA 3′-UTR fusion gene was not detected on a Coomassie brilliant blue-stained gel (data not shown). In the presence of 0.1 mM IPTG, the LacZ protein was overproduced in the ΔrneG mutant both in glucose and in sodium acetate minimal medium (Fig. 6C). β-Galactosidase activities were 1.6- and 2.3-fold higher in the ΔrneG mutant than in the wild type in glucose and in sodium acetate minimal medium, respectively (Fig. 6B). These results indicate that RNase E/G-mediated degradation of the aceA mRNA is 3′-UTR dependent.
Growth of the ΔrneG mutant on sodium acetate as the sole carbon source.
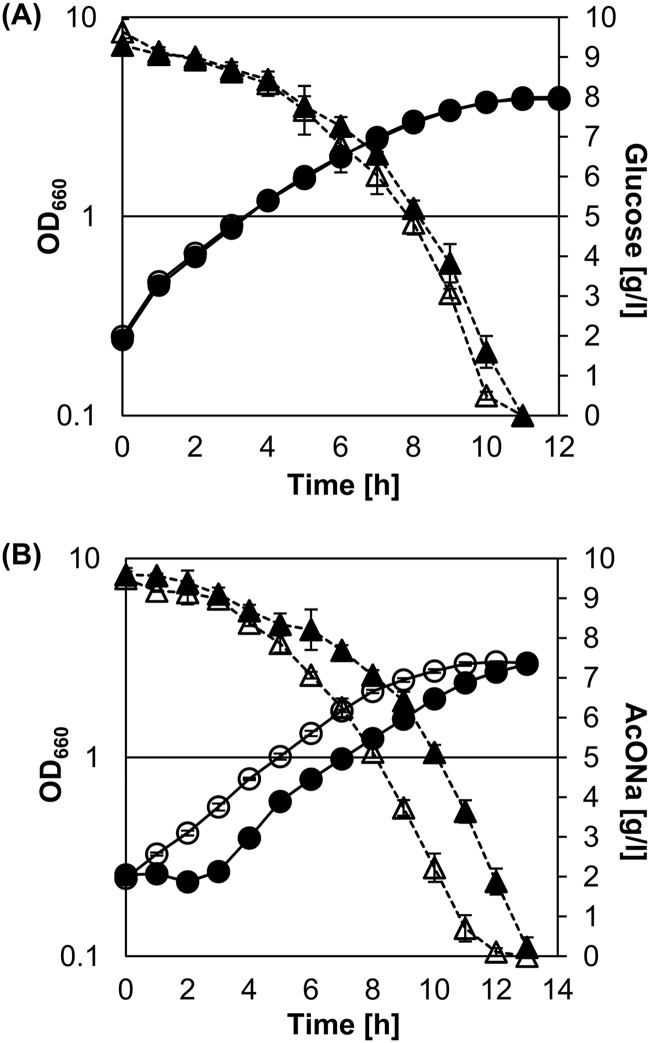
We examined the growth of the wild type and the ΔrneG mutant on sodium acetate as the sole carbon and energy source. Overnight cultures of the wild type and the ΔrneG mutant grown on CGC minimal medium containing 1% glucose were washed and then inoculated into fresh CGC minimal medium containing either 1% glucose or 1% sodium acetate. Cell growth was monitored by measuring the OD660. The growth pattern and glucose consumption rate of the ΔrneG mutant on glucose minimal medium were very similar to those of the wild type (Fig. 7A). On the other hand, the growth of the wild type on CGC minimal medium containing 1% sodium acetate started after a lag phase of 2 to 3 h, while such a lag phase was not observed for the ΔrneG mutant (Fig. 7B). However, the growth rates were almost identical between the wild-type and ΔrneG mutant strains. The doubling times in sodium acetate medium were 147 ± 5.0 min in the wild type and 153 ± 4.0 min in the ΔrneG mutant. The ΔrneG mutant consumed sodium acetate faster than did the wild type, while the sodium acetate consumption rate of the ΔrneG mutant was almost the same as in the wild type (Fig. 7B). These results suggest that RNase E/G is involved in the adaptation to the change of carbon source from glucose to acetate.
Fig 7.
Growth and carbon consumption of the ATCC 31831 (wild type) and D2281 (ΔrneG) strains. For the growth curve, overnight cultures of the wild type (filled circles) and the ΔrneG mutant (open circles) grown on CGC minimal medium containing 1% glucose were washed and then inoculated into fresh CGC minimal medium containing either 1% glucose (A) or 1% sodium acetate (B). Cell growth was monitored by measuring the OD660. The consumption (filled triangles, ATCC 31831; open triangles, D2281) of glucose (A) and sodium acetate (B) was determined via an enzyme electrode glucose sensor and CE, respectively. All values are from at least three independent cultivations, and the error bars represent the standard deviations.
DISCUSSION
In this study, we searched for in vivo target RNAs of RNase E/G other than the 5S rRNA precursor. We found that ICL was overproduced in the ΔrneG mutant strain compared with the wild-type strain when cells were grown on sodium acetate minimal medium. The aceA mRNA accumulated in the ΔrneG mutant cells. The rifampin chase experiment showed that accumulation of aceA mRNA was due to its increased stability. These results indicate that RNase E/G is involved, at least in part, in the degradation of aceA mRNA. qRT-PCR analysis also suggests that RNase E/G specifically degrades aceA mRNA, although this does not exclude the possibility that RNase E/G degrades mRNAs other than the aceA mRNA. Since ICL is a key enzyme in the glyoxylate cycle, we conclude that RNase E/G is a novel regulator of the glyoxylate cycle in C. glutamicum. In order to identify other target RNAs, it is necessary to carry out comprehensive transcriptome and/or proteome analyses in the ΔrneG mutant cells.
What is the physiological meaning of the regulation of aceA expression by RNase E/G? In the case of E. coli, the flux between the TCA cycle and the glyoxylate cycle is well balanced by several factors. First, the genes of the glyoxylate cycle are induced only when acetate or fatty acids are the sole carbon source (17). In E. coli, the ICL and MS genes are organized as an operon together with the aceK gene encoding ICD kinase/phosphatase (17). AceK phosphorylates ICD; this lowers ICD turnover and prevents a high flux of isocitrate through the TCA cycle (10, 16). Since dephosphorylation of ICL leads to loss of catalytic activity (41), phosphorylation is assumed to increase the flux of isocitrate through the glyoxylate cycle in E. coli (11).
In contrast to the case with E. coli, the induction of the C. glutamicum aceA and aceB genes in the presence of acetate occurs independently of the presence or absence of an additional carbon and energy source (17). In addition, the aceA and aceB genes are not organized as an operon. Instead, the two genes are clustered on the chromosome and transcribed in divergent directions (17). Furthermore, there is no evidence for phosphorylation control of ICD in C. glutamicum (15). Overall, the flux between the TCA cycle and the glyoxylate cycle has to be well balanced, and the regulation of the aceA and aceB genes in C. glutamicum is assumed to be quite different from that seen in other bacteria. In the case of C. glutamicum, we showed that the expression of ICL is negatively regulated by RNase E/G. This is the first report showing that the glyoxylate cycle is regulated at the mRNA level. A biochemical study suggested that the inhibition or activation of MS plays only a minor role in controlling the carbon flow in the glyoxylate cycle (17); therefore, it is reasonable that RNase E/G specifically degrades aceA mRNA. We assume that the glyoxylate cycle is switched off instantly when acetate is all consumed. Therefore, RNase E/G-mediated degradation of aceA mRNA may permit quick changes in cell metabolism. As shown in Fig. 7B, the growth of the wild type and the ΔrneG mutant on sodium acetate minimal medium suggests that RNase E/G is involved in the adaptation to the change of carbon source from glucose to acetate, although further studies are necessary to clarify this hypothesis.
We were unable to detect new 5′ termini corresponding to the sites of RNase E/G cleavage in the 5′-UTR (Fig. 4A). In addition, there was no significant difference in the expression of LacZ protein from the aceA 5′-UTR-lacZ fusion gene between the wild type and the ΔrneG mutant (Fig. 6A and C). These results indicate that the RNase E/G-mediated degradation of aceA mRNA is 5′-UTR independent. Rather, the results of 3′ RACE analysis and the β-galactosidase activity assay indicate that degradation of aceA mRNA by RNase E/G is 3′-UTR dependent. The secondary structure of the 3′-UTR of aceA mRNA suggests a possible mechanism for the degradation of aceA mRNA in C. glutamicum (Fig. 5). RNase E/G cleavage truncates the aceA mRNA at its 3′ end, leaving the 5′ terminus intact. The preferred RNase E/G cleavage site is located in the single-stranded AU-rich region between two stem-loops (Fig. 5). This situation resembles the mechanisms involved in the cleavage of the E. coli cspA and rpsO mRNAs. In E. coli, RNase E cleaves cspA mRNA at a single site between two stem-loops (22). RNase E also controls the stability of the rpsO mRNA by the removal of a stable 3′ terminator stem-loop (8, 12). Since the RNase E cleavage site of cspA mRNA was determined by an in vitro RNase E assay (22, 29), it is necessary to perform an in vitro RNase E/G cleavage assay for aceA.
The strong stem-loop structure found at the 3′ end of many bacterial mRNAs, which functions as the transcription terminator structure, is resistant to 3′-to-5′ exonucleolytic degradation (39, 44). As shown in Fig. 4B, a stable 3′ end of the aceA mRNA was not detected in the wild type even if the amount of aceA transcript was increased by the introduction of the plasmid pNaceA. This suggests that RNase E/G cleavage generates a fragment with an unprotected 3′ end which is degraded rapidly by 3′-to-5′ exoribonucleases. In the case of E. coli, after the initial endoribonucleolytic cleavage, the degradation products are rapidly degraded by 3′-to-5′ exoribonucleases such as polynucleotide phosphorylase (PNPase) (2). After RNase E cleavage, the rpsO mRNA lacking the 3′ terminator stem-loop becomes a substrate for PNPase (7). Since C. glutamicum has a PNPase homolog (NCgl1900), this PNPase homolog may degrade aceA mRNA promptly after RNase E/G cleavage. Future studies will be necessary to understand how different sequences and structures located at the 3′ end affect the access of RNase E/G to its internal target site.
In the wild type, β-galactosidase activity expressed from the lacZ-aceA 3′-UTR fusion gene was about 1.5-fold lower in sodium acetate medium than in glucose medium, while such repression was not seen in the ΔrneG mutant (Fig. 6B). When C. glutamicum cells are grown on acetate, a large amount of aceA mRNA is synthesized. Therefore, it is possible that expression of the rneG gene increases to degrade the aceA mRNA efficiently when grown on acetate. It is also possible that some unknown factor(s) also participates in the 3′-UTR-dependent degradation of aceA mRNA. It is known that an RNA chaperone, Hfq, helps RNase E-dependent degradation of mRNAs in E. coli. Hfq facilitates the pairing of small RNAs with their target mRNAs (47). Although C. glutamicum does not have an Hfq homolog, it is possible that an unidentified functional counterpart of Hfq and/or a small RNA, which may be induced when grown on acetate, is involved in the 3′-UTR-dependent degradation of aceA mRNA by the C. glutamicum RNase E/G.
ACKNOWLEDGMENTS
We thank Ayako Takada for technical assistance in mass spectrometry.
This work was supported in part by a grant-in-aid for Scientific Research (B) (20380047 to M.W.) from the Japan Society for Promotion of Science, a grant from the Global COE Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Ministry of Economy, Trade and Industry of Japan entrusted by the New Energy and Industrial Technology Development Organization.
Footnotes
Published ahead of print 5 October 2012
REFERENCES
- 1. Anupama K, Krishna Leela J, Gowrishankar J. 2011. Two pathways for RNase E action in Escherichia coli in vivo and bypass of its essentiality in mutants defective for Rho-dependent transcription termination. Mol. Microbiol. 82:1330–1348 [DOI] [PubMed] [Google Scholar]
- 2. Arraiano CM, et al. 2010. The critical role of RNA processing and degradation in the control of gene expression. FEMS Microbiol. Rev. 34:883–923 [DOI] [PubMed] [Google Scholar]
- 3. Baker KE, Mackie GA. 2003. Ectopic RNase E sites promote bypass of 5′-end-dependent mRNA decay in Escherichia coli. Mol. Microbiol. 47:75–88 [DOI] [PubMed] [Google Scholar]
- 4. Bechhofer DH. 2011. Bacillus subtilis mRNA decay: new parts in the toolkit. Wiley Interdiscip. Rev. RNA 2:387–394 [DOI] [PubMed] [Google Scholar]
- 5. Becker J, Wittman C. 2012. Bio-based production of chemicals, materials and fuels—Corynebacterium glutamicum as versatile cell factory. Curr. Opin. Biotechnol. 23:631–640 [DOI] [PubMed] [Google Scholar]
- 6. Bott M. 2007. Offering surprises: TCA cycle regulation in Corynebacterium glutamicum. Trends Microbiol. 15:417–425 [DOI] [PubMed] [Google Scholar]
- 7. Braun F, Hajnsdorf E, Régnier P. 1996. Polynucleotide phosphorylase is required for the rapid degradation of the RNase E-processed rpsO mRNA of Escherichia coli devoid of its 3′ hairpin. Mol. Microbiol. 19:997–1005 [DOI] [PubMed] [Google Scholar]
- 8. Braun F, Le Derout J, Régnier P. 1998. Ribosomes inhibit an RNase E cleavage which induces the decay of the rpsO mRNA of Escherichia coli. EMBO J. 17:4790–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown TDK, Jones-Mortimer MC, Kornberg HL. 1977. The enzymatic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J. Gen. Microbiol. 102:327–336 [DOI] [PubMed] [Google Scholar]
- 10. Cozzone AJ. 1998. Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annu. Rev. Microbiol. 52:127–164 [DOI] [PubMed] [Google Scholar]
- 11. da Silva Cruz AH, et al. 2011. Phosphorylation is the major mechanism regulating isocitrate lyase activity in Paracoccidioides brasiliensis yeast cells. FEBS J. 278:2318–2332 [DOI] [PubMed] [Google Scholar]
- 12. Deana A, Belasco JG. 2005. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 19:2526–2533 [DOI] [PubMed] [Google Scholar]
- 13. Deutscher MP. 2006. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 34:659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eikmanns BJ, Metzger M, Reinscheid D, Kircher M, Sahm H. 1991. Amplification of three threonine biosynthesis genes in Corynebacterium glutamicum and its influence on carbon flux in different strains. Appl. Microbiol. Biotechnol. 34:617–622 [DOI] [PubMed] [Google Scholar]
- 15. Eikmanns BJ, Rittmann D, Sahm H. 1995. Cloning, sequence analysis, expression, and inactivation of the Corynebacterium glutamicum icd gene encoding isocitrate dehydrogenase and biochemical characterization of the enzyme. J. Bacteriol. 177:774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Mansi M, Cozzone AJ, Shiloach J, Eikmanns BJ. 2006. Control of carbon flux through enzymes of central and intermediary metabolism during growth of Escherichia coli on acetate. Curr. Opin. Microbiol. 9:173–179 [DOI] [PubMed] [Google Scholar]
- 17. Gerstmeir R, et al. 2003. Acetate metabolism and its regulation in Corynebacterium glutamicum. J. Biotechnol. 104:99–122 [DOI] [PubMed] [Google Scholar]
- 18. Ghora BK, Apirion D. 1978. Structural analysis and in vitro processing to p5 rRNA of a 9S RNA molecule isolated from an rne mutant of E. coli. Cell 15:1055–1066 [DOI] [PubMed] [Google Scholar]
- 19. Grundy F, Turinsky AJ, Henkin TM. 1994. Catabolite regulation of Bacillus subtilis acetate and acetoin utilization genes by CcpA. J. Bacteriol. 176:4527–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hajnsdorf E, Steier O, Coscoy L, Teysset L, Régnier P. 1994. Roles of RNase E, RNase II and PNPase in the degradation of the rpsO transcripts of Escherichia coli: stabilizing function of RNase II and evidence for efficient degradation in an ams pnp rnb mutant. EMBO J. 13:3368–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han SO, Inui M, Yukawa H. 2008. Effect of carbon source availability and growth phase on expression of Corynebacterium glutamicum genes involved in the tricarboxylic acid cycle and glyoxylate bypass. Microbiology 154:3073–3083 [DOI] [PubMed] [Google Scholar]
- 22. Hankins JS, Zappavigna C, Prud'homme-Gènèreux A, Mackie GA. 2007. Role of RNA structure and susceptibility to RNase E in regulation of a cold shock mRNA, cspA mRNA. J. Bacteriol. 189:4353–4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hermann T. 2003. Industrial production of amino acids by coryneform bacteria. J. Biotechnol. 104:155–172 [DOI] [PubMed] [Google Scholar]
- 24. Jiang X, Diwa A, Belasco JG. 2000. Region of RNase E important for 5′-end-dependent RNA cleavage and autoregulated synthesis. J. Bacteriol. 182:2468–2475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaberdin VR, Bläsi U. 2006. Translation initiation and the fate of bacterial mRNAs. FEMS Microbiol. Rev. 30:967–979 [DOI] [PubMed] [Google Scholar]
- 26. Kaga N, Umitsuki G, Nagai K, Wachi M. 2002. RNase G-dependent degradation of the eno mRNA encoding a glycolysis enzyme enolase in Escherichia coli. Biosci. Biotechnol. Biochem. 66:2216–2220 [DOI] [PubMed] [Google Scholar]
- 27. Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H. 2009. Identification and functional analysis of the gene cluster for L-arabinose utilization in Corynebacterium glutamicum. Appl. Environ. Microbiol. 75:3419–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim HJ, Kim TH, Kim Y, Lee HS. 2004. Identification and characterization of glxR, a gene involved in regulation of glyoxylate bypass in Corynebacterium glutamicum. J. Bacteriol. 186:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kime L, Jourdan SS, Stead JA, Hidalgo-Sastre A, McDowall KJ. 2010. Rapid cleavage of RNA by RNase E in the absence of 5′-monophosphate stimulation. Mol. Microbiol. 76:590–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kinoshita S, Udaka S, Shimono M. 1957. Studies on the amino acid fermentation. I. Production of L-glutamic acid by various microorganisms. J. Gen. Appl. Microbiol. 3:193–205 [PubMed] [Google Scholar]
- 31. Kushner SR. 2004. mRNA decay in prokaryotes and eukaryotes: different approaches to a similar problem. IUBMB Life 56:585–594 [DOI] [PubMed] [Google Scholar]
- 32. Lee K, Bernstein JA, Cohen SN. 2002. RNase G complementation of rne null mutation identifies functional interrelationships with RNase E in Escherichia coli. Mol. Microbiol. 43:1445–1456 [DOI] [PubMed] [Google Scholar]
- 33. Li Z, Pandit S, Deutscher MP. 1999. RNase G (CafA protein) and RNase E are both required for the 5′ maturation of 16S ribosomal RNA. EMBO J. 18:2878–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lin-Chao S, Wong TT, McDowall KJ, Cohen SN. 1994. Effects of nucleotide sequence on the specificity of rne-dependent and RNase E-mediated cleavages of RNA I encoded by the pBR322 plasmid. J. Biol. Chem. 269:10797–10803 [PubMed] [Google Scholar]
- 35. Mackie GA. 1998. Ribonuclease E is a 5′-end-dependent endonuclease. Nature 395:720–723 [DOI] [PubMed] [Google Scholar]
- 36. Maeda T, Sakai T, Wachi M. 2009. The Corynebacterium glutamicum NCgl2281 gene encoding an RNase E/G family endoribonuclease can complement the Escherichia coli rng::cat mutation but not rne-1 mutation. Biosci. Biotechnol. Biochem. 73:2281–2286 [DOI] [PubMed] [Google Scholar]
- 37. Maeda T, Wachi M. 2012. Corynebacterium glutamicum RNase E/G-type endoribonuclease encoded by NCgl2281 is involved in the 5′ maturation of 5S rRNA. Arch. Microbiol. 194:65–73 [DOI] [PubMed] [Google Scholar]
- 38. McDowall KJ, Lin-Chao S, Cohen SN. 1994. A + U content rather than a particular nucleotide order determines the specificity of RNase E cleavage. J. Biol. Chem. 269:10790–10796 [PubMed] [Google Scholar]
- 39. McLaren RS, Newbury SF, Dance GS, Causton HC, Higgins CF. 1991. mRNA degradation by processive 3′-5′ exoribonucleases in vitro and the implications for prokaryotic mRNA decay in vivo. J. Mol. Biol. 221:81–95 [PubMed] [Google Scholar]
- 40. Miller JH. 1972. Experiments in molecular genetics, p 352–355 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 41. Robertson EF, Reeves HC. 1989. Phosphorylation of isocitrate lyase in Escherichia coli. Biochimie 71:1065–1070 [DOI] [PubMed] [Google Scholar]
- 42. Sakai T, Nakamura N, Umitsuki G, Nagai K, Wachi M. 2007. Increased production of pyruvic acid by Escherichia coli RNase G mutants in combination with cra mutations. Appl. Microbiol. Biotechnol. 76:183–192 [DOI] [PubMed] [Google Scholar]
- 43. Sato H, et al. 2008. Distinct roles of two anaplerotic pathways in glutamate production induced by biotin limitation in Corynebacterium glutamicum. J. Biosci. Bioeng. 106:51–58 [DOI] [PubMed] [Google Scholar]
- 44. Spickler C, Mackie GA. 2000. Action of RNase II and polynucleotide phosphorylase against RNAs containing stem-loops of defined structure. J. Bacteriol. 182:2422–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Teramoto H, Inui M, Yukawa H. 2011. Transcriptional regulators of multiple genes involved in carbon metabolism in Corynebacterium glutamicum. J. Biotechnol. 154:114–125 [DOI] [PubMed] [Google Scholar]
- 46. Tock MR, Walsh AP, Carroll G, McDowall KJ. 2000. The CafA protein required for the 5′-maturation of 16S rRNA is a 5′-end-dependent ribonuclease that has context-dependent broad sequence specificity. J. Biol. Chem. 275:8726–8732 [DOI] [PubMed] [Google Scholar]
- 47. Vogel J, Luisi BF. 2011. Hfq and its constellation of RNA. Nat. Rev. Microbiol. 9:578–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wachi M, Umitsuki G, Shimizu M, Takada A, Nagai K. 1999. Escherichia coli cafA gene encodes a novel RNase, designated as RNase G, involved in processing of the 5′ end of 16S rRNA. Biochem. Biophys. Res. Commun. 259:483–488 [DOI] [PubMed] [Google Scholar]