Abstract
This study examined factors associated with teens’ adherence to a multiple health behavior cancer preventive intervention. Analyses identified predictors of trial enrollment, run-in completion, and adherence (intervention initiation, number of sessions completed). Of 104 teens screened, 73% (n = 76) were trial-eligible. White teens were more likely to enroll than non-whites (χ2 [1] df = 4.49, p = 0.04). Among enrolled teens, 76% (n = 50) completed the run-in; there were no differences between run-in completers and non-completers. A majority of run-in completers (70%, n = 35) initiated the intervention, though teens who initiated the intervention were significantly younger than those who did not (p < 0.05). The mean number of sessions completed was 5.7 (SD = 2.6; maximum = 8). After adjusting for age, teens with poorer session engagement (e.g., less cooperative) completed fewer sessions (B = -1.97, p = 0.003, R2 = 0.24). Implications for adolescent cancer prevention research are discussed.
Keywords: adolescents, multiple health behavior change, cancer prevention, intervention adherence
Introduction
Cancer is one of the leading causes of death in the U.S. (Jemal et al., 2005). While many factors contribute to risk for cancer (e.g., inherited traits and environmental influences), many types of cancer are associated with behavioral risk factors, such as cigarette smoking, alcohol use, poor diet, and physical inactivity (Mokdad et al., 2005; Ezzati et al., 2002). Indeed, a substantial proportion of cancer-related morbidity and mortality is preventable by modifying lifestyle and behavioral risks (Mokdad et al., 2005; Ezzati et al., 2002). Research also suggests that behavioral risk factors for cancer tend to originate early in life (Tercyak & Tyc, 2006; Werch, 2007). During this time, habits related to lifestyle behaviors such as smoking, alcohol use, diet, and physical activity can become established and may persist into adulthood (Tercyak & Tyc, 2006; Werch, 2007). Consequently, childhood presents a critical window of opportunity in which to encourage cancer risk-reducing behaviors and to promote cancer-protective ones before maladaptive habits are firmly entrenched (Tercyak & Tyc, 2006; Werch, 2007).
Evidence suggests that unhealthy behaviors associated with later cancer risk often co-occur, and that few individuals in the U.S. meet criteria for healthy lifestyle (Prochaska et al., 2008). This clustering of unhealthy behaviors may further accelerate the disease process of cancer. Consequently, there is an urgent need to address multiple behavioral risk factors in the context of comprehensive cancer prevention and control (Tercyak & Tyc, 2006; Prochaska et al., 2008; Werch, 2007). Multiple health behavior change (MHBC) interventions are those which are designed to address two or more health behaviors, concurrently or sequentially, within a defined timeframe (Prochaska et al., 2008). MHBC interventions have several advantages over interventions targeting a single health behavior, given limited opportunities for health promotion, including the ability to efficiently manipulate related behaviors and the potential for cascading positive health behavior changes across lifestyles (Prochaska et al., 2008; Prochaska et al., 2010).
MHBC intervention science is still developing, and several questions regarding optimal MHBC intervention strategies remain unanswered (Goldstein et al., 2004; Prochaska et al., 2008). For example, methodological considerations such as the ideal settings in which to administer MHBC interventions, and whether behaviors are best addressed concurrently or sequentially, are unclear (Prochaska et al., 2008). Importantly, much MHBC intervention research conducted to date has focused on adults; scant evidence exists regarding effective MHBC strategies among young people (Prochaska et al., 2008; Prochaska and Sallis, 2004; Sanchez et al., 2007).
Adherence to complex behavioral interventions among adolescents is an issue that has received relatively little attention in the cancer prevention research literature. The World Health Organization defines adherence as the extent to which a person's behavior corresponds with the recommendations of a healthcare provider (World Health Organization, 2003). A recent review of behavioral interventions for physical activity targeting teens concluded that very few studies report information regarding intervention adherence among young people (van Sluijs, McMinn, & Griffin, 2007). Research examining interventions for adolescent smoking behaviors further suggests that certain subgroups of teens, including racial/ethnic minority youths, may be more difficult to engage in behavioral intervention trials (Diviak et al., 2006; Audrain, Tercyak, Goldman, & Bush, 2002). Evidence regarding MHBC intervention adherence among young people, and the potential factors influencing intervention adherence, remains limited but is important to address.
For adolescents, the primary care setting may be a good venue to deploy MHBC interventions (McDonald and Kaplan, 2002). A majority of U.S. youths younger than age 18 visit a primary care provider for routine medical care at least once a year (U.S. Department of Health and Human Services et al., 2009), and clinical practice guidelines encourage pediatric primary care providers to address multiple cancer-related lifestyle risks during preventive visits (Sege & De Vos, 2010). While researchers have pointed out the need to develop multiple risk behavior intervention strategies for the primary care setting (Goldstein et al., 2004; Orleans, 2004; Pronk et al., 2004), practical barriers inhibit implementation of MHBC interventions within the primary care context, such as lack of time and resources to implement them (Pronk et al., 2004). In light of these barriers, MHBC interventions administered among young people as an adjunct to standard primary medical care could be proposed as a viable solution.
To date, there have been few studies seeking to develop MHBC cancer prevention interventions for adolescents administered ancillary to primary medical care, despite evidence suggesting this may be an important context in which to implement MHBC interventions among young people. Moreover, there has been little research to help health professionals understand what factors may influence adolescents’ enrollment and adherence in MHBC intervention programs organized in this setting. Such knowledge is important to inform strategies seeking to enhance the external validity of future MHBC research among adolescents. In order to fill this research gap, we examined factors associated with adolescents’ enrollment in and intervention adherence to the Healthy for Life Program (HELP), a manualized MHBC cancer preventive education and counseling intervention administered as an adjunct to standard medical care among adolescents age 13– 21. Here, we investigate differences in sociodemographic characteristics across stages of trial recruitment and enrollment and examine if such characteristics, along with theoretical constructs (e.g., cancer prevention self-efficacy) and health educator-reported intervention engagement, are associated with adherence to HELP.
Method
Design
The study was a small-scale randomized controlled trial designed to evaluate the efficacy of a manualized, telephone-based prevention intervention consisting of education and counseling in addition to standard preventive medical care, compared with education and standard medical care only. The setting was an adolescent medicine clinic housed within a large, tertiary-care hospital in Washington, D.C. The clinic has a sizable and diverse patient population, with approximately 2,100 adolescents age 13 – 21 seen for routine (i.e., well-visit) check-ups annually. Institutional review board approval was obtained prior to the study and the trial was registered in ClinicalTrials.gov (NCT00459238).
Participant Recruitment
Research staff members were trained to identify potential participants visiting the clinic and to screen them for eligibility. Inclusion criteria were: age 13 – 21 years with access to a cellular and/or landline telephone and free of an illness or disability that would limit their participation, such as conditions that restricted diet and physical activity.
Potentially-eligible teens were informed about the trial at the time of their medical visit by a clinic staff member and, if interested, were provided additional information about the study by a research staff member who was present. All potentially-eligible teens were given a trial enrollment packet by the research staff member, which included detailed information about the trial and two copies of a teen consent (for those ≥ 18 years of age) or a parental consent form and a teen assent form (for those < 18 years of age). Teens and/or their parent were asked to complete a brief eligibility screen and, if eligible, read and signed the trial consent/assent forms. For teens attending the clinic without a parent, and for those who wanted more time to decide about participation, a research staff member collected their names and contact information, provided them with a pre-addressed stamped envelope, and followed-up by telephone within 7 days to complete trial enrollment.
Consenting participants completed an in-person pre-baseline assessment at the clinic that consisted of a short questionnaire about smoking behavior and biochemical verification of smoking status. Trial participants then completed the baseline assessment within 1 month (Median =30 days) of study enrollment. Baseline assessments were administered by telephone by a trained research staff member. The pre-baseline and baseline behavioral assessments served as the trial's run-in (Ulmer et al., 2008); only those successfully completing the run-in were subsequently randomized to reduce possible attrition (Ulmer et al., 2008).
Teens were randomly allocated to either an education (“Education”) or education and counseling (“Counseling”) condition. To promote trial participation and engagement, appointment reminders for all study sessions and interviews were sent by postal mail, and up to 10 attempts were made to reach participants by telephone for all intervention sessions and interviews. Follow-up assessments were also administered via telephone at 1- and 3-months post-intervention; this analysis focuses on baseline data only. Participants were provided with modest incentives (e.g., $5-$10 gift cards to popular media outlets) to complete intervention sessions and study assessments.
Intervention Description
The Healthy for Life Program (HELP) was designed to increase knowledge about lifestyle-based cancer risks, foster healthy attitudes toward one's body, promote family health history taking, and reduce multiple behavioral risks for cancer (i.e., diet, physical inactivity, smoking, and alcohol use). Intervention content was delivered for both study conditions via telephone by two masters-level pediatric health educators who were trained by the study team; all intervention content was manualized. The total time of participation for both the Education and Counseling conditions was approximately 270 minutes, divided across up to 8 × 45-minute sessions administered weekly for 8 weeks. Content for both conditions was delivered using a combination of educational tactics including didactics, visual materials mailed to participants, verbal demonstrations, written action plans, and instructions to review written action plans following the conclusion of treatment.
Enrolled teens were mailed an intervention kit several days in advance of their first telephone session. The kit included a calendar to record dates and times for phone calls and an age-appropriate workbook with self-help materials that were developed as part of the intervention, including a family health history form; information about cancer, cigarette smoking, alcohol use, and nutrition; and other health education-related materials developed for the study.
Education Condition
Telephone-based cancer education, in addition to standard medical care, was the comparison condition in the trial. The Education content concentrated on refraining from tobacco and alcohol use and promoting age-appropriate awareness of cancer screening upon reaching adulthood. The content also included a focus on a healthy diet, including adequate fruit, vegetable and fiber intake, and reduced fat intake.
Counseling Condition
The Counseling intervention combined psychological, health education, and health promotion counseling, and relied on the principles of motivational counseling for teen health behavior change (Colby et al., 2005). Grounded in Social Cognitive Theory and the Health Belief Model, the Counseling intervention addressed benefits and barriers of assuming greater responsibility for one's health and well-being (see Colby et al., 2005 for a theoretical review), knowledge, skills, self-efficacy, and personal, social, and environmental resources to promote lasting behavior change (Bandura, 1997). The intervention sought to influence health-promoting and cancer risk-reducing behaviors by enhancing teens’ motivation through highlighting where their behaviors fell at baseline (ipsative), with respect to their personal goals (goal), and with respect to public health recommendations (normative). The Counseling condition also incorporated motivational-type counseling to enhance desire to change, engage in behavioral rehearsal (e.g., cigarette refusal skills), provide behavioral feedback, improve self-efficacy, and to discuss the pros and cons of behavior change and barriers to behavior change. Counseling mirrored the Education condition in terms of participant engagement and extent of contact with health educators; only the motivational and affective content differed between the two conditions.
Measures
Trial Status
Trial recruitment and enrollment status was tracked using criteria from the Consolidated Standards of Reporting Trials (CONSORT) (Schulz et al., 2010).
Intervention Adherence
We examined two outcome variables representing intervention adherence. Among participants who completed the trial run-in (n = 50), we created a dichotomous intervention initiation variable reflecting whether teens completed zero (0) versus one or more (1+) intervention session(s). Among participants completing 1+ intervention sessions (n = 35), we created a continuous intervention adherence variable reflecting the total number of intervention sessions completed (range 1 – 8).
Multiple Cancer Risk Factors
Similar to prior MHBC research (Emmons et al., 1994; Emmons et al., 2005; Lopez et al., 2007; Tercyak et al., 2006), a total multiple cancer risk factor index was operationalized using a continuous variable based on nine individual cancer risk factors. The index was based on teens’ self-reported nutrition (< 5 servings of fruits and vegetables each day), physical activity (< 3 days per week with 20 minutes or more of vigorous physical activity), overweight or obese status, lifetime alcohol use, intentions to use alcohol, lifetime smoking, intentions to smoke, no or low future cancer screening intentions upon reaching adulthood, and family history of cancer (immediate or extended family member). Overweight/obese status was defined as Body Mass Index ≥ 25 calculated using a standard formula based on self-reported height and weight (Ogden, Carroll, and Flegal, 2008). Behavioral items were drawn from psychometrically-sound assessments of adolescent health risk behaviors (Brener et al., 2004), and cancer screening and family history items were created for the purpose of this study. Each cancer risk factor was operationalized using a dichotomous variable (0=absent, 1=present) and a summary score (range 0 – 9) was computed to reflect the total cancer risk factors (Emmons et al., 1994; Emmons et al., 2005; Lopez et al., 2007; Tercyak et al., 2006).
Cancer Knowledge
Teens’ knowledge about cancer causes and prevention was assessed using 22 true/false items (Price et al., 1988). A continuous variable was computed by summing participants’ correct responses, with higher scores reflecting greater cancer knowledge (range 0 – 22, Kuder-Richardson 20 = 0.72).
Perceived Barriers and Benefits
Perceived benefits of adopting cancer-protective health behaviors, including participating in cancer screening upon reaching adulthood, were assessed using five items with a 4-point Likert scale response adapted from previous research to be age- and content-appropriate for teens (Yeomans-Kinney et al., 1995). Items were preceded by the statement: “Here are some possible benefits of leading a healthier lifestyle now (while you are young). Indicate how strongly you agree or disagree with each of the following possible benefits.” Example items included: “Prevent cancer and heart disease in the future” and “Stay healthy now and in the future.” The items were summed to create an overall benefits score, with higher values reflecting greater perceived benefits (range 5 – 20, Cronbach's α = 0.68).
Similarly, perceived barriers were also assessed using five items with 4-point Likert scale response adapted from prior work (Friedman et al., 1994). Items were preceded by the statement: “Here are some possible barriers to leading a healthier lifestyle now (while you are young). Indicate how strongly you agree or disagree with each of the following possible barriers.” Example items included: “Don't have enough time” and “Don't believe it is important.” A summary variable was calculated based on participants’ responses, with higher values reflecting more perceived barriers to preventive and screening behaviors (range 5 – 20, Cronbach's α = 0.70). A variable reflecting the ratio of perceived benefits:perceived barriers for cancer prevention was then created and analyzed (Janz & Becker, 1984).
Prevention Self-Efficacy
In addition to measuring perceived benefits/barriers to cancer prevention, we also examined participants’ confidence in their ability to take cancer preventive actions. Self-efficacy for engaging in health-promoting behaviors and preventing cancer was evaluated through seven 4-point Likert scale items derived from earlier research (Friedman et al., 1994). Items were preceded by the statement “Tell me how confident you are in your ability to prevent cancer by...” Examples of health-promoting behaviors included: “Eating healthy,” “Becoming and staying physically active,” “Not smoking,” and “Not drinking alcohol.” Responses to the items were summed to reflect an overall self-efficacy score, with higher values indicating greater prevention self-efficacy (range 7-28, Cronbach's α = 0.70).
Response Bias
Response bias was assessed using 14 slightly negative true-false items adapted from the Lie Scale of the Minnesota Multiphasic Personality Inventory-Adolescent (Hays & McCallum, 2005). “True” responses indicate more honest reporting; more than half of participants (58%) answered 70% of questions with “true” responses. Responses to the items were summed to create a continuous response bias score, with higher values indicating more honest reporting (range 0 – 14, Kuder-Richardson 20 = 0.61).
Intervention Engagement Measures
After completing all intervention sessions, health educators evaluated intervention engagement for each study participant for whom they administered intervention sessions. Eight 4-point Likert-type items were used to create two intervention engagement measures based on health educators’ report. Probing (3 items, α = 0.78) included items such as “Subject required a lot of probing” and “Subject was reserved (too shy).” Difficulty (5 items, α = 0.84) included items such as “Subject was disengaged or detached,” “Subject was distracted by frequent interruptions,” and “Subject did not want to learn or participate.” For both the Probing and Difficulty subscales, a continuous variable (range 1 – 4) was created based on the mean response across the items that comprised each subscale.
Of the 35 participants who initiated intervention, six completed assessments for Probing and Difficulty measures that used a less sensitive, truncated (i.e., yes/no) response format. For these individuals, sample mean values for the Likert-type Probing and Difficulty subscale items sampled were imputed. All analyses were replicated with these six participants excluded from the sample and the results were unchanged. Therefore, we included these six participants in the analysis reported.
Sociodemographics
Sociodemographics assessed included participant gender, age, and race. For participants who completed baseline assessments, census tract household income was estimated using geo-coding based on teens’ home address (Federal Financial Institutions Examination Council, 2010).
Statistical Analysis
Analyses examined differences in sociodemographic characteristics across stages of trial recruitment and enrollment and, among those enrolled in the trial, factors predicting intervention adherence. We assessed differences in age, gender, and race based on stages of trial recruitment and enrollment using bivariate statistics (e.g., χ2 tests, t tests). Through bivariate analyses (i.e., χ2 tests, t tests, Pearson's r correlations), factors associated with intervention initiation and adherence were also examined. Finally, regression modeling was used to examine factors associated with intervention initiation and adherence in multivariate analyses. Predictor variables that were associated with intervention initiation and adherence in bivariate analyses at p < 0.15 were considered for inclusion in these models.
Results
Trial Status
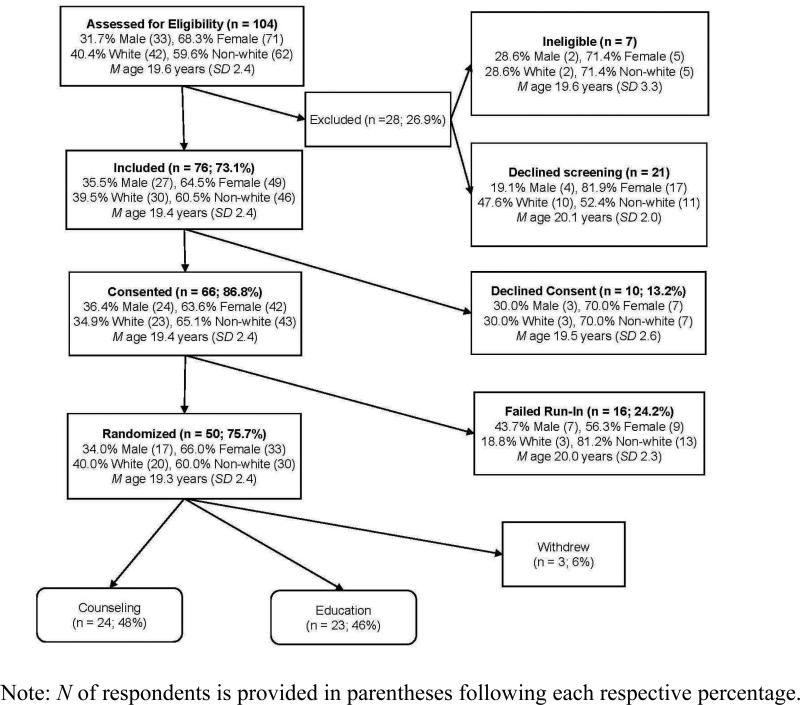
Trial status across stages of participant recruitment and enrollment is displayed in Figure 1. In total, 104 adolescents were screened for eligibility, with 76 (73%) meeting trial inclusion criteria. Of these adolescents, 66 (87%) provided informed consent and 50 participants (76%) completed the trial run-in and were randomly allocated to either the Education or Counseling condition. Of the 50 run-in completers, 3 (6%) withdrew from further study after randomization but prior to the start of the intervention and were lost to follow-up, 23 were allocated to Education (46%), and 24 were allocated to Counseling (48%).
Figure 1.
Healthy for Life Program (HELP) participant flow through screening, baseline, and randomization process
Eligible teens declining to provide informed consent were significantly more likely (p < 0.05) to be non-white compared with eligible teens who consented. Consented teens who did not complete the trial run-in were more likely to be non-white compared with run-in completers, although this difference only approached conventional statistical significance (p < 0.10).
Baseline Participant Characteristics
Baseline participant characteristics of teens completing the trial run-in (n = 50) are displayed in Table 1. Participants averaged 16.6 years of age (Standard Deviation [SD] = 2.3) and a majority were non-white (59.6%) and female (68.1%). Overall, participants had scores that reflected relatively high cancer knowledge, prevention self-efficacy, and perceived benefits, and few perceived barriers (Table 1). Participants reported a mean (M) of 4.6 (SD = 1.6) out of 9 possible cancer risk factors. Participants’ responses also reflected relatively low levels of response bias, indicating honest reporting (Table 1).
Table 1.
Participant Characteristics by Intervention Initiation
| Study Sample (N = 50) | Initiated Intervention (n = 35) | Did Not Initiate Intervention (n = 15) | ||||
|---|---|---|---|---|---|---|
| Demographics | m/n | sd/% | m/n | sd/% | m/n | sd/% |
| Age (m/sd)* | 16.6 | 2.3 | 16.2 | 2.1 | 17.7 | 2.3 |
| Race/Ethnicity (n/%) | ||||||
| White | 20 | 40.0 | 14 | 40.0 | 6 | 40.0 |
| Non-white | 30 | 60.0 | 21 | 60.0 | 9 | 60.0 |
| Gender (n/%) | ||||||
| Male | 16 | 32.0 | 10 | 28.6 | 6 | 40.0 |
| Female | 34 | 68.0 | 25 | 71.4 | 9 | 60.0 |
| Household Income ($) (m/sd) | 106, 248 | 69,941 | 115,205 | 70,296 | 85,350 | 66,721 |
| Theoretical Predictors (m/sd) | ||||||
| Cancer Knowledge (α = 0.72, range 0 – 22) | 17.2 | 3.1 | 16.9 | 3.4 | 17.9 | 2.1 |
| Prevention Benefits:Barriers | 1.94 | 0.69 | 1.84 | 0.42 | 2.19 | 1.09 |
| Prevention Self-Efficacy (α = 0.70, range 7 – 28) | 21.9 | 3.2 | 22.1 | 3.0 | 21.4 | 3.6 |
| Response Bias (m/sd) (α = 0.61, range 0 – 14) | 11.0 | 2.1 | 11.2 | 1.7 | 10.6 | 2.8 |
| Total Cancer Risk Factors (m/sd) (range 0 – 9) | 4.6 | 1.6 | 4.4 | 1.5 | 5.0 | 1.9 |
| Intervention Engagement Measures (m/sd) | ||||||
| Probing (3 items α = 0.78, range 1 – 4) | 1.6 | 0.66 | ||||
| Difficulty (5 items α = 0.84, range 1 – 4) | 1.7 | 0.62 | ||||
| Intervention Adherence | ||||||
| Sessions Completed (range 1 – 8) | 5.7 | 2.6 | ||||
Mean age was significantly (p < 0.05) lower among those who initiated the intervention compared with those who did not initiated the intervention.
Intervention Adherence
A majority (75%) of teens completing the run-in initiated the intervention (i.e., completed 1+ sessions); on average, participants who initiated the intervention completed 5.7 (SD = 2.6) of 8 possible intervention sessions. The mean number of sessions completed did not differ significantly between participants randomized to Education (M = 5.2, SD = 2.7) or Counseling (M = 6.2, SD = 2.5, p = 0.44), and the average total hours spent in intervention did not differ between study groups (Education M = 5.5, SD = 2.7, Counseling M = 6.4, SD = 2.5, p = 0.34). In bivariate analyses, participants completing the trial run-in and initiating the intervention were significantly younger (n =35, M = 16.2, SD = 2.1) than those who completed the run-in but did not initiate the intervention (n = 15, M = 17.7, SD = 2.3, p < 0.05; Table 2).
At the bivariate level, factors associated with the number of intervention sessions completed were participant age (Pearson's r = 0.26, p = 0.14) and health educator-reported intervention difficulty (Pearson's r = -0.52, p = 0.002). In a linear regression model, completion of more intervention sessions was associated with less difficulty based on health educators’ report, after adjusting for participant age (B = -1.97, Standard Error B = 0.62, p = 0.003, Adj. R2 = 0.24).
Discussion
This study examined factors associated with teens’ enrollment and intervention adherence into the Healthy for Life Program (HELP), a manualized multiple health behavior change cancer preventive education and counseling intervention administered by telephone and ancillary to standard medical care among adolescents age 13 – 21. The results highlight potentially important implications for future research in this area of investigation. Findings from one prior adolescent MHBC intervention study focusing on nutrition and physical activity behaviors suggests that, while generally receptive to the intervention content, teens in the MHBC intervention arm were more likely to indicate that the intervention was “too long” compared with those in the single behavior and comparison arms of the study (Prochaska & Sallis, 2004). As the authors point out, the added demand of participating in more intense MHBC interventions may overwhelm some teens and adversely affect their adherence (Prochaska & Sallis, 2004).
Our results suggest there may be specific subgroups of teens for whom participation in an intensive, eight session MHBC intervention program may be demanding. We found that non-white teens eligible for participation were less likely to enroll than white teens, and teens who initiated the trial were more likely to be younger. These sociodemographic characteristics may represent proxies for other underlying factors that affect enrollment and initiation into a MHBC intervention program. For example, with increasing age adolescents are more likely to have employment outside of the home (U.S. Bureau of Labor Statistics, 2009). Older teens may also have other competing time-consuming obligations and demands (e.g., work studies, extracurricular activities) that limit the time they can devote to health promotion activities. The reasons underlying differences in participation between white and non-white teens may also include cultural and socioeconomic factors (Diviak et al., 2006), such as lack of interest, misunderstanding about what is involved in research participation, and mistrust (Audrain et al., 2002; Yancy, Ortega, & Kumanyika, 2006).
For future MHBC intervention studies, it is critical to understand why older and non-white teens may be less likely to participate and to develop appropriate strategies to enhance their participation. In particular, research seems needed to develop and evaluate motivational strategies for participant recruitment, enrollment, and adherence, especially among those who appear to be more difficult to engage (Nigg et al., 2002; Patrick et al., 2006). This may include deeper examinations of age- and culturally-appropriate intervention strategies to enhance participation, such as involving members of the target population in trial recruitment/enrollment procedures and emphasizing consistency between program objectives and potential participants’ goals to lead healthy a lifestyle (Yancy, Ortega, & Kumanyika, 2006; Chang, Brown, & Nitzke, 2009).
HELP employed a simple behavioral run-in to reduce potential drop-outs following randomization and to ensure enrolled teens were committed to participating in the intervention (Ulmer et al., 2008). Overall, the run-in method appeared to be effective. Among teens completing the run-in, three-quarters initiated the intervention and, on average, these teens completed nearly 6 out of 8 possible intervention sessions. In total, only 24% of those who provided informed consent failed the behavioral run-in and were further excluded from the trial.
The findings also indicate, however, that our run-in may have been less effective among older teens, as they were less likely to initiate the intervention following randomization. This finding may be a result of sampling bias if older teens were less likely to visit the clinic. There are also inherent disadvantages of using a behavioral run-in for intervention trials, such as the possibility that the run-in eliminates potential participants who systematically differ from run-in completers (Ulmer et al., 2008). Future studies can build from this work by exploring alternative run-in methods of varying content and intensity, particularly those geared towards increasing MHBC intervention adherence among older adolescents and maximizing external validity of MHBC intervention studies more broadly.
To our knowledge, this study is among the first to examine health educator-reported measures of teens’ intervention adherence to a MHBC intervention trial focused on cancer prevention. Results suggest that health educator-reported difficulty was associated with the number of sessions completed among teens who initiated HELP, where more perceived difficulty was associated with significantly fewer completed sessions, after accounting for the influence of participant age. Intervention difficulty captured both participant characteristics (i.e., disengaged or detached; unprepared; did not want to learn) and practical factors (i.e., difficult to reach/schedule; frequent interruptions) affecting intervention progress. Future studies can expand on this work by examining how individual attributes (e.g., being unprepared) or setting (e.g., frequent interruptions) affect program adherence and outcomes. Doing so could be important to examine among teens prior to implementing MHBC interventions in an effort to enhance intervention engagement by facilitators and address practical limitations to participation, possibly through the use of an orientation session (Germann, Kirschenbaum, & Rich, 2006).
This trial's findings should be interpreted in light of important study limitations. First, the study is based on a small sample of adolescents from recruited through an adolescent medicine clinic housed at an academic medical center, which likely limited the power of this study to detect significant effects in analyses. Moreover, while the sample is demographically diverse, participants were from relatively high income households. Thus, generalizations of the results to broader populations are strongly cautioned. Second, all measures were based on self-reported behavior, and while the results did not indicate that participants were prone to response bias, they should be interpreted with this in mind. Some measures were also developed for the purposes of this research, and their reliability and validity should be examined in greater detail. Finally, analyses of theoretical and cancer risk factor predictors of intervention initiation and adherence were limited to data from participants who completed a trial run-in. Theoretical predictor and cancer risk factor data were not collected among others.
Despite these limitations, through the Healthy for Life Program we successfully designed and implemented a complex, cancer prevention MHBC intervention trial for teens within a primary care clinical setting. The recruitment and enrollment methods successfully engaged eligible teens, and the flexible, telephone-based intervention delivery format enhanced adherence among most teens who initiated the intervention. Our findings also point to potentially important areas for future MHBC cancer prevention research. Non-white and older teens were more difficult to engage in the intervention and greater health educator-reported difficulty with participants was also associated with completion of fewer intervention sessions. Additional research to develop effective motivational strategies to enhance recruitment and enrollment for MHBC intervention studies among hard-to-reach teens, and to more closely examine intervention engagement factors affecting teens’ adherence, seems warranted. Research examining strategies to improve training for health educators to enhance participant engagement in MHBC interventions may also help improve adherence.
Acknowledgments
The authors would like to thank the physicians, nurses, and staff of the clinical setting where this research took place. We are also grateful to the interventionists and consultants who contributed to the project, along with the adolescents and families who participated.
Funding Source:
This study was funded by Grant # CA119686 from the National Cancer Institute at the National Institutes of Health (PI: Kenneth Tercyak, PhD).
Footnotes
This is the pre-publication, author-produced version of a manuscript accepted for publication in Health Education & Behavior.
References
- Audrain J, Tercyak KP, Goldman P, Bush A. Recruiting adolescents into genetic studies of smoking behavior. Cancer Epidemiology, Biomarkers, & Prevention. 2002;11:249. [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. W.H. Freeman; New York: 1997. [Google Scholar]
- Brener ND, Kann L, Kinchen SA, Grunbaum JA, Whalen L, et al. Methodology of the youth risk behavior surveillance system. MMWR. 2004;53:1–13. [PubMed] [Google Scholar]
- Chang MW, Brown R, Nitzke S. Participant recruitment and retention in a pilot program to prevent weight gain in low-income overweight and obese mothers. BMC Public Health. 2009;9:424. doi: 10.1186/1471-2458-9-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Monti PM, O'Leary TT, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in medical settings. Addictive Behaviors. 2005;30:865–874. doi: 10.1016/j.addbeh.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Divak KR, Wahl SK, O'Keefe JJ, Mermelstein RJ, Flay BR. Recruitment and retention of adolescents in a smoking trajectory study: Who participated and lessons learned. Substance Use & Misuse. 2006;41:175–182. doi: 10.1080/10826080500391704. [DOI] [PubMed] [Google Scholar]
- Driskell MM, Dyment S, Mauriello L, Castle P, Sherman K. Relationships among multiple behaviors for childhood and adolescent obesity prevention. Preventive Medicine. 2008;46:209–215. doi: 10.1016/j.ypmed.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Emmons KM, Marcus BH, Linnan L, Rossi JS, Abrams DB. Mechanisms in multiple risk factor interventions: smoking, physical activity, and dietary fat intake among manufacturing workers. Working Well Research Group. Preventive Medicine. 1994;23:481–489. doi: 10.1006/pmed.1994.1066. [DOI] [PubMed] [Google Scholar]
- Emmons KM, McBride CM, Puleo E, Pollak KI, Clipp E, Kuntz K, et al. Project PREVENT: a randomized trial to reduce multiple behavioral risk factors for colon cancer. Cancer Epidemiology, Biomarkers & Prevention. 2005;14:1453–1459. doi: 10.1158/1055-9965.EPI-04-0620. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ, Comparative Risk Assessment Collaborating Group Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Federal Financial Institutions Examination Council [August 25, 2010];Geocoding System. 2010 Available: http://www.ffiec.gov/geocode/default.aspx.
- Friedman LC, Nelson DV, Webb JA, Hoffman LP, Baer PE. Dispositional optimism, self-efficacy, and health beliefs as predictors of breast self-examination. American Journal of Preventive Medicine. 1994;10:130–135. [PubMed] [Google Scholar]
- Germann JN, Kirschenbaum DS, Rich BH. Use of an orientation session may help decrease attrition in a pediatric weight management program for low-income minority adolescents. Journal of Clinical Psychology in Medical Settings. 2006;13:169–179. [Google Scholar]
- Goldstein MG, Whitlock EP, DePue J, Planning Committee of the Addressing Multiple Behavioral Risk Factors in Primary Multiple behavioral risk factor interventions in primary care. Summary of research evidence. American Journal of Preventive Medicine. 2004;27:61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Hays S, McCallum RS. A comparison of the pencil-and-paper and computer-administered Minnesota Multiphasic Personality Inventory-Adolescent. Psychology in the Schools. 2005;42:605–613. [Google Scholar]
- Janz NK, Becker MH. The Health Belief Model: A decade later. Health Education Quarterly. 1984;11:1–47. doi: 10.1177/109019818401100101. [DOI] [PubMed] [Google Scholar]
- Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- Keller S, Maddock JE, Hannover W, Thyrian JR, Basler HD. Multiple health risk behaviors in German first year university students. Preventive Medicine. 2008;46:189–195. doi: 10.1016/j.ypmed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Lopez ML, Iglesias JM, del Valle MO, Comas A, Fernandez JM, et al. Impact of a primary care intervention on smoking, drinking, diet, weight, sun exposure, and work risk in families with cancer experience. Cancer Causes and Control. 2007;18:525–535. doi: 10.1007/s10552-007-0124-0. [DOI] [PubMed] [Google Scholar]
- McDonald TP, Kaplan D. Adolescent preventive services for girls. Current Women's Health Reports. 2002;2:450–456. [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Correction: actual causes of death in the United States, 2000. JAMA. 2005;293:293–294. doi: 10.1001/jama.293.3.293. [DOI] [PubMed] [Google Scholar]
- Nigg CR, Allegrante JP, Ory M. Theory-comparison and multiple-behavior research: common themes advancing health behavior research. Health Educatioin Research. 2002;17:670–679. doi: 10.1093/her/17.5.670. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among U.S. children and adolescents, 2003-2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Orleans CT. Addressing multiple behavioral health risks in primary care. Broadening the focus of health behavior change research and practice. American Journal of Preventive Medicine. 2004;27:1–3. doi: 10.1016/j.amepre.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Patrick K, Calfas KJ, Norman GJ, Zabinski MF, Sallis JF, Rupp J, et al. Randomized controlled trial of a primary care and home-based intervention for physical activity and nutrition behaviors: PACE+ for adolescents. Archives of Pediatrics & Adolescent Medicine. 2006;160:128–136. doi: 10.1001/archpedi.160.2.128. [DOI] [PubMed] [Google Scholar]
- Price JH, Desmond SM, Wallace M, Smith D, Stewart PM. Differences in black and white adolescents’ perceptions about cancer. The Journal of School Health. 1988;58:66–70. doi: 10.1111/j.1746-1561.1988.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Nigg CR, Spring B, Velicer WF, Prochaska JO. The benefits and challenges of multiple health behavior change in research and in practice. Preventive Medicine. 2010;50:26–29. doi: 10.1016/j.ypmed.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Sallis JF. A randomized controlled trial of single versus multiple health behavior change: promoting physical activity and nutrition among adolescents. Health Psychology. 2004;23:314–318. doi: 10.1037/0278-6133.23.3.314. [DOI] [PubMed] [Google Scholar]
- Prochaska JJ, Spring B, Nigg CR. Multiple health behavior change research: an introduction and overview. Preventive Medicine. 2008;46:181–188. doi: 10.1016/j.ypmed.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronk NP, Peek CJ, Goldstein MG. Addressing multiple behavioral risk factors in primary care. A synthesis of current knowledge and stakeholder dialogue sessions. American Journal of Preventive Medicine. 2004;27:4–17. doi: 10.1016/j.amepre.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Norman GJ, Sallis JF, Calfas KJ, Cella J, Patrick K. Patterns and correlates of physical activity and nutrition behaviors in adolescents. American Journal of Preventive Medicine. 2007;32:124–130. doi: 10.1016/j.amepre.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sege RD, De Vos E. Evidence-based health care for children: What are we missing? Issue Brief. 2010;85:1–14. [PubMed] [Google Scholar]
- Tercyak KP, Donze JR, Prahlad S, Mosher RB, Shad AT. Multiple behavioral risk factors among adolescent survivors of childhood cancer in the Survivor Health and Resilience Education (SHARE) program. Pediatric Blood & Cancer. 2006;47:825–830. doi: 10.1002/pbc.20602. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Tyc VL. Opportunities and challenges in the prevention and control of cancer and other chronic diseases: children's diet and nutrition and weight and physical activity. Journal of Pediatric Psychology. 2006;31:750–763. doi: 10.1093/jpepsy/jsj126. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of Labor Statistics [August 11, 2010];Current Population Survey. Table 3: Employment status of the civilian noninstitutional population by age, sex, and race. 2009 from http://www.bls.gov/cps/cpsaat3.pdf.
- U.S. Department of Health. Human Services . Healthy People 2010: Understanding and improving health. 2nd ed. U.S. Government Printing Office; Washington, DC: 2000. [Google Scholar]
- U.S. Department of Health and Human Services. Health Resources and Services Administration, Maternal and Child Health Bureau . Child Health USA 2008-2009. U.S. Department of Health and Human Services; Rockville, MD: 2009. [Google Scholar]
- Ulmer M, Robinaugh D, Friedberg JP, Lipsitz SR, Natarajan S. Usefulness of a run-in period to reduce drop-outs in a randomized controlled trial of a behavioral intervention. Contemporary Clinical Trials. 2008;29:705–710. doi: 10.1016/j.cct.2008.04.005. [DOI] [PubMed] [Google Scholar]
- van Sluigs EM, McMinn AM, Griffin SJ. Effectiveness of interventions to promote physical activity in children and adolescents: Systematic review of controlled trials. BMC. 2007;335:703. doi: 10.1136/bmj.39320.843947.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CC. The Behavior-Image Model: a paradigm for integrating prevention and health promotion in brief interventions. Health Education Research. 2007;22:677–690. doi: 10.1093/her/cyl146. [DOI] [PubMed] [Google Scholar]
- Werch CE, Bian H, Moore MJ, Ames S, DiClemente CC, Weiler RM. Brief multiple behavior interventions in a college student health care clinic. The Journal of Adolescent Health. 2007;41:577–585. doi: 10.1016/j.jadohealth.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werch CE, Moore MJ, Bian H, DiClemente CC, Huang IC, Ames SC, et al. Are effects from a brief multiple behavior intervention for college students sustained over time? Preventive Medicine. 2010;50:30–34. doi: 10.1016/j.ypmed.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Adherence to long-term therapies: Evidence for action. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- Yancy AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annual Review of Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- Yeomans-Kinney A, Vernon SW, Frankowski RF, Weber DM, Bitsura JM, Vogel VG. Factors related to enrollment in the breast cancer prevention trial at a comprehensive cancer center during the first year of recruitment. Cancer. 1995;76:46–56. doi: 10.1002/1097-0142(19950701)76:1<46::aid-cncr2820760107>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]