Abstract
From a prospective cohort of 344 women who seroconverted for toxoplasmosis during pregnancy, 344 amniotic fluid, 264 placenta, and 216 cord blood samples were tested for diagnosis of congenital toxoplasmosis using the same PCR assay. The sensitivity and negative predictive value of the PCR assay using amniotic fluid were 86.3% and 97.2%, respectively, and both specificity and positive predictive value were 100%. Using placenta and cord blood, sensitivities were 79.5% and 21.2%, and specificities were 92% and 100%, respectively. In addition, the calculation of pretest and posttest probabilities and the use of logistic regression allowed us to obtain curves that give a dynamic interpretation of the risk of congenital toxoplasmosis according to gestational age at maternal infection, as represented by the three sample types (amniotic fluid, placenta, and cord blood). Two examples are cited here: for a maternal infection at 25 weeks of amenorrhea, a negative result of prenatal diagnosis allowed estimation of the probability of congenital toxoplasmosis at 5% instead of an a priori (pretest) risk estimate of 33%. For an infection at 10 weeks of amenorrhea associated with a pretest congenital toxoplasmosis risk of 7%, a positive PCR result using placenta at birth yields a risk increase to 43%, while a negative result damps down the risk to 0.02%. Thus, with a molecular diagnosis performing at a high level, and in spite of the persistence of false negatives, posttest risk curves using both negative and positive results prove highly informative, allowing a better assessment of the actual risk of congenital toxoplasmosis and finally an improved decision guide to treatment.
INTRODUCTION
Toxoplasmosis is a worldwide infection due to the protozoan parasite Toxoplasma gondii, which is generally benign but may cause severe infections in the fetus and the immunocompromised patient. A fetus can become infected and develop congenital toxoplasmosis if the mother contracts toxoplasmosis during pregnancy (7). For the pregnant woman, as for otherwise healthy adults, the parasite rarely causes any symptoms. On the contrary, congenital toxoplasmosis has a wide clinical spectrum ranging from lethal forms to subclinical forms, yet it is likely to belatedly produce ocular lesions that may eventually lead to blindness (27). The risk of transmission to the fetus, hence of congenital toxoplasmosis, increases, but its severity decreases, with gestational age at maternal infection (15). In France, in pregnant women 20 to 40 years old, the rate of infection by T. gondii presently varies from 6.1 to 7.2 per 1,000, and approximately 300 newborns annually suffer from congenital toxoplasmosis (4, 22, 40). Early diagnosis of congenital toxoplasmosis in pregnancy is crucial for proposing the most appropriate therapeutic approach in order to prevent late complications. In France and in a few European countries where toxoplasmosis is regarded as serious public health problem, a screening and monthly serologic follow-up of nonimmune pregnant patients have been established in order to reduce the frequency of congenital toxoplasmosis (2, 38, 40). A network of public regional reference laboratories exists throughout France, to which private laboratories may send their sera if the interpretation is difficult: e.g., to confirm the specificity of low IgG or IgM titers or to determine the date of infection using IgG titer kinetics or the avidity index of Toxoplasma-specific IgG. This proficiency network was formally organized with the creation of the National Reference Centre for Toxoplasmosis in 2006 (22; https://www.chu-reims.fr/professionnels/cnr-toxoplasmose-1).
The diagnosis of congenital toxoplasmosis may prove a difficult task, combining clinical features and results from a battery of serologic and molecular tests. A combination of criteria was defined by the European Research Network on Congenital Toxoplasmosis (23) in order to set up a “gold standard” for the diagnosis of this disease in Europe. Different time steps in the microbiological diagnosis of congenital toxoplasmosis, using different methods on different samples, may be individualized: during pregnancy, at birth, and during the first year of age. First, serologic tests are able to detect toxoplasmosis acquired by the mother during gestation. Accurate determination of the date of maternal infection is essential to evaluate the fetal risk for development of congenital toxoplasmosis (14). This is achieved when seroconversion is observed or by using the kinetics of specific IgG in successive serum samples and the calculation of the IgG avidity index (reviewed in reference 28). Next, depending on the term, prenatal diagnosis (PND) may be proposed for pregnant women experiencing primary infection with T. gondii: in France, this includes a molecular test using amniotic fluid and/or monthly ultrasonography examinations. At birth, molecular tests can also be performed using placenta (5, 17, 18, 29) and cord blood. Finally, at birth and during follow-up, serologic tests can confirm or rule out the diagnosis of congenital toxoplasmosis. A 1-year follow-up of the infant is recommended before definitely ruling out the diagnosis of congenital toxoplasmosis (23).
The PND of congenital toxoplasmosis has been based on PCR using amniotic fluid since the 1990s, when it superseded former methods based upon Toxoplasma isolation in fetal blood and amniotic fluid by mouse inoculation, as well as the detection of specific IgMs and IgAs in fetal blood in utero (reviewed in reference 3 and see references 25 and 26). However, most PCR assays worldwide have remained “in-house” or “laboratory-developed” assays, leading to a great heterogeneity in laboratory practices and methods (36) as well as to large variations in diagnostic performances (19, 35, 37). In congenital toxoplasmosis, the sensitivity and specificity of PCR on amniotic fluid were reported to range from 40% to 100% and from 80 to 100%, respectively (reviewed in reference 3 and see references 5, 33, 37, and 42). For molecular methods using placenta or cord blood, variable efficacies have been reported (reviewed in references 3 and 31). It should be emphasized that the molecular methods used for this diagnosis need to be highly specific but also highly sensitive since the parasite burden is often very low (12, 32), and both false-positive and false-negative results may lead to a wrong therapeutic decision in a critical situation.
In the present work, we aimed at determining the accuracy of the diagnosis of congenital toxoplasmosis by using an optimized PCR assay on amniotic fluid, placenta, and cord blood in a large prospective cohort of 344 patients who successively underwent amniocentesis and whose children were followed up after birth for at least 1 year. We analyzed the results of PCR tests according to gestational age at maternal infection and inferred accurate posttest predictive values for diagnosis. The final objectives of this work are to give practical up to date information with respect to disease risk assessment according to age at maternal infection, whether the molecular diagnosis of congenital toxoplasmosis is negative or positive.
MATERIALS AND METHODS
Mother and child management.
From January 1996 to December 2006, 344 women who contracted toxoplasmosis during pregnancy and underwent amniocentesis for molecular PND of congenital toxoplasmosis were enrolled in the study. The recruitment was regional (Languedoc-Roussillon, France). All Toxoplasma infections contracted during pregnancy and diagnosed according to state of the art serology were retained. Amniotic fluid sampling was performed after obtaining the written approval of the mother and, as recommended by the National Reference Centre for Toxoplasmosis, at least 1 month after the maternal infection for PND. Mothers were treated orally with 3 g or 9 million U of spiramycin per day while waiting for the results of PND. In addition, in order to detect possible abnormalities, an ultrasound scan was performed monthly. Signs suggestive of congenital toxoplasmosis were neurological abnormalities, such as dilatation of the lateral ventricles or intracranial calcifications. In the case of a positive PCR result and abnormal cerebral findings, and according to the parents' opinion, termination of pregnancy was performed after expert assessment by a prenatal diagnosis multidisciplinary board. Pregnancies with a positive PCR result and normal ultrasound findings continued until term with a treatment that includes sulfadoxine and pyrimethamine and rescue with folinic acid. At birth, neurological examination, ophthalmoscopy, and transfontanellar cranial ultrasonography were performed on the newborn. All neonates and, when pregnancies were terminated, products of conception were included in our study. All biological tests were performed in the Parasitology-Mycology Department of the Montpellier University Hospital, France.
Serologic tests.
The following techniques were used for detection of specific IgGs: a direct agglutination (DA) assay, the Toxoscreen DA (bioMérieux, France); indirect immunofluorescence (bioMérieux, France); and a microparticle enzyme immunoassay (MEIA) with the Toxo-IgG EIA kit (Axsym Abbott, France). The following techniques were used for detection of specific IgMs: indirect immunofluorescence (bioMérieux, France), MEIA with the Toxo-IgM EIA kit (Axsym Abbott, France), and an immunosorbent agglutination assay (ISAGA) (bioMérieux, France). Determination of the date of the maternal infection was done either after serologic conversion (i.e., the shift from a negative to a positive Toxoplasma serology with specific IgMs and IgGs) or after studying the kinetics of specific IgG titer and taking into account the result of the test for avidity of Toxoplasma-specific immunoglobulin G (Bio-Rad, France). At birth and during follow-up of the child, comparative maternal-child serology was performed with detection of specific IgGs, IgMs, and IgAs. Detection of specific IgGs was also used to measure the mother/child ratio of Toxoplasma-specific IgG (so-called “immune charge” [IC]), which was calculated by the following formula: IC = Cmother/Cchild, where Cmother = anti-T. gondii IgG titer/total IgG concentration within the serum of the mother and Cserum represents the same ratio within the serum of the child (13). The serologic surveillance of the child was continued during the first year of life until confirmation or exclusion of congenital toxoplasmosis.
Parasite detection.
Toxoplasma was detected in amniotic fluid, placenta, and cord blood by PCR and by inoculation into mice. PCR detection of Toxoplasma DNA was performed routinely, using primers B22 and B23 (8, 11) to amplify a fragment of the B1 gene (9). The assay used here was assessed in several studies as performing at a high level, as judged from its sensitivity and specificity, and no modification of sensitivity was observed throughout the period of the study (11, 35). The assay was able to detect 0.5 tachyzoite per reaction, or 2.5 tachyzoites/ml of amniotic fluid (11, 35). For each amniotic fluid sample, a 4-ml aliquot was processed fresh for DNA amplification using a method based on selective lysis of contaminating red blood cells, heat-detergent extraction, and thermolysis (20). Placentas (300 g) were ground, trypsinized, filtered, washed, and lysed with proteinase K (Euromedex, France); buffy coat portions (300 μl), isolated from cord blood after centrifugation for 10 min at 1,750 × g, were also lysed with proteinase K (Euromedex, France). Their DNA was prepared with the protein precipitation solution kit (A795A; Promega, France). In all cases, the PCR was performed using 5 μl of the extracted DNA (11). According to good practices, negative controls, positive controls, a search for inhibitors, and a decontamination step with uracil-DNA-glycosylase to prevent carryover contaminations were performed in all reactions. As regards mouse inoculation, 500 μl of amniotic fluid or placenta and cord blood were tested using the reference method in France (14). Briefly, half of the placenta preparation described above was injected intraperitoneally into five mice (1 ml per mouse), and the whole ground cord blood clot was inoculated into three mice (1 ml per mouse). Four and 8 weeks after inoculation, blood samples were examined using an agglutination test (Toxo-Screen DA; bioMérieux, France) to detect the presence of Toxoplasma-specific IgG antibodies. Brains of seropositive mice were microscopically checked for the presence of T. gondii cysts to confirm infection.
Case definition of congenital toxoplasmosis.
The criteria used in the present study to confirm or rule out the diagnosis of congenital toxoplasmosis (i.e., reference diagnosis) were those defined by the European Research Network on Congenital Toxoplasmosis (23), which included serologic tests and mouse inoculation and were complemented by the results of the immune charge (13). These criteria represent the present gold standard for diagnosis of congenital toxoplasmosis in Europe. All cases were allocated into three mutually exclusive categories: (i) definite fetal infection, (ii) unlikely to have fetal infection, or (iii) not infected. Due to the long-term follow-up of children, inclusion in the first or latter category leaves no room for doubt and was considered definitive. For this study, all definite congenital toxoplasmosis cases were confirmed after birth (or directly from the aborted fetus), when at least one of the following criteria was met (23): (i) detection of Toxoplasma gondii in amniotic fluid or in cord blood by mouse inoculation, (ii) rise in IgG titers within the first year of life or persistent positivity after that, (iii) positivity for specific IgMs and/or IgAs during the first 6 months of life, or (iv) immune charge (see definition above) of the neonate 3-fold higher than that of the mother.
It is noteworthy that the postnatal follow-up constitutes the basis for definitely confirming this diagnosis. Here, all cases of congenital toxoplasmosis diagnosed by PCR have been confirmed by one of the above criteria. A child was considered not infected when disappearance of transmitted maternal IgG was documented by negative serology within the first year of life without treatment. On the other hand, cases were considered “unlikely to have congenital toxoplasmosis” when the evidence was suggestive but incomplete: i.e., when a drop in IgG titers was verified but no follow-up was possible. In case of fetal loss, the diagnosis was assessed from ultrasonography findings and results of mouse inoculation and of pathological examination. It is noteworthy that for this study, the results of molecular diagnosis alone were not used to allocate a case into a category.
Statistical analysis.
Sensitivity, specificity, and positive and negative predictive values (PPV and NPV, respectively), as well as positive and negative likelihood ratios, were calculated for PCR results according to gestational age at maternal infection. Likelihood ratios (LRs) provide a direct estimation of how much a test result will change the odds of having a disease. The 95% confidence limits (95% CL) of sensitivity, specificity, PPV, and NPV were computed using the binomial exact method or approximate normal formula. The confidence limits of LRs were computed with the log method (34). To estimate the pretest probability (prevalence) and posttest probability (after the PCR results) of having congenital toxoplasmosis according to gestational age at maternal infection, a logistic regression model was used. Prevalence was estimated in the entire cohort. Posttest probabilities of congenital toxoplasmosis were estimated independently in the PCR-negative and PCR-positive populations: they correspond to 1 − NPV and PPV, respectively. These results were reported on curves, where the values of prevalence and posttest risks of congenital toxoplasmosis were plotted against gestational age at maternal infection. The statistical software SAS Enterprise Guide (1) version 4.39 was used for statistical analysis.
RESULTS
Clinical and biological features of the cohort.
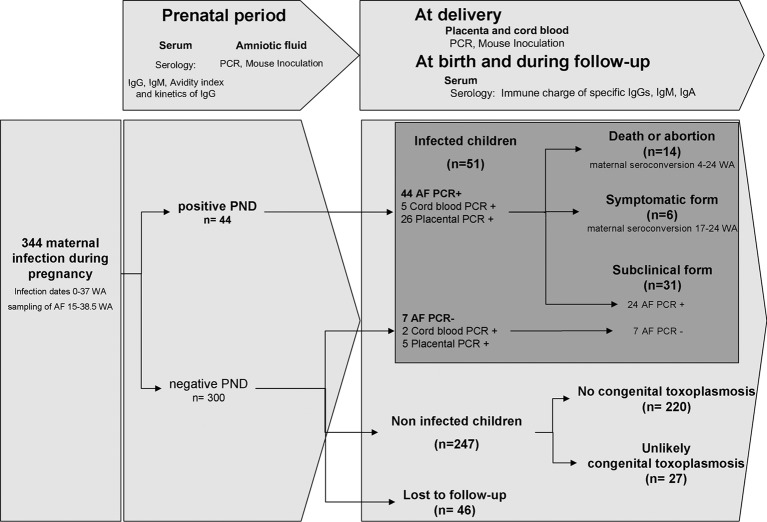
Over an 11-year period, 344 women who contracted toxoplasmosis during pregnancy and underwent amniocentesis for molecular PND of congenital toxoplasmosis in the Montpellier University Hospital (France) were prospectively enrolled in this study (Fig. 1). Their children were then followed-up for a maximum period of time in order to confirm or rule out congenital toxoplasmosis. The demographic features of the cohort are shown in Table S1 in the supplemental material. The dates of infection (according to gestational age) were accurately established using serologic tests (see Materials and Methods) for 336 (97.7%) patients (see Table S2 in the supplemental material). In the entire survey, we had 46 cases (13.4%) lost to follow-up; 16 (4.7%) were lost before delivery and 30 (8.7%) during the postnatal period. Out of the 344 cases, using the “gold standard” criteria defined above (see “Case definition of congenital toxoplasmosis” in Materials and Methods), 51 cases of congenital toxoplasmosis were diagnosed in 14 fetuses and 37 live-born children, yielding a global transmission rate of 14.8%. Symptomatic forms were seen in only 5.8% (20/344) of all pregnancies (see Table S2), all of them showing a positive PCR using amniotic fluid during PND. The outcomes of all pregnancies are presented in Fig. 1. Fourteen pregnancies were terminated, 8 in the first trimester and 6 in the second trimester. Among the 37 live-born children with congenital toxoplasmosis, only six presented a clinically symptomatic form (one case of intracranial calcifications, three cases of unilateral macular chorioretinitis, and two cases of neuro-ocular symptoms with intracranial calcifications and unilateral macular chorioretinitis); 24 children presented a subclinical form of the disease.
Fig 1.
Description and outcome of the cases of Toxoplasma gondii maternal infection analyzed in this study shown as a flow diagram. (Top boxes) Samples and biological tests used in this study. Below are shown the descriptions of cases. PND, prenatal diagnosis; AF, amniotic fluid.
Overall levels of performance of amniotic fluid testing and dynamic interpretation of PCR results according to gestational age at infection.
The levels of performance of the different tests used in the study have been calculated based on all of the biological results at the end of follow-up and according to the gold standard criteria defined by the European Research Network on Congenital Toxoplasmosis (23). The detection of Toxoplasma DNA by PCR in amniotic fluid was positive in 44 of the 344 samples and false negative in 7 of the 51 congenital toxoplasmosis cases. The overall performance levels of molecular PND, confirmed by comparison with other tests, were as follows: sensitivity, 86.3%; specificity, 100%; PPV, 100%; and NPV, 97.2% (Table 1). Mouse inoculation with amniotic fluid was performed in 18 out of these 44 cases and found positive in 8 cases, yielding a sensitivity of 44.4%; the specificity was 100% (see Table S3 in the supplemental material).
TABLE 1.
Performance levels of molecular diagnosis of congenital toxoplasmosis using amniotic fluid, placenta, and cord blood overall and by date of maternal infection
| Sample type and date of maternal infection | No. of cases with congenital toxoplasmosis |
No. of cases with no congenital toxoplasmosis |
Sensitivity, % (95% CL) | Specificity, % (95% CL) | Positive predictive value, % (95% CL) | Negative predictive value, % (95% CL) | Positive likelihood ratio (95% CL) | Negative likelihood ratio (95% CL) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR+ | PCR− | PCR+ | PCR− | |||||||
| Amniotic fluid | ||||||||||
| 0–6 WAa,b | 2 | 0 | 0 | 63 | 91 (59–100) | 100 (97–100) | 100 (69.1–100) | 99 (96.2–99.9) | NCc | 0.091 (0.014–0.59) |
| 7–15 WA | 8 | 1 | 0 | 80 | ||||||
| 16–28 WA | 21 | 6 | 0 | 87 | 78 (62–94) | 100 (96–100) | 100 (83.9–100) | 94 (88.6–98.5) | NC | 0.222 (0.11–0.45) |
| >28 WA | 12 | 0 | 0 | 11 | 100 (74–100) | 100 (72–100) | 100 (73.5–100) | 100 (71.5–100) | NC | 0 |
| Imprecise dating | 1 | 0 | 0 | 6 | NC | NC | NC | NC | NC | NC |
| All | 44 | 7 | 0 | 247 | 86 (77–96) | 100 (99–100) | 100 (92–100) | 97 (95.2–99.3) | NC | 0.14 (0.069–0.24) |
| Placenta | ||||||||||
| 0–6 WA | 1 | 0 | 2 | 44 | 100 (48–100) | 95 (91–99) | 45 (16–74.9) | 100 (96.8–100) | NC | 20.0 (9.17–43.6) |
| 7–15 WA | 4 | 0 | 4 | 69 | ||||||
| 16–28 WA | 18 | 4 | 8 | 56 | 82 (60–95) | 87 (79–96) | 69 (51.5–87) | 93 (83.8–98.1) | 0.21 (0.09–0.51) | 6.55 (3.32–12.89) |
| >28 WA | 7 | 4 | 1 | 10 | 64 (31–89) | 91 (59–100) | 87 (47.3–99.7) | 71 (41.9–91.6) | 0.40 (0.18–0.90) | 7.0 (1.03–47.81) |
| Imprecise dating | 1 | 0 | 0 | 5 | NC | NC | NC | NC | NC | NC |
| All | 31 | 8 | 15 | 184 | 79 (67–92) | 92 (89–96) | 67 (53.8–80.9) | 96 (93.0–98.7) | 0.22 (0.12–0.41) | 10.55 (6.32–17.60) |
| Cord blood | ||||||||||
| 0–6 WA | 1 | 0 | 0 | 41 | 50 (7–93) | 100 (97–100) | 100 (15.8–100) | 98 (93.3–99.8) | 0.50 (0.19–1.33) | NC |
| 7–15 WA | 1 | 2 | 0 | 62 | ||||||
| 16–28 WA | 3 | 15 | 0 | 56 | 17 (4–41) | 100 (94–100) | 100 (29.2–100) | 80 (69.4–88.4) | 0.83 (0.68–1.02) | NC |
| >28 WA | 2 | 8 | 0 | 7 | 20 (3–57) | 100 (59–100) | 100 (15.8–100) | 47 (21.1–71.9) | 0.80 (0.59–1.09) | NC |
| Imprecise dating | 0 | 1 | 0 | 3 | NC | NC | NC | NC | NC | NC |
| All | 7 | 26 | 0 | 169 | 21 (7–35) | 100 (98–100) | 100 (59.0–100) | 87 (81.9–91.4) | 0.79 (0.66–0.94) | NC |
WA, weeks of amenorrhea.
The periods 0 to 6 WA and 7 to 15 WA were distinguished due to the presence of significantly different transmission rates between them (see Table S2 in the supplemental material); however, the levels of performance of the biological tests showed no statistical differences; hence, we fused the calculations for both periods.
NC, not computable.
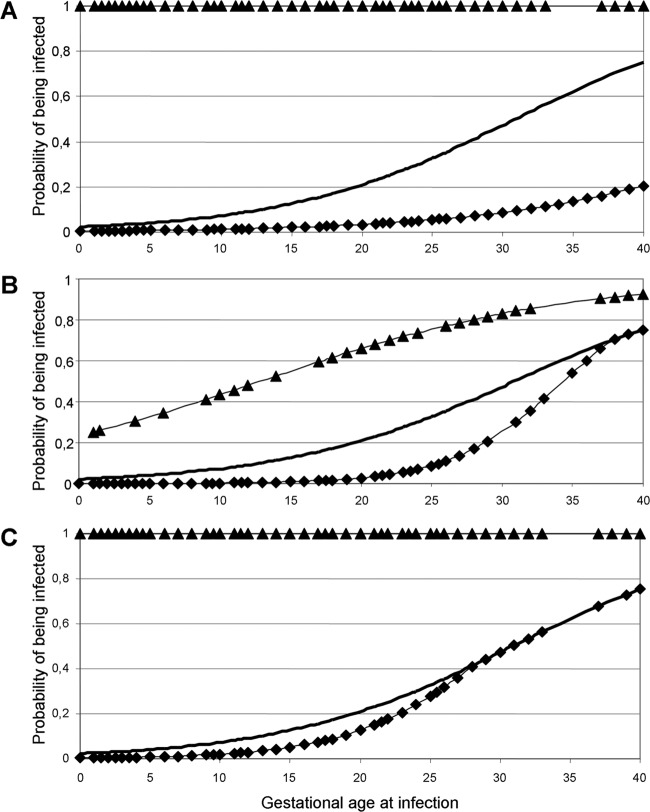
In addition, logistic regression analysis allowed us to calculate the pretest and posttest risks of congenital toxoplasmosis according to gestational age at infection and PCR results using amniotic fluid (see Materials and Methods and the legend to Fig. 2). As expected, a positive PCR on amniotic fluid was always associated with a 100% probability of giving birth to an infected child: for a maternal infection around 10 weeks of amenorrhea (WA), the pretest risk of congenital toxoplasmosis is only 7% (Fig. 2A, prevalence curve); however, if the PCR using amniotic fluid is positive, this risk obviously rises to 100% (Fig. 2A, PPV curve). More interestingly, Fig. 2A also shows the usefulness of negative PCR results. For example, for an infection at 25 WA, the risk appears reduced from 33% (Fig. 2A, prevalence curve) to 4.8% (Fig. 2A, 1 − NPV curve) if one takes into account the negative result; for a late infection (37 WA), a negative PCR result on amniotic fluid makes the risk fall from a pretest value of 68% to a posttest value of <20%. Pre- and posttest risk values can be extrapolated from the curves for any maternal infection date (Fig. 2).
Fig 2.
Assessment of the actual risk of congenital toxoplasmosis using posttest predictive values of amniotic fluid, placenta, and/or cord blood PCR results. Determination of the final diagnosis at the end of child follow-up allowed the calculation, in our cohort, of the prevalence of congenital toxoplasmosis (or pretest risk) according to gestational age at maternal infection, all along the course of pregnancy (bold curve). We then calculated the same risk but differentiating, for each case, positive and negative PCR results (posttest probabilities of being infected). Solid triangles, posttest risk curves when the PCR test was positive (PPV); solid diamonds, posttest risk curves when the PCR test was negative (1 − NPV). These curves are represented on three graphs for amniotic fluid (A), placenta (B), and cord blood (C). Results from all of the cases in this study are shown on the logistic regression curves.
Overall levels of performance of placenta testing and dynamic interpretation of PCR results according to gestational age at infection.
The overall sensitivity of PCR using placenta was 79.5% (31/39), and its specificity was 92.4% (209/227). These figures were 70.7% (29/41) and 98.6% (236/239) for mouse inoculation using placenta (see Table S3 in the supplemental material). Here again, we applied logistic regression analysis for a dynamic view using placenta PCR results (Fig. 2B). For example, for a maternal infection at 15 WA, the pretest probability of having congenital toxoplasmosis is 12.5%, but if the placenta is PCR negative, the posttest risk is 0.8%; this figure rises to 53% if the PCR is positive. For a maternal infection at 20 WA, the figures for the prevalence, on the one hand, and the risk estimates in case of a negative PCR result and a positive PCR result, on the other hand, are 21%, 3%, and 66%, respectively, and at 25 WA the risk estimates are 33%, 9%, and 74%, respectively (Fig. 2B).
Overall levels of performance of cord blood testing and dynamic interpretation of PCR results according to gestational age at infection.
Using cord blood at delivery, the sensitivity of the PCR assay was 21.2% (7/33) versus 8.8% using mouse inoculation; specificity was 100% (183/183 and 186/186) for both techniques (Table 1; see Table S3 in the supplemental material). As for amniotic fluid and placenta, one can determine the prevalence and posttest risks in case of a negative PCR and a positive PCR: for example, these are 21%, 3% and 66%, respectively, for an infection at 20 WA and 33%, 9%, and 74%, respectively, for 25 WA (Fig. 2C).
PCR testing of amniotic fluid, placenta, and cord blood in the same patients.
In our cohort of 344 patients, all three samples (amniotic fluid, cord blood, and placenta) were assayed by PCR in 213 cases, of which 199 had a complete follow-up; 34 out of the 37 live-born congenital toxoplasmosis patients had the three samples tested (see Table S4 in the supplemental material). All three samples were PCR positive in only five cases (15% of the 37 congenital toxoplasmosis cases); the dates of maternal infection for these cases were 4, 10, 24, 30, and 31 WA. Amniotic fluid was the only PCR-positive sample in six cases (18%); the dates of maternal infection were 23 and 25 WA in two cases and 31 WA in the remaining six cases. Conversely, in only two cases, both placenta and cord blood were found to be PCR positive while amniotic fluid was negative; the dates of maternal infections for those two cases were 18 and 24 WA, and the dates of PND were 24.5 and 28.5 WA, respectively. In one case of congenital toxoplasmosis, the three molecular tests were negative. Thus, the rate of false-negative results for molecular diagnosis as a whole (using the three samples) was as low as 3%.
Serologic diagnosis and follow-up.
‘State of the art” serology was used during pregnancy for dating maternal infection and with the child for assessing the definite status of the Toxoplasma infection; thus, all congenital toxoplasmosis cases diagnosed by molecular methods were confirmed at birth or during follow-up by serology. The details of this are shown in Table S2 in the supplemental material. Serology during the neonatal period was contributive for the diagnosis of congenital toxoplasmosis in 37 cases: it was positive and confirmed the positive molecular PND in 30 cases, but it was also positive for seven false-negative results of PND, allowing the diagnosis to be made. In the remaining 14 congenital toxoplasmosis cases, a termination of pregnancy occurred and serologic testing was not possible. The sensitivities of IgM and IgA detection at birth were 62.2% (23/37) and 59.5% (22/37), respectively. A positive immune charge of specific IgGs (see Materials and Methods for definition) was found in 6/37 (16.2%) cases; one of these had a negative molecular PND and was diagnosed only with this parameter.
DISCUSSION
Our study shows that an optimized PCR assay can detect 86% of congenital toxoplasmosis cases prenatally, therefore leaving only 14% undetected at this stage. We also show the interest in molecular testing of the placenta and cord blood at birth for this diagnosis. This interest is highlighted by the novel dynamic analysis of the risk of congenital toxoplasmosis that we propose, taking into account not only the classical gestational age at maternal infection but also the results of the molecular tests. Our main objective was indeed to correlate the results of molecular testing with the diagnosis of congenital toxoplasmosis in a dynamic view which would be useful to the practitioner.
Practical interest in the predictive values of molecular diagnosis of congenital toxoplasmosis in the prenatal period using amniotic fluid and at delivery using placenta and cord blood.
The calculated pre- and posttest risk curves (Fig. 2) we used here allow consideration of the results of molecular diagnosis of congenital toxoplasmosis in a novel manner; they should be helpful to obstetricians for day-to-day counsel about the risk of congenital toxoplasmosis to women who seroconverted during pregnancy. Indeed, to our knowledge, counsel is generally given according to (i) congenital toxoplasmosis prevalence or (ii) a positive PCR result on amniotic fluid and/or cord blood. A negative PND is considered of little value due to the presence of false negatives. Here, we show the respective weight of each molecular test result according to the gestational age at maternal infection whether the results are positive or negative. This gives access to a larger amount of information and specifically gives the actual risk of congenital toxoplasmosis in case of a positive (PPV curve) or a negative (1-NPV curve) PCR result at a given time point, whichever the type of sample used. Thus, if a positive PCR result using amniotic fluid and/or cord blood obviously confirms the diagnosis of congenital toxoplasmosis (23), the presence of a negative result using amniotic fluid considerably lowers the probability that transmission of the parasite occurred, particularly after 20 WA. Thus, a negative PCR result using amniotic fluid appears more helpful than generally thought, especially for midterm and late maternal infections.
Due to the large number of false-negative results, which makes the 1 − NPV curve overlap the prevalence curve, a negative cord blood PCR result is not very informative at any stage of the pregnancy. Using placenta, in spite of moderate sensitivity and specificity, both positive and negative PCR results appear highly informative due to the large distance between the negative and, particularly, positive posttest risk curves and the prevalence curve (Fig. 2C).
It should be stressed that the curves presented here may be used by any group as long as the levels of performance of the molecular diagnosis are similar to ours; without the amount of clinical data our laboratory has, this technically requires a PCR efficiency as close to 2 as possible and an analytical sensitivity of ≤0.5 tachyzoite per reaction (11, 35); otherwise, the curves would have to be built by the users.
Levels of performance of the parasitological diagnosis.
In this study, the “gold standard” for the definite diagnosis of congenital toxoplasmosis was the reference definition of criteria created by the European research Network on Congenital Toxoplasmosis (23), and all definite diagnoses of congenital toxoplasmosis were asserted by serologic follow-up after birth; thus, any falsely negative or positive molecular diagnosis could be identified. The sensitivity at 86% reported here for our PCR assay is among the highest ever published for this diagnosis for large cohorts, which varies from 65% to 92% (reviewed in reference 3, and see references 5, 33, 37, 39, and 42). It is noteworthy that, in our study, the seven cases of congenital toxoplasmosis that were not detected in utero were subclinical forms; all symptomatic cases (n = 17) were diagnosed during the prenatal period by PCR. Moreover, no false-positive results were observed, yielding 100% specificity. The specificity reported in the literature varies from 94 to 100% (reviewed in reference 3, and see references 5, 33, 37, 39, and 42). An absolute specificity is compulsory in the context of PND, as false positives may generate (i) at worse, an unwarranted decision of termination of pregnancy and (ii) unnecessary treatment, at least until delivery (and, in some cases, for the first year of age).
Placenta testing by PCR and/or mouse inoculation generally yields low sensitivities (under 60%) (5, 6, 10, 17, 18, 24, 29). Recent reports yielded conflicting results (16, 29), rendering the clinical relevance and usefulness of placenta testing for this diagnosis controversial; these two reports essentially differed by the sensitivity of the PCR assay used: 71% (20/28) versus 25% (13/51), respectively. In our experience, the search for Toxoplasma in placenta by PCR was highly sensitive (79% [31/39]), but as also shown by others (reviewed in reference 3 and see reference 31), a positive PCR using placenta was not always correlated with congenital toxoplasmosis, as was the case here in 16 patients, yielding a relatively high rate of false-positive results (6%). However, three of these were also mouse inoculation positive, indicating that these false positives were most probably due to a colonization of the placenta without transmission of the parasite to the fetus, this being better detected using a highly sensitive PCR assay. A positive placenta may be regarded as a clue for the diagnosis of congenital toxoplasmosis that needs to be confirmed by a positive cord blood and/or a positive serologic response in the newborn/infant.
With respect to cord blood, this is, to our knowledge, the first report of sensitivity and specificity of both PCR and mouse inoculation using this type of sample; only one study using mouse inoculation reported a sensitivity of 16% (4/25 cases) (24). Here, in two cases in which molecular PND and serologic screening at birth were negative, the first argument to confirm the diagnosis of congenital toxoplasmosis was a PCR-positive cord blood sample; these results allowed the start of drug treatment immediately after birth. Thus, we consider that, in spite of its low sensitivity and due to its high specificity and PPV, PCR testing of cord blood remains an important step in the diagnosis of congenital toxoplasmosis. This is even more important when PND is negative and when serology at birth is not contributive. It should also be noted that, in our experience, a positive cord blood sample was always associated with a positive placenta.
Mouse inoculation, the conventional reference method, has proven consistently less sensitive than PCR in the context of PND (reviewed in reference 3 and see references 5, 21, 24, 30, 33, and 39), which was confirmed in this study. Nevertheless, there is a general consensus in France to continue including this method in routine diagnosis, due to a few reports from some groups of congenital toxoplasmosis cases with a positive mouse inoculation and a negative PCR on amniotic fluid (5, 21, 33). Moreover, mouse inoculation is the only method to isolate strains for genotyping, evaluation of pathogenicity or drug sensitivity, etc. (1): in our hands, compared to amniotic fluid and cord blood, the placenta is the best-suited sample material for this. Our values for the sensitivity of mouse inoculation are different from those of previous reports: thus, the values 38% and 71% we report here for mouse inoculation of amniotic fluid and placenta, respectively, have to be compared with the values 71% and 52% reported by Bessieres et al. (5). Two differences from the protocol used by this center might explain these discrepancies: they use a higher inoculum of amniotic fluid and immunofluorescence instead of the Toxoscreen DA for mouse serologic screening.
Serologic screening at birth and during postnatal follow-up.
A serologic follow-up of the child using a combination of state of the art methods is the only way to assert a definite diagnosis of congenital toxoplasmosis and to rule out a falsely negative or positive molecular diagnosis. The 62.2% and 59.5% sensitivity of neonate IgM and IgA detection reported here are similar to the values of 64 to 70% for IgM and 53 to 65% for IgA reported in most series with cohorts ranging from 27 to 233 congenital toxoplasmosis cases (5, 6, 24, 30, 41). Testing of both isotypes increased sensitivity to 68%, which was also observed by Wallon et al. (41) (IgM, 67%; IgA, 54%; IgM and/or IgA, 73%), Naessens et al. (24) (IgM, 41%; IgA, 64%; IgM and/or IgA, 66%), and Bessières et al. in 2001 (6) (IgM, 50%; IgA, 60%; IgM and/or IgA, 81%) and 2009 (5) (IgM, 64%; IgA, 53%; IgM and/or IgA, 73%).
With respect to follow-up, we report a low percentage of loss during follow-up (13%) and a long follow-up period (1 year). In our cohort, the postnatal serologic follow-up did not detect any further case of congenital toxoplasmosis than those detected by molecular methods and neonatal serology. Nevertheless, in seven cases, the confirmation of a positive molecular PND was not obtained at birth but only during long-term follow-up.
In total, our experience confirms the usefulness and accuracy of a high-performance-level molecular diagnosis to detect congenital toxoplasmosis, both prenatally and at birth. The novelty of our study relies upon the consideration of the curves of pre- and posttest risks obtained from this molecular diagnosis for estimation of the actual risk of congenital toxoplasmosis throughout the course of pregnancy. We therefore obtain a dynamic interpretation according to gestational age at maternal infection that is highly informative; this should help microbiologists, obstetricians, and pediatricians to better assess the actual risk of congenital toxoplasmosis after a correct molecular test has been performed and thus guide the decision for treatment.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sylvie Douzou, Céline Fraissinet, Florence Michel, Delphine Gilbert, and Bounleth Sanichanh for technical help in the routine diagnosis of toxoplasmosis. We also acknowledge Valérie Macioce for help in writing the manuscript.
Footnotes
Published ahead of print 3 October 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Ajzenberg D, et al. 2002. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J. Infect. Dis. 186:684–689 [DOI] [PubMed] [Google Scholar]
- 2. Anonymous 1992. Congenital toxoplasmosis, Copenhagen, January 17, 1992. Proceedings. Scand. J. Infect. Dis. Suppl. 84:1–96 [PubMed] [Google Scholar]
- 3. Bastien P. 2002. Molecular diagnosis of toxoplasmosis. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl 1):S205–S215 [DOI] [PubMed] [Google Scholar]
- 4. Berger F, Goulet G, Le Strat Y, Desenclos J. 2008. Toxoplasmosis in pregnant women in France: trends in seroprevalence and incidence, and associated factors, 1995–2003. Bull. Epidémiol. Hebdomadaire 14-15:117–121 (In French.) [Google Scholar]
- 5. Bessieres MH, et al. 2009. Diagnosis of congenital toxoplasmosis: prenatal and neonatal evaluation of methods used in Toulouse University Hospital and incidence of congenital toxoplasmosis. Mem. Inst. Oswaldo Cruz 104:389–392 [DOI] [PubMed] [Google Scholar]
- 6. Bessieres MH, et al. 2001. Neonatal screening for congenital toxoplasmosis in a cohort of 165 women infected during pregnancy and influence of in utero treatment on the results of neonatal tests. Eur. J. Obstet. Gynecol. Reprod. Biol. 94:37–45 [DOI] [PubMed] [Google Scholar]
- 7. Boyer K, et al. 2011. Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin. Infect. Dis. 53:1081–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bretagne S, et al. 1993. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J. Infect. Dis. 168:1585–1588 [DOI] [PubMed] [Google Scholar]
- 9. Burg JL, Grover CM, Pouletty P, Boothroyd JC. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cassaing S, et al. 2006. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J. Clin. Microbiol. 44:720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chabbert E, Lachaud L, Crobu L, Bastien P. 2004. Comparison of two widely used PCR primer systems for detection of Toxoplasma in amniotic fluid, blood, and tissues. J. Clin. Microbiol. 42:1719–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa JM, Ernault P, Gautier E, Bretagne S. 2001. Prenatal diagnosis of congenital toxoplasmosis by duplex real-time PCR using fluorescence resonance energy transfer hybridization probes. Prenat. Diagn. 21:85–88 [DOI] [PubMed] [Google Scholar]
- 13. Desmonts G. 1971. Congenital toxoplasmosis: problems in early diagnosis, p 137–149 In Hentsch D. (ed), Toxoplasmosis. Hans Huber Publishers, Bern, Switzerland [Google Scholar]
- 14. Desmonts G, Couvreur J. 1974. Toxoplasmosis in pregnancy and its transmission to the fetus. Bull. N. Y. Acad. Med. 50:146–159 [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn D, et al. 1999. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet 353:1829–1833 [DOI] [PubMed] [Google Scholar]
- 16. Filisetti D, Cocquerelle V, Pfaff A, Villard O, Candolfi E. 2010. Placental testing for Toxoplasma gondii is not useful to diagnose congenital toxoplasmosis. Pediatr. Infect. Dis. J. 29:665–667 [DOI] [PubMed] [Google Scholar]
- 17. Fricker-Hidalgo H, et al. 2007. Value of Toxoplasma gondii detection in one hundred thirty-three placentas for the diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 26:845–846 [DOI] [PubMed] [Google Scholar]
- 18. Fricker-Hidalgo H, et al. 1998. Detection of Toxoplasma gondii in 94 placentae from infected women by polymerase chain reaction, in vivo, and in vitro cultures. Placenta 19:545–549 [DOI] [PubMed] [Google Scholar]
- 19. Guy E, et al. 1996. Interlaboratory comparison of polymerase chain reaction for the detection of Toxoplasma gondii DNA added to samples of amniotic fluid. Eur. J. Clin. Microbiol. Infect. Dis. 15:836–839 [DOI] [PubMed] [Google Scholar]
- 20. Hohlfeld P, et al. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695–699 [DOI] [PubMed] [Google Scholar]
- 21. Jenum PA, Holberg-Petersen M, Melby KK, Stray-Pedersen B. 1998. Diagnosis of congenital Toxoplasma gondii infection by polymerase chain reaction (PCR) on amniotic fluid samples. The Norwegian experience. APMIS 106:680–686 [PubMed] [Google Scholar]
- 22. King L, et al. 2008. Congenital toxoplasmosis: implementation of a surveillance system in France. Bull. Epidémiol. Hebdomadaire 14-15:122–123 (In French.) [Google Scholar]
- 23. Lebech M, et al. 1996. Classification system and case definitions of Toxoplasma gondii infection in immunocompetent pregnant women and their congenitally infected offspring. European Research Network on Congenital Toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 15:799–805 [DOI] [PubMed] [Google Scholar]
- 24. Naessens A, et al. 1999. Diagnosis of congenital toxoplasmosis in the neonatal period: a multicenter evaluation. J. Pediatr. 135:714–719 [DOI] [PubMed] [Google Scholar]
- 25. Pratlong F, et al. 1994. Fetal diagnosis of toxoplasmosis in 190 women infected during pregnancy. Prenat. Diagn. 14:191–198 [DOI] [PubMed] [Google Scholar]
- 26. Pratlong F, et al. 1996. Antenatal diagnosis of congenital toxoplasmosis: evaluation of the biological parameters in a cohort of 286 patients. Br. J. Obstet. Gynaecol. 103:552–557 [DOI] [PubMed] [Google Scholar]
- 27. Remington J, McLeod R, Wilson C, Desmonts G. 2010. Toxoplasmosis, p 918–1041 In Remington JS, Klein JO, Wilson CB, Nizet V, Maldonado Y. (ed), Infectious diseases of the fetus and newborn infant, 7th ed Saunders-Elsevier, Philadelphia, PA [Google Scholar]
- 28. Remington JS, Thulliez P, Montoya JG. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Robert-Gangneux F, et al. 2010. Clinical relevance of placenta examination for the diagnosis of congenital toxoplasmosis. Pediatr. Infect. Dis. J. 29:33–38 [DOI] [PubMed] [Google Scholar]
- 30. Robert-Gangneux F, et al. 1999. Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J. Clin. Microbiol. 37:2893–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robert-Gangneux F, et al. 2011. The placenta: a main role in congenital toxoplasmosis? Trends Parasitol. 27:530–536 [DOI] [PubMed] [Google Scholar]
- 32. Romand S, et al. 2004. Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am. J. Obstet. Gynecol. 190:797–802 [DOI] [PubMed] [Google Scholar]
- 33. Romand S, et al. 2001. Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet. Gynecol. 97:296–300 [DOI] [PubMed] [Google Scholar]
- 34. Simel DL, Samsa GP, Matchar DB. 1991. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J. Clin. Epidemiol. 44:763–770 [DOI] [PubMed] [Google Scholar]
- 35. Sterkers Y, et al. 2010. Multicentric comparative analytical performance study for molecular detection of low amounts of Toxoplasma gondii from simulated specimens. J. Clin. Microbiol. 48:3216–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sterkers Y, Varlet-Marie E, Marty P, Bastien P. 2010. Diversity and evolution of methods and practices for the molecular diagnosis of congenital toxoplasmosis in France: a 4-year survey. Clin. Microbiol. Infect. 16:1594–1602 [DOI] [PubMed] [Google Scholar]
- 37. Thalib L, et al. 2005. Prediction of congenital toxoplasmosis by polymerase chain reaction analysis of amniotic fluid. Br. J. Obstet. Gynaecol. 112:567–574 [DOI] [PubMed] [Google Scholar]
- 38. Thulliez P. 1992. Screening programme for congenital toxoplasmosis in France. Scand. J. Infect. Dis. Suppl. 84:43–45 [PubMed] [Google Scholar]
- 39. Vidigal PV, et al. 2002. Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR. Rev. Soc. Bras. Med. Trop. 35:1–6 [DOI] [PubMed] [Google Scholar]
- 40. Villena I, et al. 2010. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveill. 15:19600 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19600 [DOI] [PubMed] [Google Scholar]
- 41. Wallon M, et al. 1999. Diagnosis of congenital toxoplasmosis at birth: what is the value of testing for IgM and IgA? Eur. J. Pediatr. 158:645–649 [DOI] [PubMed] [Google Scholar]
- 42. Wallon M, et al. 2010. Accuracy of real-time polymerase chain reaction for Toxoplasma gondii in amniotic fluid. Obstet. Gynecol. 115:727–733 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.