LETTER
Extensively drug-resistant tuberculosis (XDR-TB), which is TB resistant to isoniazid and rifampin plus one fluoroquinolone and a second-line injectable drug, represents an obstacle for the treatment and control of TB. Previously, we reported four XDR-TB cases and a high proportion of the Beijing genotype among multidrug-resistant TB (MDR-TB) isolates in the state of Valle del Cauca, Colombia (3), where a MDR-TB hot spot had been identified (7). According to the information of the local TB program, to date 21 XDR-TB cases have been diagnosed in the country, 14 of which were from this state.
With the approval of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) Review Board, we characterized the XDR-TB cases detected in Valle del Cauca in the period 2001 to 2009, including their clinical and epidemiological features.
Resistance profiles were identified using the proportion method on 7H10 agar (6) and confirmed by a supranational laboratory. Molecular characterization of the isolates was performed by spoligotyping (5) and 24-locus variable-number tandem-repeat (VNTR) typing (8). GenoType MTBDRplus and -sl assays (Hain Lifescience GmbH) were applied to identify mutations associated with resistance to first- and second-line anti-TB drugs. Shared types and lineages of Mycobacterium tuberculosis isolates were determined using the SITVIT2 database (property of Institute Pasteur de la Guadeloupe).
In total, 10 XDR-TB patients were identified. Their median age was 30 years, and two cases (representing friends) were epidemiologically linked. All patients suffered from either bilateral disease or compromised lung function, and one underwent pneumonectomy. Three patients were labeled as primary resistant cases as they had no history of TB, suggesting active transmission of XDR-TB. The remaining patients had been intermittently exposed to second-line drugs. Six patients had a fatal outcome, one of them being HIV positive (Table 1).
TABLE 1.
Phylogenetic and epidemiological data of XDR-TB isolates from Valle del Cauca, Colombiam
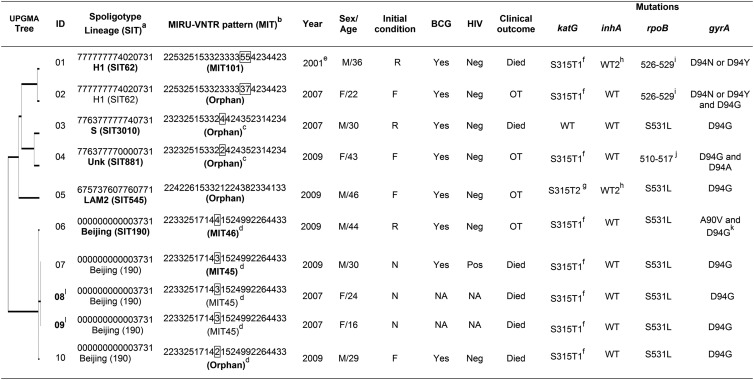
aSpoligotypes are shown in octal format; lineages are designated according to the SITVIT2 database.
bMIT numbers represent mycobacterial interspersed repetitive unit (MIRU) international types determined using 24 loci. The 8 unique profiles are defined as MIT101, -45, and -46 and five different orphan patterns.
cStrains 03 and 04, which differ at a single MIRU locus (4 and 2 copies for MIRU-40, respectively), showed related spoligotype binary patterns SIT3010 and SIT881; hence, it is possible that SIT881 evolved by loss of a single block of spacers 25 to 31 either directly from SIT3010 or from a linked ancestor.
dAll the Beijing SIT190 strains (ID 06, 07, 08, 09, and 10) are highly related, since they represent a single locus variant in the 24-locus typing scheme (4, 3, 3, 3, and 2 copies for MIRU-39, respectively).
eDiagnosed in 2001 but classified as an XDR-TB case until 2007 according to the WHO definition.
fS315T1: base exchange at codon 315, AGC to ACC.
gS315T2: base exchange at codon 315, AGC to ACA.
hFor this isolate, there was no hybridization either in the WT2 probe which analyzed nucleic acid in the position 8 or in either of the two mutations described.
iThis gene region is defined by the absence of WT7 probe which could represent any of these mutations: H526R, H526P, H526Q, H526N, H526L, H526S, and H526C.
jIsolate with an absence in the hybridization probes WT2 and WT2/WT3 that could have represented any of these mutations: Q513L, Q513P, and del514–516.
kHeteroresistant strain.
lEpidemiologically linked cases.
Abbreviations: BCG, Mycobacterium bovis; bacillus Calmette-Guérin vaccination SIT, spoligotype international type; ID, identification number; Unk, unknown; NA, data not available; M, male; F (in column 6), female; R, relapse; F (in column 7), failure of treatment with first-line drugs; N, new case; OT, on treatment; Neg, negative; Pos, positive; WT, wild type. Numbers in column 6 represent age in years.
Shared types/lineages SIT190/Beijing, SIT62/H1, SIT881/unknown, SIT545/LAM2, and SIT3010/S were identified by spoligotyping. These genotypes, combined with the VNTR 24-locus results, yielded eight unique profiles, with MIRU-39 being the only locus that discriminated between the highly related Beijing/SIT190 strains (Table 1). The Beijing genotype was the most frequent (5/10), and one specific subtype, MIT45, was especially present among younger patients (≤30 years old), new TB cases, and fatal outcomes (Table 1).
Non-Beijing strains 03 and 04 differed only in a single locus (MIRU-40) and also revealed the highly related spoligopatterns SIT3010 and SIT881 (Table 1). It is conceivable either that SIT881 evolved directly from SIT3010 or that they both derived from a common ancestor.
The distribution of the 5 XDR-TB shared types and lineages identified by spoligotyping in this state compared to the worldwide distribution revealed their high geographical specificity for South America and for Colombia in particular (Table 2).
TABLE 2.
Worldwide distribution of the M. tuberculosis shared types (SIT) present among the 10 Colombian XDR-TB isolates as determined using the SITVIT2 database
| SIT (clade) octal no. (SITVIT2 database no.) | Spoligotype | Distribution (%) in regions with ≥3% of a given SIT(s)a | Distribution (%) in countries with ≥3% of a given SIT(s)b |
|---|---|---|---|
| SIT62 (H1) 777777774020731 (497) | ■■■■■■■■■■■■■■■■■■■■■■■■■◽◽◽◽◽◽■◽◽◽◽■■■◽■■■ | AMER-S 40.24, EURO-S 13.68, AMER-N 13.68, EURO-W 8.05, AFRI-E 4.02, AFRI-W 3.62 | COL 37.63, USA 13.68, ITA 8.25, ESP 4.22, FXX 3.62, GMB 3.42 |
| 190 (Beijing) 000000000003731 (179) | ◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽◽■■■■■◽■■■ | ASIA-E 35.2, AMER-N 32.96, AMER-S 16.76, ASIA-SE 5.59 | USA 32.96, CHN 24.58, COL 16.2, JPN 6.7, KOR 3.35 |
| 545 (LAM2) 675737607760771 (8) | ■■◽■■■■◽■■■■◽■■■■■■■◽◽◽◽■■■■■■■■◽◽◽◽■■■■■■■ | AMER-S 50.0, EURO-W 25.0, AMER-N 25.0 | COL 50.0, USA 25.0, FXX 25.0 |
| 881 (unknown) 776377770000731 (72) | ■■■■■■■■◽◽■■■■■■■■■■■■■■◽◽◽◽◽◽◽◽◽◽◽◽■■■◽■■■ | AMER-S 76.0, AMER-N 16.0, EURO-S 8.0 | COL 72.0, USA 16.0, VEN 4.0, ITA 4.0, ESP 4.0 |
| 3010 (S) 776377777740731 (9) | ■■■■■■■■◽◽■■■■■■■■■■■■■■■■■■■■■◽◽◽◽◽■■■◽■■■ | AMER-S 77.78, EURO-S 11.11, AMER-N 11.11 | COL 66.67, USA 11.1, PER 11.1, ITA 11.1 |
Worldwide distribution is reported for regions with SITs representing ≥3% of their total number in the SITVIT2 database. The definition of macrogeographical regions and subregions (http://unstats.un.org/unsd/methods/m49/m49regin.htm) is according to the United Nations. Regions: AFRI (Africa), AMER (Americas), ASIA (Asia), EURO (Europe), and OCE (Oceania), subdivided into E (eastern), M (middle), C (central), N (northern), S (southern), SE (southeastern), and W (western). Furthermore, CARIB (the Caribbean) belongs to the Americas, while Oceania is subdivided in 4 subregions, AUST (Australasia), MEL (Melanesia), MIC (Micronesia), and POLY (Polynesia). Note that in our classification scheme, Russia has been assigned a new subregion by itself (northern Asia) instead of including it among the rest of eastern Europe. That assignment reflects its geographical localization as well as due to the similarity of specific TB genotypes circulating in Russia (a majority of Beijing genotypes) to those prevalent in central, eastern, and southeastern Asia.
The 3-letter country codes are according to http://en.wikipedia.org./wiki/ISO_3166–1_alpha-3; countrywide distribution is shown only for SITs representing ≥3% of their total number in the SITVIT2 database.
The most frequent mutations associated with resistance to isoniazid and rifampin were S315T1 (80%) and S531L (70%) in the katG and rpoB genes, respectively. These mutations were previously shown to be related to MDR-TB transmission in Europe (2). A1401G (100%) and D94G (90%) were the most common mutations in the rrs and gyrA genes, associated with resistance to aminoglycoside-cyclic peptides and fluoroquinolone, respectively (Table 1).
Most of the XDR-TB patients had been treated with moxifloxacin, and all isolates had mutations in the gyrA gene; however, nine were phenotypically susceptible to this drug; suggesting alternative mutations or other resistance mechanisms. Moreover, one isolate (ID 03) did not exhibit concordance between the phenotypic and genotypic susceptibility profiles for isoniazid, emphasizing the importance of phenotypic drug susceptibility testing as the basis for treatment.
The presence of the same mutations in the Beijing isolates, in addition to the highly similar VNTR patterns, suggests the clonal expansion of this particular Beijing lineage. Haarlem SIT62/H1 was the second most frequent (2/10) sublineage, as in previous observations in Colombia (Table 2) (3). Multiple mutational events and clonal expansion of one or more hypervirulent and resistant strains (e.g., Beijing and Haarlem) may be occurring in Valle del Cauca (1, 4).
The observed genetic relatedness between most of our XDR-TB isolates (Table 1), as well as their high geographical specificity (Table 2), highlights the emergence of XDR-TB strains within a pool of actively transmitted M. tuberculosis clinical isolates, which is highly alarming. Moreover, despite continuous efforts, a need remains to advance drug susceptibility testing and individualized treatment availability, especially since these patients have a prolonged infectious stage, resulting in a greater dissemination of these strains.
ACKNOWLEDGMENTS
This work was supported by CIDEIM, the Secretary of Health of Valle del Cauca, and the Young Researcher program from COLCIENCIAS (contracts 159-2008 and 797-2009).
We thank the personnel of the Secretary of Health from Buenaventura and Valle del Cauca, Hain Lifescience GmbH, and bioMérieux for their great contribution to this work. We also acknowledge the help of David Couvin in the SITVIT2 database project (Institut Pasteur de Guadeloupe), which was supported by a research grant from the Regional Council of Guadeloupe.
We declare no potential conflict of interest relevant to this article.
Footnotes
Published ahead of print 19 September 2012
Contributor Information
Sonia L. Villegas, Centro Internacional de Entrenamiento e Investigaciones Médicas—CIDEIM Cali, Colombia
Carolina Mehaffy, Colorado State University Fort Collins, Colorado, USA.
Liliana Forero, Secretaría Departamental de Salud del Valle Cali, Colombia.
Cesar Moreira, Secretaría de Salud Pública Municipal Buenaventura, Colombia.
Nalin Rastogi, WHO Supranational TB Reference Laboratory Institute Pasteur de la Guadeloupe, Abymes Guadeloupe, France.
Dick van Soolingen, Tuberculosis Reference Laboratory National Institute for Public Health and the Environment (RIVM) Bilthoven, The Netherlands Department of Pulmonary Diseases/Department of Clinical Microbiology Radboud University Nijmegen Medical Centre Nijmegen, The Netherlands.
REFERENCES
- 1. Dalla Costa ER, et al. 2009. Correlations of mutations in katG, oxyR-ahpC and inhA genes and in vitro susceptibility in Mycobacterium tuberculosis clinical strains segregated by spoligotype families from tuberculosis prevalent countries in South America. BMC Microbiol. 9:39 doi:10.1186/1471-2180-9-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Devaux I, et al. 2010. Surveillance of extensively drug-resistant tuberculosis in Europe, 2003–2007. Euro Surveill. 15:pii=19518. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19518 [PubMed] [Google Scholar]
- 3. Ferro BE, Nieto LM, Rozo JC, Forero L, van Soolingen D. 2011. Multidrug-resistant Mycobacterium tuberculosis, Southwestern Colombia. Emerg. Infect. Dis. 17:1259–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ioerger TR, et al. 2009. Genome analysis of multi- and extensively-drug-resistant tuberculosis from KwaZulu-Natal, South Africa. PLoS One 4:e7778 doi:10.1371/journal.pone.0007778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamerbeek J, et al. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kent PT, Kubica GP. 1985. Public health mycobacteriology: guide for the level III laboratory. CDC, Atlanta, GA [Google Scholar]
- 7. Moreira CA, et al. 2004. Initial drug resistance as a threat for tuberculosis control: the case of Buenaventura, Colombia. Biomedica 24(Supp 1):73–79 (In Spanish.) [PubMed] [Google Scholar]
- 8. Supply P, et al. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]


