Abstract
In two outbreaks of food-borne botulism in France, Clostridium botulinum type A was isolated and characterized from incriminated foods. Botulinum neurotoxin type A was detected in the patients' sera by mouse bioassay and in vitro endopeptidase assay with an immunocapture step and identification of the cleavage products by mass spectrometry.
TEXT
Botulism is a rare disease in France (annual incidence of 0.2 to 0.5 per million inhabitants), consisting mainly of type B food-borne botulism due to the consumption of homemade canned foods or pork products or, in some cases, commercial food preparations (4, 15). However, severe outbreaks of type A botulism have been identified in recent years in different areas of France (13, 15, 17, 18). Detection of botulinum neurotoxin (BoNT) in biological samples, mainly in serum, is the most direct way to confirm a diagnosis of botulism. Since the toxicity of BoNTs is extremely high, the mouse bioassay remains the standard method, because it is able to detect minute amounts of BoNT. But new in vitro methods are in development as alternatives to the mouse bioassay to provide information on toxicity within a matter of hours instead of days (3, 14).
C. botulinum strains were isolated from food samples using egg yolk agar medium supplemented with d-cycloserine, sulfamethoxazole, and trimethoprim (14). The bont gene was PCR amplified with overlapping primer pairs and sequenced (9).
The immunocapture test was performed with protein G magnetic beads coupled to immunopurified rabbit polyclonal anti-BoNT/A-Hc antibodies at 0.2 mg/ml as described previously (1) (Fig. 1A and B). Five-microliter amounts of coated beads were incubated in 500 μl of human serum or toxin preparation diluted in 500 μl HEPES buffer containing 1 mg/ml bovine serum albumin (BSA) for 1 h at room temperature and washed three times in HEPES-BSA buffer. The beads were suspended in 25 μl of reaction buffer containing the peptide substrate as described in reference 2. The mixtures were incubated at 37°C for 4 h. Detection of the C-terminal (C-ter) and N-terminal (N-ter) peptides resulting from substrate cleavage was modified from Barr's protocol (2). Briefly, 25 μl of water containing 2% trifluoroacetic acid and 1 μg/ml of internal standard as defined in reference 2 were added to the bead suspensions. After centrifugation, the supernatant (40 μl) was injected into a liquid chromatography system coupled to a triple quadrupole mass spectrometer (LC-MS/MS). Chromatography was achieved on a Zorbax SB C18 column (150 by 2.1 mm inner diameter) with a gradient elution of mobile phase A (water with 0.1% formic acid) and mobile phase B (acetonitrile with 0.1% formic acid). The mass spectrometer was a TSQ Quantum Ultra (Thermo Scientific, San Jose, CA) operated in the positive ion mode and coupled to the HPLC column via an electrospray interface. Successive dilutions of the cleaved C-ter peptide were injected into the LC-MS/MS system to check linearity and limit of detection (LOD). The ratio of the peak area of C-ter peptide to the coeluted internal standard, i.e., the normalized peak area, was monitored and found at 1 × 10−3 at the lowest detectable C-ter peptide dilution showing a signal-to-noise ratio of 10.
Fig 1.
Specificity of the anti-BoNT/A-Hc antibodies and typical LC-MS/MS chromatograms obtained for N-ter and C-ter peptides resulting from botulinum neurotoxin A activity after immunocapture-Endopep-MS assay in serum samples. (A) SDS-PAGE of semipurified BoNT/A (lane 1; 1.5 μg) dissociated in heavy (H) and light (L) chains (the preparation also contains OrfX2 protein, as determined by N-terminal sequencing [MNNLK]) and recombinant C-terminal part of BoNT/A H chain (Hc) (lane 2; 1.5 μg). (B) Western blot with crude rabbit anti-BoNT/A serum (1/10,000 dilution) and immunopurified anti-recombinant BoNT/A-Hc antibodies (1 μg/ml). Anti-BoNT/A-Hc antibodies recognize the H chain and recombinant Hc protein from BoNT/A but not the L chain. (C to F) LC-MS/MS chromatograms of N-ter and C-ter peptides. Negative-control serum sample (C); positive-control serum sample spiked with 4 MLD50/ml of BoNT/A (D); serum sample from patient 912 (E); serum sample from patient 943 (F). Two selected reaction monitoring (SRM) transitions of N-ter (Nter-1 and Nter-2) and C-ter (Cter-1 and Cter-2) peptides are represented. The last SRM transition corresponds to the internal standard (I.S.).
In vivo assays were performed as previously described (16).
In outbreak 1 (2008), two persons from northwest France with a generalized and complete paralysis were hospitalized in intensive care unit 1 day after eating industrially produced chicken enchiladas (Table 1) (13). They needed long-term intubation/mechanical ventilation for 4 and 5 months, respectively. BoNT serotype A (BoNT/A) was detected in the serum of both patients by the mouse bioassay with estimated titers of 8 and 16 50% minimal lethal dose (MLD50)/ml. The remaining chicken and vegetable mix contained a high level of BoNT/A and a C. botulinum type A strain (Table 1). The isolated strain (2008-148) was initially identified as subtype A1 based on bont gene sequencing. However, the amino acid sequence identity of BoNT/A of this strain was 93.8% similar to that of strain Hall (subtype A1) and 94.3% similar to that of strain H04402 065 (subtype A5) (5). Strain 2008-148 is more closely related, though not identical, to subtypes A1 and A5 than to the other subtypes (Fig. 2).
TABLE 1.
Detection of BoNT/A in patient's serum samples and contaminated food from two botulism outbreaks by mouse bioassay and immunocapture-Endopep-MS assaya
| Sample | Toxin titer (MLD50/ml) in mouse bioassay | BoNT/A detection by Endopep-MS assayb (C-ter peptide) (×10−3) | Strain isolated |
|---|---|---|---|
| Outbreak 1 | |||
| Patient's serum 16853 | 8 | 3.85 | |
| Patient's serum 16851 | 16 | 1.05 | |
| Commercial enchiladas | 280,000d | ND | C. botulinum A1/A5 (2010-148) |
| Outbreak 2 | |||
| Patient's serum 908 | 4 | 3.47 | |
| Patient's serum 912 | 4 | 5.57 | |
| Patient's serum 913 | 4 | 1.03 | |
| Patient's serum 943 | 4 | 6.77 | |
| Patient's serum 939c | 8 | 1.88 | |
| Intestinal content 1c | BLD | ND | Negative |
| Intestinal content 2c | BLD | ND | Negative |
| Mixed salad with homemade canned beans | 200d | ND | C. botulinum A2 (2010-969) |
| 5 Sera from asymptomatic persons | BLD | BLD |
Typical BoNT/A detection by Endopep-MS assay is shown in Fig. 1E and F. BLD, below limit of detection; ND, not done.
Normalized C-terminus peptide peak areas (SRM transition, 324.8/422.2).
Samples from the patient who died.
MLD50/g.
Fig 2.
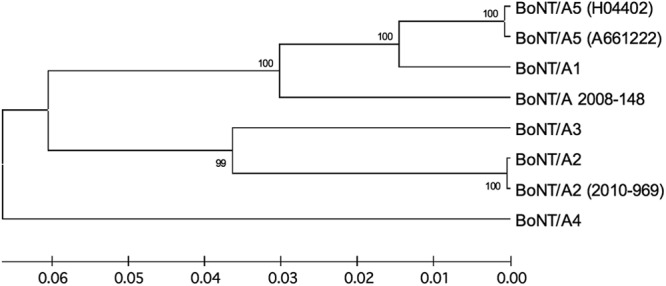
Evolutionary relationships of BoNT subtypes, including those of strains 2008-148 and 2010-969. The evolutionary history was inferred using the unweighted pair group method with Arithmetic Mean (UPGMA). The optimal tree with a sum of branch lengths of 0.27558317 is shown. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method and are measured in units of the number of amino acid substitutions per site. The analysis involved 8 amino acid sequences. All positions containing gaps and missing data were eliminated. Evolutionary analyses were conducted in MEGA5 (20).
Outbreak 2 occurred in Corsica in 2010 and included 5 persons presenting signs of severe intoxication (Table 1). Three patients required mechanical ventilation for 37 to 78 days, and a young person died from cardiac/respiratory failure (17). The patients had consumed a salad prepared with homemade canned beans, which contained BoNT/A and a C. botulinum type A strain. The person who died had a toxin titer of 8 MLD50/ml, and neither BoNT/A nor C. botulinum was detected in two intestinal content samples (Table 1). The isolated C. botulinum strain (2010-969) from the salad was classified as subtype A2 by bont sequencing (100% sequence identity with strain Mascarpone). It is assumed that food contamination reflects the prevalence of C. botulinum types in the environment where the food is prepared (6). This is consistent with the fact that C. botulinum A2 is frequent in southern Italy (8) and that the contaminated food from Corsica, which is close to Italy, also contains a subtype A2 strain.
The Endopep-MS assay was reported to be a sensitive and reliable method of BoNT/A detection and identification in biological samples (2, 10, 12) and could be an alternative in vitro method instead of the classical mouse bioassay. An immunocapture step was included to increase the sensitivity of the Endopep-MS assay, as previously found (7, 12). To avoid any loss of BoNT/A enzymatic activity with anti-L antibodies (11), we used polyclonal antibodies raised against the BoNT/A-Hc domain and further purified by immunoaffinity with the recombinant antigen protein (Fig. 1A and B).
The sensitivity of the Endopep-MS assay combined with immunocapture was determined with BoNT/A diluted in aqueous buffer or in human serum in comparison with the mouse bioassay. As shown in Table 2, both methods gave roughly equivalent levels of sensitivity with samples containing limiting amounts of BoNT/A. In the dilutions containing approximately 1 MLD50, the toxin was detected by the immunocapture-Endopep-MS assay with a normalized C-ter peptide signal of around the signal at the LOD (Table 2). All the 7 patients' sera which were found to contain BoNT/A by mouse bioassay, with estimated titers ranging from 4 to 16 MLD50/ml (2 to 8 MLD50/mouse) (Table 1), yielded a positive signal in immunocapture-Endopep-MS assay from around 1 to 7 times the signal at LOD (Table 1 and Fig. 1). However, the results with naturally contaminated sera did not parallel the mouse bioassay titration (Table 1). This could be due to several explanations: the incremental nature of the mouse results working above the LOD but below the limit of quantification, different toxin subtypes/variants, patient's serum limitations, etc. The immunocapture-Endopep-MS assay was specific, since any of the 5 control sera yielded a positive signal, and the polyclonal antibodies raised against BoNT/A1 Hc were able to efficiently capture both BoNT/A2 and BoNT/A1-A5. Another advantage of the in vitro assay is that it allows unambiguous differentiation between botulism and autoimmune neuropathies like Guillain-Barre syndrome. Indeed, the initial clinical symptoms of Guillain-Barre syndrome can be similar to those of botulism (19), and in our experience, sera from certain of these patients induce respiratory distress in mice, which can be confused with symptoms following injection of BoNT. Indeed, an immunocapture-Endopep-MS for BoNT/B has been found useful to differentiate type B botulism from Guillain-Barre syndrome (7).
TABLE 2.
Sensitivity of the immunocapture-Endopep-MS assay for BoNT/A detection in aqueous buffer or in human serum in comparison with the sensitivity of the mouse bioassaya
| Dilution | Estimated LD50 per mouse | BoNT/A in aqueous buffer |
BoNT/A in human serum |
||
|---|---|---|---|---|---|
| Mouse bioassay (no. of deaths/total no. of mice) | C-ter peptide ± SD (×10−3) | Mouse bioassay (no. of deaths/total no. of mice) | C-ter peptide ± SD (×10−3) | ||
| 1 | 25 | 7/8 | 8.05 ± 1.3 | ||
| 1:2 | 12.5 | 7/8 | 4.07 ± 0.4 | 5/6 | 3.51 ± 0.6 |
| 1:4 | 6. 25 | 6/8 | 2.75 ± 0.4 | 5/6 | 2.08 ± 0.5 |
| 1:8 | 3.12 | 3/8 | 2.19 ± 0.5 | 2/6 | 1.09 ± 0.1 |
| 1:16 | 1.56 | 4/8 | 1.2 ± 0.06 | 0/6 | BLD |
| 1:32 | 0.78 | 0/8 | BLD | 0/6 | BLD |
| 1:64 | 0.39 | 0/6 | BLD | ||
Culture supernatant of strain Hall calibrated to an estimated titer of 50 MLD50/ml was serially 2-fold diluted in phosphate-buffered saline (PBS)-gelatin (BoNT/A in aqueous buffer). One volume of a pool of human serum was mixed with 1 volume of C. botulinum type A culture supernatant, and then serial 2-fold dilutions in PBS-gelatin were performed (BoNT/A in human serum). Mice were injected intraperitoneally with 0.5 ml of toxin dilutions. The estimated MLD50 per mouse according to each dilution of the starting toxin preparation is indicated in the second column. Results are expressed as the number of dead mice versus total number of challenged mice and are from three independent experiments. Immunocapture was performed with 0.5 ml of each preparation or dilution and then subjected to LC-MS. The results are expressed as the mean normalized C terminus peptide peak areas (SRM transition, 324.8/422.2) ± standard deviations and are from three independent experiments. BLD, below limit of detection.
Nucleotide sequence accession numbers.
The GenBank accession numbers for strains 2008-148 and 2010-969 are JQ954969 and JQ954970, respectively.
ACKNOWLEDGMENTS
The work was supported by funding from IP and CEA.
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Becher F, Duriez E, Volland H, Tabet JC, Ezan E. 2007. Detection of functional ricin by immunoaffinity and liquid chromatography-tandem mass spectrometry. Anal. Chem. 79:659–665 [DOI] [PubMed] [Google Scholar]
- 2. Boyer AE, et al. 2005. From the mouse to the mass spectrometer: detection and differentiation of the endoproteinase activities of botulinum neurotoxins A-G by mass spectrometry. Anal. Chem. 77:3916–3924 [DOI] [PubMed] [Google Scholar]
- 3. Cai S, Singh BR, Sharma S. 2007. Botulism diagnostics: from clinical symptoms to in vitro assays. Crit. Rev. Microbiol. 33:109–125 [DOI] [PubMed] [Google Scholar]
- 4. Carlier JP, Espié E, Popoff MR. 2007. Le botulisme humain en France, 2003–2006, p 261–264 In Bulletin Epidemiologique Hebdomadaire, no. 31–32. Institut de Veille Sanitaire, Paris, France: http://opac.invs.sante.fr/doc_num.php?explnum_id=3629 [Google Scholar]
- 5. Carter AT, et al. 2009. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BMC Genomics 10:115 doi:10.1186/1471-2164-10-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dodds KL. 1993. Clostridium botulinum in the environment, p 21–51 In Hauschild AHW, Dodds KL. (ed), Clostridium botulinum: ecology and control in foods. Marcel Dekker, Inc., New York, NY [Google Scholar]
- 7. Ferracci G, et al. 2011. A label-free biosensor assay for botulinum neurotoxin B in food and human serum. Anal. Biochem. 410:281–288 [DOI] [PubMed] [Google Scholar]
- 8. Franciosa G, Floridi F, Maugliani A, Aureli P. 2004. Differentiation of the gene clusters encoding botulinum neurotoxin type A complexes in Clostridium botulinum type A, Ab, and A(B) strains. Appl. Environ. Microbiol. 70:7192–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hill KK, et al. 2007. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 189:818–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalb SR, Goodnough MC, Malizio CJ, Pirkle JL, Barr JR. 2005. Detection of botulinum neurotoxin A in a spiked milk sample with subtype identification through toxin proteomics. Anal. Chem. 77:6140–6146 [DOI] [PubMed] [Google Scholar]
- 11. Kalb SR, et al. 2009. Extraction and inhibition of enzymatic activity of botulinum neurotoxins/A1, /A2, and /A3 by a panel of monoclonal anti-BoNT/A antibodies. PLoS One 4:e5355 doi:10.1371/journal.pone.0005355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalb SR, et al. 2006. The use of Endopep-MS for the detection of botulinum toxins A, B, E, and F in serum and stool samples. Anal. Biochem. 351:84–92 [DOI] [PubMed] [Google Scholar]
- 13. King LA. 2008. Two severe cases of botulism associated with industrially produced chicken enchiladas, France, August 2008. Euro Surveill. 13:18978. [DOI] [PubMed] [Google Scholar]
- 14. Lindström M, Korkeala H. 2006. Laboratory diagnosis of botulism. Clin. Microbiol. Rev. 19:298–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazuet C, Bouvet P, King LA, Popoff MR. 2011. Le botulisme humain en France, 2007–2009, p 49–53 In Bulletin Epidemiologique Hebdomadaire, no. 6. Institut de Veille Sanitaire, Paris, France: http://www.invs.sante.fr/beh/2011/06/beh_06_2011.pdf [Google Scholar]
- 16. Mazuet C, Dano J, Popoff MR, Creminon C, Volland H. 2010. Characterization of botulinum neurotoxin type A neutralizing monoclonal antibodies and influence of their half-lives on therapeutic activity. PLoS One 5:e12416 doi:10.1371/journal.pone.0012416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oriot C, et al. 2011. One collective case of type A foodborne botulism in Corsica. Clin. Toxicol. (Phila.). 49:752–754 [DOI] [PubMed] [Google Scholar]
- 18. Pingeon J, et al. 2011. Two outbreaks of botulism associated with consumption of green olive paste, France, September 2011. Euro Surveill. 16:20035. [DOI] [PubMed] [Google Scholar]
- 19. Susuki K, et al. 2001. Guillain-Barre syndrome mimicking botulism. J. Neurol. 248:720–721 [DOI] [PubMed] [Google Scholar]
- 20. Tamura K, et al. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]