Abstract
Nasopharyngeal sampling is used for detecting bacteria commonly involved in upper respiratory tract infections, but it requires training and may not always be well tolerated. We sampled children (n = 66) of ages 0 to 4 years, with rhinorrhea, by using a nasopharyngeal swab, a nasal swab, and nose blowing/wiping into a paper tissue. Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus were cultured at similar rates across methods with high concordance (80 to 97%), indicating that they are reliably detected by alternative means.
TEXT
Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus are bacterial pathogens commonly involved in upper respiratory tract infections in young children. The recommended sampling site for S. pneumoniae and M. catarrhalis is the nasopharynx (10, 11), while for H. influenzae and S. aureus this is the naso- and oropharynx (4) and the nasal vestibule (17), respectively. However, sampling these sites requires training and may not always be well tolerated by the child, especially when conducted repeatedly.
At the time of an upper respiratory tract infection, secretions from the nasopharynx, paranasal sinuses, and nasal cavity accumulate and discharge spontaneously from the nose. Theoretically, bacteria residing at various niches should be detectable in these nasal secretions.
We compared conventional culture results of nasal secretions collected by blowing or wiping the nose into a paper tissue to the current standards for detection of S. pneumoniae, H. influenzae, H. catarrhalis, and S. aureus in a group of children with rhinorrhea as a symptom of an upper respiratory tract infection.
Children (n = 66) of ages 0 to 4 years with nasal secretions as symptom of an upper respiratory tract infection were eligible for the present cross-sectional study. Children were recruited from the outpatient clinic of the ear-nose-throat department of the Wilhelmina Children's Hospital, University Medical Center Utrecht, the Netherlands, from two local day care centers, and from a population of children who had previously participated in a randomized trial (14). Children with craniofacial abnormalities were excluded, as were children who had received antimicrobial therapy in the previous 2 weeks.
The study was approved by the Institutional Review Board of the University Medical Center Utrecht (available at http://www.umcutrecht.nl/metc) and undertaken in accordance with the European Guidelines for Good Clinical Practice, which incorporate the provisions of the Declaration of Helsinki. Written informed consent was obtained from both parents.
The sequence of sampling was randomized. Pediatric swabs with a flocked nylon fiber tip were used (Eswab 482CE; Copan, Brescia, Italy). A sample from the nasal vestibule (nasal swab) was taken by inserting a swab 1 cm into the nostril and rotating it three times. A nasopharyngeal sample was obtained according to World Health Organization guidelines (11). Nasal secretions were collected with a paper tissue as described by Leach and colleagues (9) by blowing or wiping the nose. The first few paper tissues were discarded, and disposable (nonsterile) gloves were worn during and hands disinfected between procedures. After swabbing visible discharge, the paper tissue was placed as a whole in a container with 10 ml of phosphate-buffered saline (PBS). All swabs were immediately inoculated in 1 ml of modified liquid Amies transport medium, stored at room temperature, and transferred to the laboratory. All samples were cultured within 24 h on Trypticase soy agar supplemented with 5% defibrinated sheep blood with and without 5 mg/liter gentamicin, Hektoën enteric agar 2, and mannitol salt agar by dipping the swab into the medium for each new plate separately. For the paper tissue samples, a cotton swab was used to inoculate the plates. Identification of S. pneumoniae, H. influenzae, M. catarrhalis, and S. aureus was based on colony morphology and conventional methods of determination (16). Growth was measured semiquantitatively.
To detect a concordance rate for each bacterium of at least 80% with 90% power and a two-sided alpha of 5%, a sample size of 66 subjects was required for this study. Concordance rates between sampling methods and the specificities and sensitivities of the alternative methods were calculated. Cohen's kappa statistic was computed to take chance agreement between pairs (alternative versus standard sampling method) into account. A value of 1 indicates full agreement, while a value of 0 indicates merely chance. A P value of <0.05 was considered statistically significant. Data were analyzed with the statistical software package SPSS, version 18.0, and Episheet (13).
The study was conducted between April 2010 and November 2011. The mean age of the 66 participants was 2.4 years, and 40 (61%) were boys. Concordance between sampling methods ranged from 80% to 97% (Table 1). The agreement between pairs ranged from good (kappa statistic, 0.57) to excellent (0.93). S. pneumoniae was cultured even more frequently from a paper tissue directly than from a nasopharyngeal swab (P = 0.039). Likewise, the detection rate for S. aureus was highest for culture of a paper tissue (Table 1). H. influenzae and M. catarrhalis were detected at comparable rates across sampling methods. The density of pneumococci was highest in cultures of a paper tissue directly compared to each of the other sampling methods (Fig. 1).
TABLE 1.
Detection of bacterial pathogens in children with visible nasal secretions: prevalence, concordance, sensitivity, and specificity between sampling methodsa
| Species and sampleb | No. (%) of positive cultures | P valuec | Concordanced (95% CI) | Cohen's kappa | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| Streptococcus pneumoniae | ||||||
| Nasopharyngeal swab | 42 (64) | Reference | NA | NA | NA | NA |
| Nasal swab | 42 (64) | 1.000 | 88 (78–94) | 0.74 | 91 (77–97) | 83 (62–95) |
| Swab from a paper tissuee | 42 (65) | 1.000 | 80 (69–88) | 0.57 | 85 (70–94) | 71 (49–87) |
| Paper tissue directly | 49 (74) | 0.039 | 86 (76–93) | 0.69 | 98 (86–100) | 67 (45–84) |
| Haemophilus influenzae | ||||||
| Nasopharyngeal swab | 46 (70) | Reference | NA | NA | NA | NA |
| Nasal swab | 44 (67) | 0.500 | 97 (90–99) | 0.93 | 96 (84–99) | 100 (80–100) |
| Swab from a paper tissue | 42 (64) | 0.125 | 94 (86–98) | 0.86 | 91 (78–97) | 100 (80–100) |
| Paper tissue directly | 43 (65) | 0.250 | 95 (88–99) | 0.90 | 94 (81–98) | 100 (80–100) |
| Moraxella catarrhalis | ||||||
| Nasopharyngeal swab | 47 (71) | Reference | NA | NA | NA | NA |
| Nasal swab | 49 (74) | 0.687 | 91 (82–96) | 0.77 | 96 (84–99) | 79 (54–93) |
| Swab from a paper tissue | 45 (68) | 0.727 | 88 (78–94) | 0.71 | 89 (76–96) | 84 (60–96) |
| Paper tissue directly | 45 (68) | 0.727 | 88 (78–94) | 0.71 | 89 (76–96) | 84 (60–96) |
| Staphylococcus aureus | ||||||
| Nasopharyngeal swab | 22 (33) | 0.344 | 85 (75–92) | 0.64 | 83 (58–96) | 85 (72–94) |
| Nasal swab | 18 (27) | Reference | NA | NA | NA | NA |
| Swab from a paper tissue | 15 (23) | 0.508 | 86 (76–93) | 0.64 | 67 (41–86) | 94 (82–98) |
| Paper tissue directly | 25 (38) | 0.065 | 83 (73–91) | 0.63 | 89 (64–98) | 81 (67–91) |
Abbreviations: CI, confidence interval; NA, not applicable.
Nasopharyngeal swab was considered the gold standard for detecting S. pneumoniae, H. influenzae, and M. catarrhalis; nasal swab was considered the gold standard for detecting S. aureus.
McNemar's test for paired samples; compared with the respective gold standards for detection.
Concordance, proportion of “true” positives and negatives compared with the respective gold standards for detection.
Culture result for pneumococcus of one swab from a paper tissue was missing.
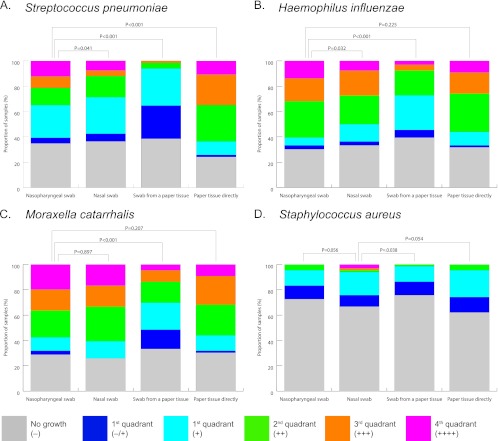
Fig 1.
Semiquantitative culture results for bacterial pathogens with different sampling methods. Conventional culture results were obtained for S. pneumoniae (A), H. influenzae (B), M. catarrhalis (C), or S. aureus (D) in children with an upper respiratory tract infection. Colonies were graded according to the distance from the point of inoculation, ranging from − (no growth) to ++++ (growth at maximum distance). Sampling methods were compared to the “gold standard” by using the Wilcoxon signed-rank test for paired samples. Nasopharyngeal sampling was considered the standard for S. pneumoniae, H. influenzae, and M. catarrhalis (4, 10, 11) and nasal sampling for S. aureus (17).
This is the first study comparing culture of nasal secretions, collected by wiping or blowing the nose into a paper tissue, with the current standards for detecting bacterial pathogens in children with an upper respiratory tract infection. Bacteria were generally detected at similar rates across sampling methods. Most importantly, concordance between sampling methods was high, indicating that in children with rhinorrhea, culture of a paper tissue collected by simply blowing or wiping the nose reliably detects four common bacterial pathogens. This may have major implications since it is a familiar and, especially for older children, a less unpleasant procedure than nasopharyngeal sampling. Moreover, it does not require training.
Nasopharyngeal sampling has been previously compared with other sampling techniques (1, 3, 4, 6, 12, 15), but no studies compared this with culture of a paper tissue directly. While Leach et al. found that detecting pneumococci in a swab from nasal secretions collected by blowing the nose into a paper tissue was highly sensitive for children with visible secretions, this was not compared either with a nasopharyngeal sample or with culture of the paper tissue as a whole (9). The latter is attractive since it does not require expensive swabs and is even simpler to perform.
We used PBS to transport the paper tissue. PBS is a water-based salt solution commonly used in research, but contrary to the case with modified liquid Amies, it is not specifically formulated to sustain viability of microorganisms. We postulate that the higher pneumococcal detection rate is related to the (abundant) presence of mucus in samples collected by a paper tissue. The physiologic composition of mucus produced during an upper respiratory tract infection may provide a good substrate for bacteria to abide in (2, 8). Our results support the concept that in children with an upper respiratory tract infection, nasal secretions contain bacteria whose detection during asymptomatic episodes would be more constrained to a specific ecologic niche. Moreover, pneumococci reside in the nasopharynx, an anatomical site that normally prevents easy dispersion. Changes in the upper respiratory tract during infection may therefore be linked to enhanced bacterial transmission.
Our study has some limitations. First, the specific criterion for rhinorrhea to be present limits the generalizability of our findings. Importantly, our results cannot be extrapolated to assessment of colonization in asymptomatic children (11). Second, mild respiratory infections are mostly attributed to a viral infection (5). The presence of bacterial pathogens may change during such an infection (7).
Our data show that culturing nasal secretions from a paper tissue reliably detects bacterial pathogens in children with rhinorrhea. This sampling method provides valuable information for studies of bacterial transmission or surveillance in children with upper respiratory tract infections.
ACKNOWLEDGMENTS
We have no conflicts of interest to declare related to this study.
Most of all, we are indebted to all participating children and their parents, who made this study possible. Additionally, we thank all cooperating institutes for their dedication to this project, especially day care centers of Saartje Kinderopvang, Utrecht, the Netherlands. In particular, we thank M. Rovers for methodological support in design of the study, L. Vos for sample collection, and A. Wyllie for laboratory assistance.
Footnotes
Published ahead of print 10 October 2012
REFERENCES
- 1. Agoritsas K, et al. 2006. Evaluation of the Quidel QuickVue test for detection of influenza A and B viruses in the pediatric emergency medicine setting by use of three specimen collection methods. J. Clin. Microbiol. 44:2638–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bevins CL, Salzman NH. 2011. The potter's wheel: the host's role in sculpting its microbiota. Cell. Mol. Life Sci. 68:3675–3685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Converse GM, Dillon HC. 1977. Epidemiological studies of Streptococcus pneumoniae in infants: methods of isolating pneumococci. J. Clin. Microbiol. 5:293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenberg D, et al. 2004. Relative importance of nasopharyngeal versus oropharyngeal sampling for isolation of Streptococcus pneumoniae and Haemophilus influenzae from healthy and sick individuals varies with age. J. Clin. Microbiol. 42:4604–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heikkinen T, Järvinen A. 2003. The common cold. Lancet 361:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ipp M, Carson S, Petric M, Parkin PC. 2002. Rapid painless diagnosis of viral respiratory infection. Arch. Dis. Child. 86:372–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ishizuka S, et al. 2003. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J. Infect. Dis. 188:1928–1939 [DOI] [PubMed] [Google Scholar]
- 8. Jonsson H, Ström E, Roos S. 2001. Addition of mucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 204:19–22 [DOI] [PubMed] [Google Scholar]
- 9. Leach AJ, Stubbs E, Hare K, Beissbarth J, Morris PS. 2008. Comparison of nasal swabs with nose blowing for community-based pneumococcal surveillance of healthy children. J. Clin. Microbiol. 46:2081–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy TF, Parameswaran GI. 2009. Moraxella catarrhalis, a human respiratory tract pathogen. Clin. Infect. Dis. 49:124–131 [DOI] [PubMed] [Google Scholar]
- 11. O'Brien KL, Nohynek H, World Health Organization Pneumococcal Vaccine Trials Carriage Working Group 2003. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr. Infect. Dis. J. 22:e1–e11 [DOI] [PubMed] [Google Scholar]
- 12. Rapola S, Salo E, Kiiski P, Leinonen M, Takala AK. 1997. Comparison of four different sampling methods for detecting pharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children. J. Clin. Microbiol. 35:1077–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rothman K. 25 October 2011. Episheet: spreadsheets for the analysis of epidemiologic data. October 2011 version. http://www.epidemiolog.net/studymat/
- 14. van den Bergh MR, et al. 2011. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine and DTPa-IPV-Hib when coadministered as a 3-dose primary vaccination schedule in the Netherlands: a randomized controlled trial. Pediatr. Infect. Dis. J. 30:e170–e178 [DOI] [PubMed] [Google Scholar]
- 15. van der Veen EL, Rovers MM, Leverstein-van Hall MA, Sanders EAM, Schilder AGM. 2006. Influence of sampling technique on detection of potential pathogens in the nasopharynx. Arch. Otolaryngol. Head Neck Surg. 132:752–755 [DOI] [PubMed] [Google Scholar]
- 16. Versalovic J. 2011. Manual of clinical microbiology, 10th ed ASM Press, Washington, DC [Google Scholar]
- 17. Williams RE. 1963. Healthy carriage of Staphylococcus aureus: its prevalence and importance. Bacteriol. Rev. 27:56–71 [DOI] [PMC free article] [PubMed] [Google Scholar]