Abstract
The GIX antigen, which is expressed on the surface of thymocytes of certain mouse strains, is an antigenic determinant of the major envelope glycoprotein of murine leukemia virus (gp70). Although GIX is expressed in some mouse strains that appear to be free of virus, the antigen can also be induced in GIX- mice by infection with particular murine leukemia viruses (termed GIX+). We have investigated the envelope gene products from two closely related viruses that differ in their GIX phenotype. Analysis of the envelope protein precursors by polyacrylamide gel electrophoresis and endoglycosidase treatment indicated that the GIX+ viral protein contained six oligosaccharide chains, whereas the GIX- viral protein contained seven. The observed differences in gel electrophoretic mobilities and glycopeptide profiles of the respective glycosylated envelope gene cleavage products (gp70) may be accounted for by the presence of an additional oligosaccharide chain on the gp70 of the GIX- virus. No differences between the apparent molecular weights of the nonglycosylated product of the envelope gene (p15E) were detected. These results suggest that the GIX- virus codes for an extra glycosylation site relative to the GIX+ virus, and this oligosaccharide chain is present both on the envelope gene precursor (Prenv) and on the major cleavage product (gp70). Recent nucleotide sequence analyses of selected RNase T1 oligonucleotides from the genomes of viruses that differ in GIX phenotype have similarly suggested that there may be a correlation between the GIX- phenotype and an extra glycosylation site [Donis-Keller, H., Rommelaere, J., Ellis, R. W. & Hopkins, N. (1980) Proc. Natl. Acad. Sci. USA 77, 1642-1645]. The results of these two different approaches raise the possibility that the presence of an additional oligosaccharide chain on gp70 may, either directly or indirectly, mask the expression of the GIX antigen on the surfaces of thymocytes and virus-infected cells.
Full text
PDF

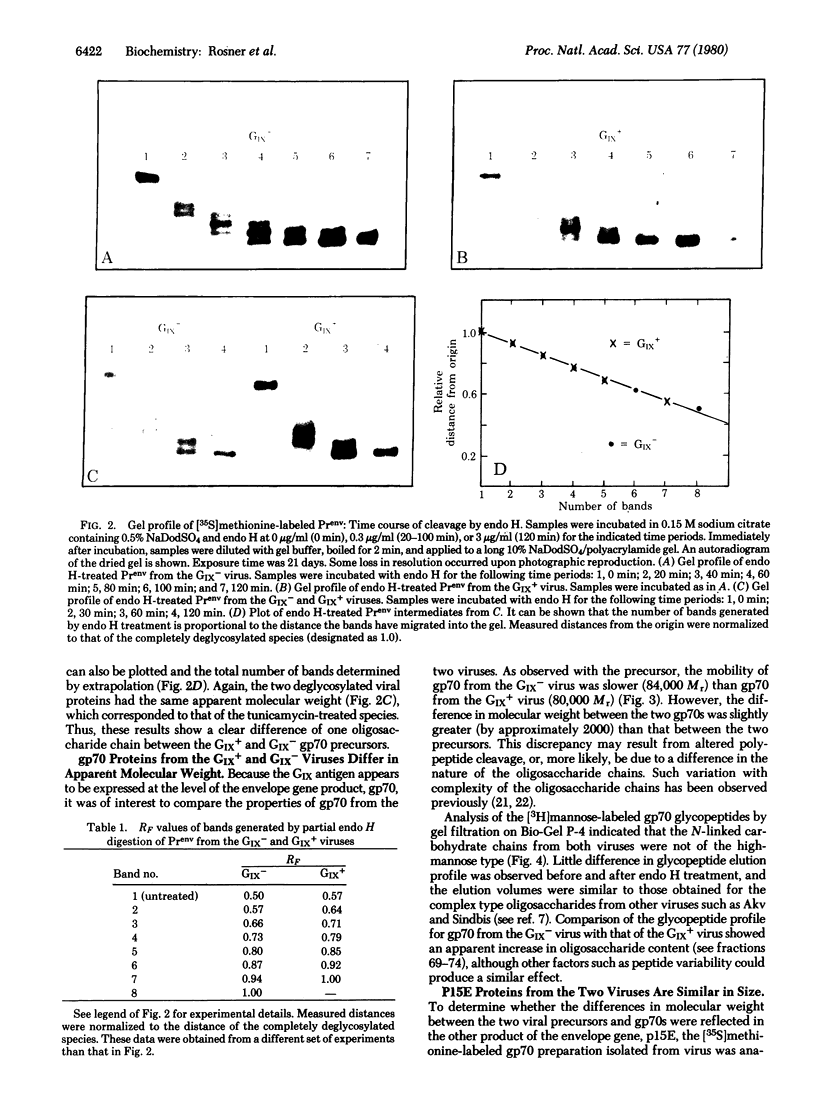


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyse E. A. The Gx system in relation to C-type viruses and heredity. Immunol Rev. 1977 Jan;33:125–145. doi: 10.1111/j.1600-065x.1977.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Rommelaere J., Ellis R. W., Hopkins N. Nucleotide sequences associated with differences in electrophoretic mobility of envelope glycoprotein gp70 and with GIX antigen phenotype of certain murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1642–1645. doi: 10.1073/pnas.77.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., Hopkins N., Fleissner E. Biochemical analysis of murine leukemia viruses isolated from radiation-induced leukemias of strain BALB/c. J Virol. 1980 Feb;33(2):661–670. doi: 10.1128/jvi.33.2.661-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faller D. V., Hopkins N. T1 oligonucleotides that segregate with tropism and with properties of gp70 in recombinants between N- and B-tropic murine leukemia viruses. J Virol. 1978 Apr;26(1):153–158. doi: 10.1128/jvi.26.1.153-158.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G., Buchhagen D. L., Klenk H. D., Fleissner E. Presence of murine leukemia virus envelope proteins gp70 and p15(E) in a common polyprotein of infected cells. J Virol. 1976 Nov;20(2):501–508. doi: 10.1128/jvi.20.2.501-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Schindler J., Gottlieb P. D. Evidence for recombination between N- and B-tropic murine leukemia viruses. J Virol. 1977 Mar;21(3):1074–1078. doi: 10.1128/jvi.21.3.1074-1078.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McLellan W. L., August J. T. Analysis of the envelope of Rauscher murine oncornavirus: in vitro labeling of glycopeptides. J Virol. 1976 Dec;20(3):627–636. doi: 10.1128/jvi.20.3.627-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Karshin W. L., Jamjoom G. A., Arlinghaus R. B. A fucose-deficient glycoprotein precursor to Rauscher leukemia virus gp69/71. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2326–2330. doi: 10.1073/pnas.73.7.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata Y., Ikeda H., Stockert E., Boyse E. A. Relation of GIX antigen of thymocytes to envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):188–197. doi: 10.1084/jem.141.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter A., Fleissner E. The presence of disulfide-linked gp70-p15(E) complexes in AKR murine leukemia virus. Virology. 1977 Dec;83(2):417–422. doi: 10.1016/0042-6822(77)90187-8. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Grinna L. S., Robbins P. W. Differences in glycosylation patterns of closely related murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):67–71. doi: 10.1073/pnas.77.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Schindler J., Hynes R., Hopkins N. Evidence for recombination between N- and B-tropic murine leukemia viruses: analysis of three virion proteins by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1977 Sep;23(3):700–700. doi: 10.1128/jvi.23.3.700-.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Keegstra K. Glycoproteins of Sindbis virus: priliminary characterization of the oligosaccharides. J Virol. 1974 Sep;14(3):522–530. doi: 10.1128/jvi.14.3.522-530.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert E., Old L. J., Boyse E. A. The G-IX system. A cell surface allo-antigen associated with murine leukemia virus; implications regarding chromosomal integration of the viral genome. J Exp Med. 1971 Jun 1;133(6):1334–1355. doi: 10.1084/jem.133.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Synthesis and glycosylation in vitro of glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1516–1520. doi: 10.1073/pnas.74.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Vitetta E. S., Fleissner E., Boyse E. A. Biochemical evidence linking the GIX thymocyte surface antigen to the gp69/71 envelope glycoprotein of murine leukemia virus. J Exp Med. 1975 Jan 1;141(1):198–205. doi: 10.1084/jem.141.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zaane D., Dekker-Michielsen J. A., Bloemers H. P. Virus-specific precursor polypeptides in cells infected with Rauscher leukemia virus: synthesis, identification, and processing. Virology. 1976 Nov;75(1):113–129. doi: 10.1016/0042-6822(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]









