Abstract
A longitudinal study combining multilocus sequence typing with molecular evolutionary analysis determined the distribution, population structure, and evolution of antibiotic resistance in Neisseria gonorrhoeae isolates in Saskatchewan that were collected between 2005 and 2008. Of 195 gonococcal isolates examined, 29 sequence types (STs) were identified with 3 major circulating strains (ST-1 through ST-3) comprising 52% of all gonococcal isolates studied. The prevalences, persistence, distribution patterns, and clonalities of these isolates strongly suggest that gonorrhea endemicity within this broad geographic region was driven by these 3 circulating strains. ST-1 exhibited a significantly (P = 0.001) higher prevalence throughout the study than did the others, accounting for ∼25% of the tested isolates each year. The spatial distributions of the gonococcal strains indicated that ST-1 in 2007 entered a linear component of the sexual network, reaching the remote north and resulting in the further spread and maintenance of infection. Ciprofloxacin and azithromycin resistances were observed in distantly related gonococcal lineages, clearly indicating the convergent acquisition of these antibiotic-resistant phenotypes. In addition, all ciprofloxacin- and azithromycin-resistant lineages were found at the edges of the minimum spanning tree, far from the major lineages, suggesting that these antibiotic phenotypes were most likely introduced into the province. In contrast, resistance to penicillin was found mostly in the endemic gonococcal lineages, suggesting that penicillin resistance was probably acquired in Saskatchewan as a result of spontaneous mutations in already-established lineages. Tetracycline resistance was present in all STs except one, indicating its ubiquitous nature in the gonococcal population studied.
INTRODUCTION
Neisseria gonorrhoeae, a fastidious Gram-negative diplococcus, is an obligate human pathogen that causes the disease gonorrhea. Complications from the spread of the organism along the mucosal surfaces may result in endometritis, salpingitis, and peritonitis in females (3), which can lead to infertility or ectopic pregnancy (27). In Canada, between 1999 and 2008, the overall rate of gonococcal infection increased by 116.5%, from 17.6 to 38 cases per 100,000 (20). Saskatchewan has had the highest infection rate of all the Canadian provinces, with an increase from 45.2 cases per 100,000 in 2000 to 131.3 cases per 100,000 in 2008, resulting in a 290.4% increase (173.4% above the national average) (20). Antimicrobial resistance in N. gonorrhoeae is a major public health problem, as treatment options are becoming increasingly limited. Recently, N. gonorrhoeae isolates with decreased susceptibilities (MIC ≥ 0.25) to third-generation cephalosporins (cefixime and ceftriaxone) have been identified in Japan, Europe, the United States, and Canada (4, 15, 16, 19, 29, 30). These antibiotics are the last remaining options for treating gonorrhea in a single-dose fashion, a preferred mode of treatment for gonococcal infections.
Strain typing can be successfully used to identify specific strains that are spreading globally or to identify temporal and geographic changes in strain types and the emergence and transmission of individual strain phenotypes (31). The multilocus sequence typing (MLST) genotyping method is based on obtaining the DNA sequences of various housekeeping genes that are under stabilizing selection (32) (i.e., nonsynonymous substitutions are likely to be purged relatively rarely). As these genes evolve slowly, the genetic relatedness of a bacterial population can be ascertained over the long term more confidently than with genotyping methods based on antigen-encoding genes that are under positive selection pressure and that change rapidly over the short term (32, 34). A combination of MLST with molecular evolutionary analysis, such as eBURST (12) and the minimum spanning tree, provides reliable insight into the evolutionary pathways and transmission routes of gonococcal isolates. In addition, the emergence and evolution of antibiotic-resistant N. gonorrhoeae strains can be better resolved using MLST with the analytical methods discussed above to reveal their evolutionary paths and clonal complexes (CCs).
The aim of this research was to characterize and analyze gonococcal isolates from the province of Saskatchewan that were collected between 2005 and 2008 to uncover the distribution patterns and population structures of these strains and to gain insight into the evolution of antibiotic-resistant phenotypes. This investigation is a unique longitudinal population-based study in which 195 consecutively obtained N. gonorrhoeae isolates over a 4-year period were examined by MLST and analyzed by eBURST and the minimum spanning tree methods to investigate gonococcal strain relationships and the evolution of antibiotic-resistant phenotypes.
MATERIALS AND METHODS
Bacterial isolates.
The Saskatchewan Disease Control Laboratory (SDCL) serves as the microbiological reference center for the province of Saskatchewan. Clinics and hospitals across the province submit N. gonorrhoeae specimens to the SDCL for identification and confirmation. From 2005 to 2008, the SDCL received 195 consecutive gonococcal isolates for culture, representing 195 unique patients. Over 98% of the isolates were urogenital in origin. Primary identification was undertaken at the SDCL using standard microbiological methods (9, 41). Information, such as city of isolation and the age and sex of the patients, was available for each isolate. Isolates were stored at −80°C in brain heart infusion medium (Difco BD Biosciences, Oakville, ON, Canada) containing 20% glycerol and were shipped on dry ice to Saskatoon for molecular typing. They were cultured on Difco GC medium base (Becton, Dickinson and Company, Sparks, MD) supplemented with 1% modified Kellogg's supplement GC medium base (GCMB) followed by incubation at 35°C with 5 to 7% CO2 in a humid environment for 18 to 24 h (9, 41). Isolates were reconfirmed as N. gonorrhoeae using the oxidase test and Gram staining (37).
Antimicrobial susceptibility testing.
The MICs of the N. gonorrhoeae isolates to penicillin, tetracycline, ciprofloxacin, spectinomycin, cefixime, and ceftriaxone were assessed, in duplicate, by the agar dilution method as described previously (9, 41). All antimicrobial agents were purchased from Sigma-Aldrich (Oakville, ON, Canada). WHO strains B, C, and F and strain ATCC 49226 were used as reference strains for antimicrobial susceptibility testing (9, 28, 35). Antimicrobial susceptibility criteria followed CLSI recommendations (9); interpretative criteria for azithromycin resistance have been described previously (7). β-Lactamase production was determined using nitrocefin (Calbiochem, EMD Chemicals Inc., Darmstadt, Germany). N. gonorrhoeae isolates identified as having plasmid-mediated tetracycline resistance (i.e., TRNG) based on MICs of ≥16 mg/liter were confirmed by tetM analysis with WHO strain M as a positive control (40).
Multilocus sequencing typing.
Growth from an overnight culture on GC medium base was collected using a sterile cotton swab and resuspended in 1 ml of 0.9% saline. After centrifugation at 10,000 × g for 1 min, the supernatant was removed, and genomic DNA was extracted using the Qiagen DNeasy tissue kit (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions.
All gonococcal isolates were typed using the MLST method described by Tazi et al. (26), and sequence types (STs) were randomly assigned. The following seven markers were used: fumC, gdh, glnA, gnd, pilA, pyrD, and serC. The oligonucleotide primers used in this study were purchased from Life Technologies–Invitrogen (Burlington, ON, Canada). Amplicons were purified using a PCR purification kit (Qiagen, Mississauga, ON, Canada), and their nucleotide sequences on both strands were determined using an Applied Biosystems 3730X1 DNA analyzer (Plant Biotechnology Institute, National Research Council of Canada, Saskatoon, SK, Canada). DNA sequences were analyzed using DNASTAR Lasergene 7 (DNASTAR Inc., USA). DNA sequences for each locus were compared, and every unique sequence was assigned as a distinct allele using Sequence Output (www.mlst.net). Each unique combination of allele numbers was assigned as a different sequence type (ST) which unambiguously defined strains of N. gonorrhoeae.
Phylogenetic and eBURST analysis.
MEGA4 (25) was used to construct phylogenetic trees from concatenated sequences of the housekeeping genes. Evolutionary distances were computed using the maximum composite likelihood method (24). The neighbor-joining algorithm (22) was used to generate the initial phylogenetic tree. To predict ancestral profiles and clonal complexes, each genotype identified in this study was analyzed by eBURST (12). For identification of the clonal complexes, the most stringent default group definition was used, where all STs must be single-locus variants of at least one ST in the clonal complex.
Minimum spanning tree.
The minimum sum of the weights of the edges (genetic distances) was used to create a minimum spanning tree from the MLST profile data. Prim's algorithm was implemented to determine the links between different STs. The highest priority was given to STs with the most numerous single-locus variants. The two STs with the greatest number of single-locus and then double-locus variants were linked first, preferably using intermediate STs.
Statistical analyses.
Data were analyzed using SAS (SAS Institute Inc., Cary, NC) by applying the Marascuilo procedure test to determine if there was a significant difference between the ratios for every pair of STs. Also, a trend in changes in the prevalence of gonococcal strains over the 4-year period was evaluated using the Chi-square multiple-hypothesis test.
Nucleotide sequence accession numbers.
Unique DNA sequences were deposited in GenBank with accession numbers HM359031 through HM359049 and HM359053 through HM359071.
RESULTS
Identified sequence types and clonal complexes of N. gonorrhoeae.
In total, 2,730 DNA sequences were processed. The 195 N. gonorrhoeae isolates were resolved by MLST into 29 different STs. Most notably, 3 distinct STs were responsible for 52% (n = 106) of all the N. gonorrhoeae isolates we examined. ST-1 accounted for 23.6% (n = 46) of the isolates, followed by ST-3 (15.9%, n = 31) and ST-2 (12.3%, n = 24). Seventy-four isolates were resolved into 11 STs (ST-4, ST-5, ST-6, ST-8, ST-11, ST-14, ST-15, ST-16, ST-20, ST-21, and ST-25); these STs accounted for 1.5% (n = 3) to 7.2% (n = 14) of the isolates examined. Lastly, 10 STs (ST-7, ST-12, ST-13, ST-17, ST-18, ST-22, ST-23, ST-26, ST-27, and ST-28) were represented by a single isolate, and five STs (ST-9, ST-10, ST-19, ST-24, and ST-29) were represented by two isolates each, indicating that these STs were sporadic and minor.
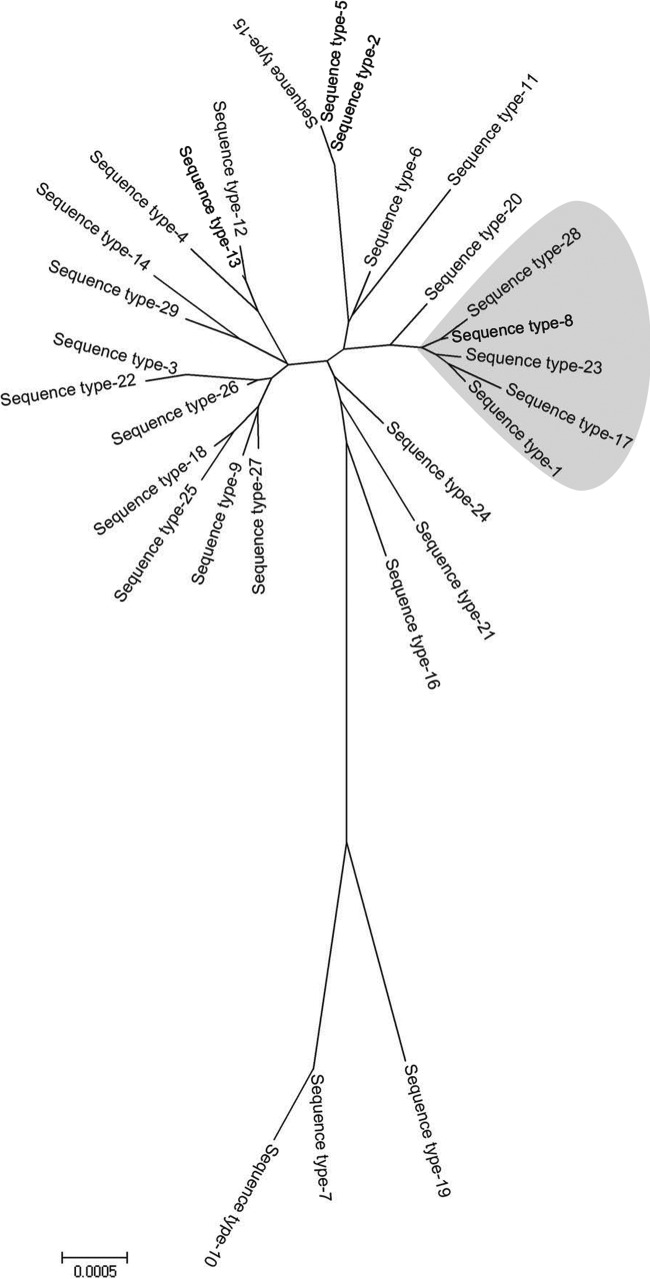
The genetic relatedness between the 29 STs is presented in a minimum evolution tree (Fig. 1) that also indicates the major clonal complex. The population snapshot of all 195 N. gonorrhoeae isolates generated by the eBURST analysis revealed the existence of one major and eight minor clonal complexes (CCs). ST-1 was identified as the ancestral strain for the major CC that also involved ST-8, ST-17, ST-23, and ST-28, as also indicated in the minimum evolution tree. The eight minor CCs comprised one triplet (STs 27, 28, and 6) and seven doublets (STs 2 and 5, 18 and 25, 13 and 12, 24 and 29, 21 and 19, 3 and 22, and 7 and 10). Seven different STs (ST-14, ST-15, ST-11, ST-20, ST-16, ST-4, and ST-9) were identified as singletons and could not be assigned to any existing CC.
Fig 1.
Minimum evolution tree of the concatenated sequences from the 29 sequence types (STs) comprising 195 N. gonorrhoeae isolates. The 5 lineages shaded in gray represent the major clonal complex identified in the population of N. gonorrhoeae isolates from Saskatchewan over the study period. The evolutionary history was inferred using the minimum evolution method (21). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The minimum evolution tree was searched using the close-neighbor-interchange algorithm (18) at a search level of 1.
Distribution of sequence types.
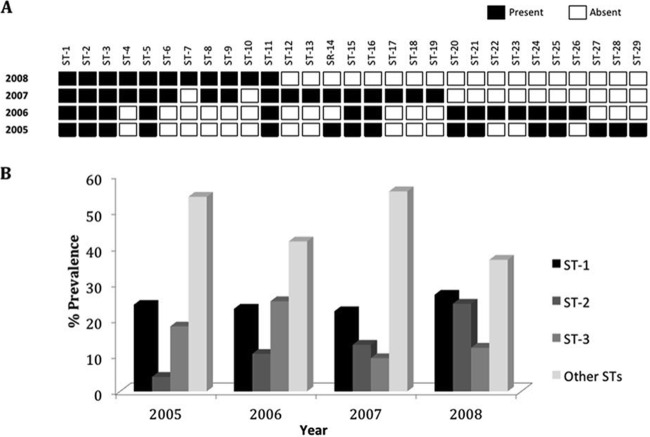
Longitudinal data collected from 2005 to 2008 clearly showed the existence of two different classes of gonococcal strains. One class, comprising ST-1, ST-2, ST-3, ST-5, and ST-11, was characterized by the constant circulation of the STs throughout the period of testing (Fig. 2A), while the other class of strains was characterized by the STs' sporadic occurrence (Fig. 2A). Circulating sequence types (i.e., ST-1, ST-2, and ST-3) showed significantly higher prevalences (P ≤ 0.05) than any sporadic strain identified in this study (Fig. 2A and B). In examining trends in the prevalences of gonococcal strains, separately for each year, only ST-1 showed continuously higher significance (P ≤ 0.05) than any other ST (Fig. 2B). The strains associated with the sporadic sequence types rapidly disappeared (Fig. 2A), and the sporadic sequence types from 2005 and 2006 were completely replaced in 2008 with new sequence types.
Fig 2.
Longitudinal distribution of Neisseria gonorrhoeae strains. (A) Occurrence of the STs over a 4-year period. Solid squares indicate the presence of certain STs, while open squares indicate their absence. (B) Prevalence rates of the most predominant circulating strains compared to the prevalence rates of all other STs identified during the 2005-2008 study period in Saskatchewan.
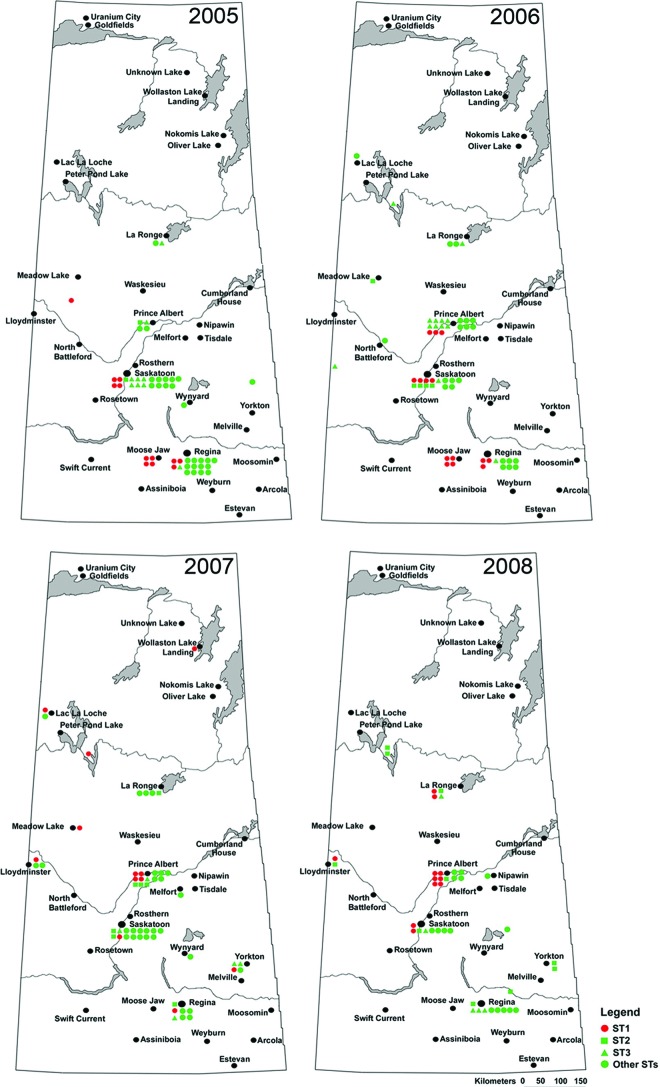
The geographic distribution of the gonococcal sequence types across the province during the study period indicated a distinct migration route for ST-1 (Fig. 3). Isolates belonging to ST-1 were first identified in the southern part of the province (Fig. 3) and migrated toward the north over the study period. In 2008, the last year of this study, ST-1 isolates were predominantly identified in the northern part of the province (Fig. 3). Other STs showed more nondistinct migration.
Fig 3.
Spatial distribution of Neisseria gonorrhoeae strains in Saskatchewan during the 2005-2008 study period. The most predominant ST-1 strain is shown in red to emphasize its importance. The other two circulating strains, ST-2 and ST-3, are shown in green squares and triangles, respectively. All of the other 26 sequence types identified in this study are indicated by green circles. (Reprinted from reference 33.)
Sequence types and antibiotic resistance.
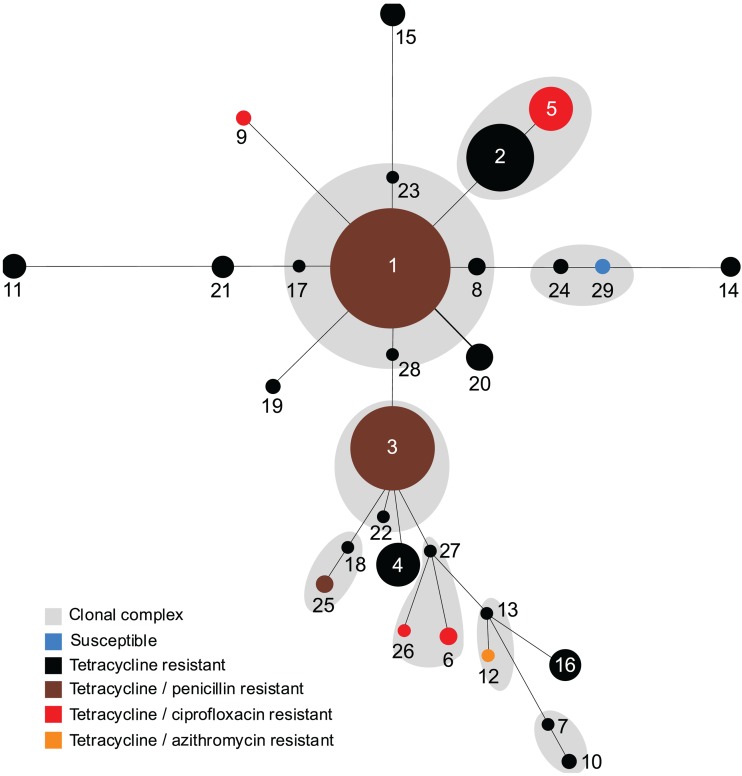
During the period of this study, all gonococcal isolates were found to be susceptible to cefixime, ceftriaxone, and spectinomycin. The majority of isolates (77.9%, n = 152) were resistant to tetracycline, with 94.1% (n = 143/152) of these isolates being chromosomally resistant and 5.9% (n = 9) carrying plasmid-mediated tetracycline resistance (TRNG). Tetracycline resistance was identified in all STs except ST-29 (Fig. 4), while TRNG isolates were identified in ST-20 (7/7), followed by ST-25 (1/3) and ST-26 (1/1). Resistance to penicillin (3.0%, 6/195), ciprofloxacin (2.6%, 5/195), and azithromycin (0.5%, 1/195) was low, and no penicillinase-producing N. gonorrhoeae (PPNG) isolates were detected. The majority of isolates resistant to penicillin (5/6) were found in the circulating strains ST-1 (n = 3) and ST-3 (n = 2), whereas one penicillin-resistant isolate was identified in sporadic strain ST-25. All ciprofloxacin-resistant isolates (n = 5) were found in sporadic sequence types, including ST-6, ST-9, ST-26, and ST-5. Also, the single azithromycin-resistant isolate (n = 1) was found in sporadic sequence type ST-12. All of these sporadic isolates were positioned on the edges of the minimum spanning tree (Fig. 4).
Fig 4.
Minimum spanning tree based on the multilocus sequence typing allelic profiles depicting the antibiotic-resistant phenotype distribution across Neisseria gonorrhoeae lineages and their clonal complexes. Each circle represents a unique ST. The number of isolates of each ST is directly proportional to the area of the circle. The blue circle represents the ST susceptible to all tested antibiotics, while black indicates resistance to tetracycline alone, brown indicates resistance to penicillin and tetracycline, red indicates resistance to ciprofloxacin and tetracycline, and orange indicates resistance to azithromycin and tetracycline. Gray shadings represent the clonal complexes which consist of single-locus variants.
DISCUSSION
To reveal the distribution patterns and population structures of N. gonorrhoeae isolates from the province of Saskatchewan, we performed a longitudinal study over a 4-year period using MLST, a neutral typing method (14, 32, 34) (i.e., a method capable of buffering frequent mutations), and molecular evolutionary analyses. By using MLST, we were able to minimize some limitations associated with typing methods, such as porB and N. gonorrhoeae multiantigen sequence typing (NG-MAST), that may overdiscriminate related isolates. For example, in a recent study (34), we showed that the concatenated sequences of the housekeeping genes (MLST) retain the phylogenetic signals of the circulating gonococcal strains and can identify them over a long period of time. On the other hand, the hypervariable porB DNA sequence is subject to frequent mutation and recombination, further producing a distorting effect among genetically related isolates that may lead to a failure to identify the predominant related strains of N. gonorrhoeae that circulate within the community over an extended period of time (34). A unique characteristic of using MLST analysis is its ability to provide a buffer against the distorting effects of mutations and recombinations, further resulting in the preservation of clonal stability of N. gonorrhoeae isolates. Therefore, MLST is a suitable method for fine-scaled epidemiological investigations designed to monitor the spread of gonococcal isolates over an extended period of time.
The present population-based study shows that the majority of gonococcal infections in Saskatchewan between 2005 and 2008, a region with one of the highest gonorrhea infection rates worldwide (36), were mainly caused by three distinct circulating gonococcal strains. Another study examining the population genetics of N. gonorrhoeae isolates in Liverpool, United Kingdom, also identified a couple of gonococcal strains, ST-1596 and ST-1583, with profound geographic persistence, indicating that these gonococcal strains may have a fitness advantage which enables them to persist over a long period of time (2). However, these persistent gonococcal strains found in Liverpool did not show the high prevalences that the three major circulating strains showed in our study. In comparing the gonococcal population from Saskatchewan to one from Shanghai (26), we found that the main characteristic of gonococcal isolates from Saskatchewan is a high degree of genetic homogeneity. For instance, 195 gonococcal isolates from this study were resolved into 29 different STs, while the population of 96 isolates from Shanghai was resolved into 76 STs. In addition, the gonococcal population from Saskatchewan showed temporal clustering, whereas no temporal clustering was observed among the isolates from Shanghai. These studies suggest the existence of two distinct gonococcal populations. The gonococcal population from Shanghai was very diverse, with no predominant strains, whereas the gonococcal population from Saskatchewan, Canada, was homogenous, with the existence of several predominant strains that spread throughout the province.
The spatial distribution of the gonococcal sequence types showed that the most predominant type, ST-1, was initially identified in the south-central part of the province (Fig. 3), mainly in the most populated areas of the province. Over the next 2 years (2005-2006), infections associated with ST-1 remained in the same areas, showing characteristics of endemic infection. By 2007, strain ST-1 exhibited a distinct northward motion, reaching remote places which are accessible only by airplane or boat. This type of ST-1 distribution reflects the linear component of a sexual network (38). Using routinely collected case/contact information by public health authorities, Wylie and Jolly (38) identified two types of components, radial and linear, within a large sexual network in the province of Manitoba, Canada. Linear-type components were characterized by a larger number of distinct geographic locations than the radial-type components. From a public health perspective, linear components represent an important reservoir for gonorrhea maintenance and spread (38). Further, Wylie et al. (39) found that the linear component included extensive interconnections present within a large sexual network, resulting in frequent bridging events between different demographic groups. For instance, in a study that examined a large linear-type component in Manitoba, Canada, involving 82 people, the authors found two prominent central figures that caused the network to span significant distances, resulting in the infection of people living in various communities scattered throughout this large region of western Canada (38). In contrast to linear components, radial components were characterized by local transmissions through direct or indirect links between members of the same community. In our study, the observation that ST-1 is frequently found over a vast area of the Saskatchewan province and connects otherwise geographically disconnected areas clearly indicates its importance in spreading and maintaining gonococcal infection. We were unable to discern in this study whether ST-1 is common in other parts of Canada or globally, because the MLST scheme used, described earlier by Tazi et al. (26), is not posted online and is therefore unavailable for international comparison.
The population snapshot of 195 gonococcal isolates created by our eBURST analysis shows the existence of a single major clonal complex together with several minor clonal complexes. Most notably, the highly predominant ST-1 was found as an ancestor of the major clonal complex, further suggesting that this circulating strain has been present in Saskatchewan for a long period. Unlike the gonococcal population in Shanghai (26), where all STs within the major clonal complexes did not differ in size, the founder of the major clonal complex in Saskatchewan was profoundly larger than the other STs. Our eBURST analysis suggested that, in Saskatchewan, there exists a predominant gonococcal lineage, which is well established, whereas in Shanghai the gonococcal population is more dynamic, without predominant gonococcal strains.
Emerging resistance to extended-spectrum cephalosporins (19, 29, 30), as well as resistance to fluoroquinolone (15, 41) and macrolide (8, 10, 13, 23) antibiotics, among gonococcal isolates is an ongoing challenge for the treatment and control of gonorrhea infections. In this study, we have provided insight into the antimicrobial profiles of N. gonorrhoeae isolates collected over a large geographic area using past and present antimicrobial agents recommended for gonococcal treatment (5, 6). The most prevalent antibiotic resistance was chromosomal resistance to tetracycline. The high rate of resistance to this antibiotic may be attributed to the history of tetracycline use in the cotreatment of chlamydial infections, as tetracycline has not been recommended for the primary treatment of gonorrhea in Canada since the late 1980s (11). Notably, the prevalence rate of ciprofloxacin resistance (2.6%) in gonococcal isolates from this study was significantly lower than that reported in the recent study (28.2%) carried out in Ontario, Canada (1). The depiction of ciprofloxacin resistance across the gonococcal lineages on the minimum spanning tree (Fig. 4) clearly suggests the convergent acquisition (genetically distant lineages acquiring the same antimicrobial phenotype) of drug resistance by these isolates rather than the clonal expansion. Furthermore, all gonococcal lineages resistant to ciprofloxacin were positioned at the edges of the minimum spanning tree, indicating a very distant genetic relatedness to the major gonococcal lineages, and further suggesting that ciprofloxacin resistance was most likely introduced from outside the province. Mavroidi et al. (17) found that the emergence and rapid recent increase of a quinolone-resistant strain of N. gonorrhoeae in Greece were due to the importation of a quinolone-resistant strain, ST-1901, from other countries. Although resistance to ciprofloxacin in Saskatchewan is presently low, it may emerge and spread, as we already observed the importation of several quinolone-resistant N. gonorrhoeae strains within this province. Chromosomal resistance to penicillin was observed among the major gonococcal lineages (Fig. 4), further suggesting that this phenotype was probably acquired in Saskatchewan as a result of spontaneous mutations in established lineages.
In summary, this study has revealed the structure of the gonococcal population from the province of Saskatchewan and clearly indicates the high impact of several circulating strains on the overall rates and spread of N. gonorrhoeae isolates. In addition to providing current insight into the antibiotic susceptibilities of gonococcal isolates, this work also illustrates how combining antimicrobial susceptibility data, MLST, and evolutionary analysis (eBURST and the minimum spanning tree) provides a more comprehensive view of the evolution and dissemination of antimicrobial susceptibility traits in the gonococcal population.
ACKNOWLEDGMENTS
We thank Daniela Vidovic for the preparation of figures. We also thank Evelyn Nagle for technical assistance with N. gonorrhoeae isolates and Aura Helena Corredor for her assistance with the manuscript.
This work was supported by the Saskatchewan Health Research Foundation (grant 9127, Research Alliance for the Prevention of Infectious Disease [RAPID]).
Footnotes
Published ahead of print 12 September 2012
REFERENCES
- 1. Allen VG, et al. 2011. Molecular analysis of antimicrobial resistance mechanisms in Neisseria gonorrhoeae isolates from Ontario, Canada. Antimicrob. Agents Chemother. 55:703–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bennett JS, et al. 2007. Species status of Neisseria gonorrhoeae: evolutionary and epidemiological inferences from multilocus sequence typing. BMC Biol. 5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolan G, Ehrhardt AA, Wasserheit JN. 1999. Gender perspectives and STDs, p 117–127 In Holmes KK, Mardh PA, Sparling PF, Lemon SM, Stamm WE, Piot P, Wasserheit JN. (ed), Sexually transmitted diseases, 3rd ed McGraw-Hill, New York, NY [Google Scholar]
- 4. Bolan GA, Sparling PF, Wasserheit JD. 2012. The emerging threat of untreatable gonococcal infection. N. Engl. J. Med. 366:485–487 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention 2006. Sexually transmitted diseases guidelines. MMWR Recomm. Rep. 55:1–94 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2007. Update to CDC's sexually transmitted diseases guidelines. Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb. Mortal. Wkly. Rep. 56:332–336 [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2010. Gonococcal isolate surveillance project (GISP) protocol. Isolates with alert value MICs. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/std/gisp/GISP-Protocol07-15-2010.pdf [Google Scholar]
- 8. Chisholm SA, et al. 2009. Emergence of high-level azithromycin resistance in Neisseria gonorrhoeae in England and Wales. J. Antimicrob. Chemother. 64:353–358 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2006. M7–A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Dillon JR, et al. 2001. Reduced susceptibility to azithromycin and high percentages of penicillin and tetracycline resistance in Neisseria gonorrhoeae isolates from Manaus, Brazil, 1998. Sex. Transm. Dis. 28:521–526 [DOI] [PubMed] [Google Scholar]
- 11. Division of STD Prevention and Control, Health Protection Branch, Health Canada 1998. Gonococcal infection, p 115–124 In Canadian STD guidelines. 1998 ed Division of STD Prevention and Control, Health Protection Branch, Health Canada, Ottawa, ON, Canada [Google Scholar]
- 12. Feil EJ, Li BC, Aanensen DM, Hanage WP, Spratt BG. 2004. eBURST: inferring patterns of evolutionary descent among clusters of related bacterial genotypes from multilocus sequence typing data. J. Bacteriol. 186:1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galarza PG, et al. 2009. Emergence of high level azithromycin-resistant Neisseria gonorrhoeae strains isolated in Argentina. Sex. Transm. Dis. 36:787–788 [DOI] [PubMed] [Google Scholar]
- 14. Ilina NE, et al. 2010. Molecular surveillance of clinical Neisseria gonorrhoeae isolates in Russia. J. Clin. Microbiol. 48:3681–3689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin I, Jayaraman G, Wong T, Liu G, Gilmour M, Canadian Public Health Laboratory Network 2011. Trends in antimicrobial resistance in Neisseria gonorrhoeae isolated in Canada: 2000-2009. Sex. Transm. Dis. 38:892–898 [DOI] [PubMed] [Google Scholar]
- 16. Martin I, et al. 2012. Emergence and characterization of Neisseria gonorrhoeae isolates with decreased susceptibilities to ceftriaxone and cefixime in Canada: 2001-2010. Sex. Transm. Dis. 39:316–323 [DOI] [PubMed] [Google Scholar]
- 17. Mavroidi A, et al. 2011. Analysis of emergence of quinolone-resistant gonococci in Greece by combined use of Neisseria gonorrhoeae multiantigen sequence typing and multilocus sequence typing. J. Clin. Microbiol. 49:1196–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nei M, Kumar S. 2000. Molecular evolution and phylogenetics. Oxford University Press, New York, NY [Google Scholar]
- 19. Ohnishi M, et al. 2011. Ceftriaxone-resistant Neisseria gonorrhoeae, Japan. Emerg. Infect. Dis. 17:148–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Public Health Agency of Canada 2008. Report on sexually transmitted infections in Canada. Public Health Agency of Canada, Ottawa, ON, Canada: http://www.phac-aspc.gc.ca/std-mts/report/sti-its2008/04-eng.php [Google Scholar]
- 21. Rzhetsky A, Nei M. 1992. A simple method for estimating and testing minimum evolution trees. Mol. Biol. Evol. 9:945–967 [Google Scholar]
- 22. Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 23. Starnino S, Stefanelli P. 2009. Azithromycin-resistant Neisseria gonorrhoeae strains recently isolated in Italy. J. Antimicrob. Chemother. 63:1200–1204 [DOI] [PubMed] [Google Scholar]
- 24. Tamura K, Nei M, Kumar S. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 101:11030–11035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 26. Tazi L, et al. 2010. Population dynamics of Neisseria gonorrhoeae in Shanghai, China: a comparative study. BMC Infect. Dis. 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turner CF, et al. 2002. Untreated gonococcal and chlamydial infection in a probability sample of adults. JAMA 287:726–733 [DOI] [PubMed] [Google Scholar]
- 28. Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall J. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J. Antimicrob. Chemother. 63:1142–1151 [DOI] [PubMed] [Google Scholar]
- 29. Unemo M, Golparian D, Stary A, Eigentler A. 2011. First Neisseria gonorrhoeae strain with resistance to cefixime causing gonorrhoea treatment failure in Austria, 2011. Euro Surveill. 16(43):pii:19998. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19998 [PubMed] [Google Scholar]
- 30. Unemo M, et al. 2011. High-level cefixime- and ceftriaxone-resistant N. gonorrhoeae in Europe (France): novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob. Agents Chemother. 56:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Unemo M, Dillon JR. 2011. Review and international recommendation of methods for typing Neisseria gonorrhoeae isolates and their implications for improved knowledge of gonococcal epidemiology, treatment, and biology. Clin. Microbiol. Rev. 24:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Urwin R, Maiden MCJ. 2003. Multi-locus sequence typing: a tool for global epidemiology. Trends Microbiol. 11:479–487 [DOI] [PubMed] [Google Scholar]
- 33. Vidovic S, Korber D. 2006. Prevalence of Escherichia coli O157 in Saskatchewan cattle: characterization of isolates by using random amplified polymorphic DNA PCR, antibiotic resistance profiles, and pathogenicity determinants. Appl. Environ. Microbiol. 72:4347–4355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vidovic S, Horsman GB, Liao M, Dillon JR. 2011. Influence of conserved and hypervariable genetic markers on genotyping circulating strains of Neisseria gonorrhoeae. PLoS One 6:e28259 doi:10.1371/journal.pone.0028259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization 1997. Surveillance of antibiotic susceptibility of Neisseria gonorrhoeae in the WHO western Pacific region 1992-4. WHO Western Pacific Region Gonococcal Antimicrobial Surveillance Programme. Genitourin. Med. 73:355–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization 2001. Prevalence and incidence in 2005 of selected sexually transmitted infections: methods and results. World Health Organization, Geneva, Switzerland [Google Scholar]
- 37. World Health Organization 2005. Surveillance of antibiotic resistance in Neisseria gonorrhoeae in the World Health Organization Western Pacific Region, 2003. Commun. Dis. Intell. 29:62–64 [PubMed] [Google Scholar]
- 38. Wylie LJ, Jolly A. 2001. Patterns of chlamydia and gonorrhea infection in sexual networks in Manitoba, Canada. Sex. Transm. Dis. 28:14–24 [DOI] [PubMed] [Google Scholar]
- 39. Wylie LJ, Cabral T, Jolly AM. 2005. Identification of networks of sexually transmitted infection: a molecular, geographic, and social network analysis. J. Infect. Dis. 191:899–906 [DOI] [PubMed] [Google Scholar]
- 40. Xia MS, Pang YJ, Roberts MC. 1995. Detection of two groups of 25.2 MDa Tet M plasmids by polymerase chain reaction of the downstream region. Mol. Cell. Probes 9:327–332 [DOI] [PubMed] [Google Scholar]
- 41. Yang Y, et al. 2006. Antimicrobial susceptibility and molecular determinants of quinolone resistance in Neisseria gonorrhoeae isolates from Shanghai. J. Antimicrob. Chemother. 58:868–872 [DOI] [PubMed] [Google Scholar]