Abstract
Monomicrobial necrotizing fasciitis (type II) is typically caused by group A streptococcus alone or in combination with Staphylococcus aureus. Escherichia coli has been isolated from polymicrobial or Fournier's gangrene but has rarely been reported in monomicrobial necrotizing fasciitis. We describe the clinical characteristics and outcomes of seven cases of monomicrobial E. coli necrotizing fasciitis and/or severe soft tissue infection diagnosed at a single institution during an 18-month period. Four isolates from three patients and two isolates from two patients with type I polymicrobial severe soft tissue infection (controls) were assayed by the randomly amplified polymorphic DNA (RAPD) analysis for fingerprinting and PCR amplification of primers in order to detect cytotoxic necrotizing factor 1 and 2 (cnf1 and cnf2) genes. All patients had some type of immune suppression. The limb was the most commonly involved organ. In all cases, E. coli was isolated as a monomicrobial pathogen from blood, fascia, or both. All patients died during hospitalization, three within the first 48 h. The RAPD amplification assay showed a high degree of genetic diversity among the “flesh-eating” strains and controls. The cnf1 toxin gene was identified in two out of three cases, but not in the controls. cnf2 was not detected in any of the patients. E. coli may be responsible for life-threatening necrotizing fasciitis. Further research is needed to reveal relevant risk factors, reservoirs, and modes of transmission of cnf1 E. coli.
INTRODUCTION
Escherichia coli is a common species of facultative anaerobes found in the human gastrointestinal tract and the most commonly encountered pathogen of the enterobacterial family (5). Although most strains of E. coli harmlessly reside in the lumen of the colon, a plethora of pathotypes exist in the colon, thus causing specific types of illness in both normal hosts and those with compromised defense mechanisms. Pathogenic strains differ from commensal organisms in that they produce virulence factors specific for each pathotype. Pathogenic types of E. coli can be crudely divided into diarrheagenic E. coli and extraintestinal pathogenic E. coli (ExPEC). ExPEC infections include uropathogenic E. coli, neonatal meningitis-associated E. coli, and sepsis related to E. coli. This pathogen can also cause a wide variety of other extraintestinal infections, such as nosocomial pneumonia, cholecystitis and cholangitis, peritonitis, cellulites, osteomyelitis, and infectious arthritis (10).
Monomicrobial necrotizing fasciitis (type II) is typically caused by group A streptococcus, occasionally in combination with Staphylococcus aureus (2). Among Gram negatives, Vibrio vulnificus and Aeromonas hydrophila are well-known causative agents of monomicrobial necrotizing fasciitis (12). E. coli has been isolated from polymicrobial or Fournier's gangrene, but has rarely been reported in monomicrobial necrotizing fasciitis (8).
Herein, we report a series of seven patients with necrotizing fasciitis or severe soft tissue infections caused solely by E. coli, observed in one medical center over a period of 18 months. We also describe the clinical characteristics, treatment, and outcomes of these seven cases, as well as the phenotypic and molecular characteristics of the responsible E. coli strains.
MATERIALS AND METHODS
Seven cases of monomicrobial E. coli severe soft tissue infection were diagnosed at the Rabin Medical Center, Beilinson Hospital, Petah-Tiqva, Israel, from May 2010 through October 2011.
Diagnosis of severe soft tissue infection, including necrotizing fasciitis and/or pyomyositis, was based on a typical clinical presentation (i.e., erythema, tenderness, fever, and pain in the involved skin and/or muscles) and confirmed by compatible radiologic and/or surgical findings. E. coli was considered to be the causative agent if it was the only isolated pathogen from blood and/or surgical tissue (muscle or fascia).
Clinical microbiology.
Blood cultures were processed using the Bactec 9240 blood culture system (Becton Dickinson). Standard methods were used to culture clinical specimens. Species identity was confirmed by the API 20E (bioMérieux) system. Antibiotic susceptibilities were determined by the disk diffusion method according to the standards of the Clinical and Laboratory Standards Institute (CLSI) (15). The MIC was determined by the Etest (AB Biodisk, Solna, Sweden). Extended-spectrum β-lactamase (ESBL) production was tested according to the CLSI's recommendations, using ceftazidime (30 μg) and a combination of ceftazidime-clavulanate (30/10 μg) discs (Oxoid), with a ≥5-mm difference indicating positivity.
Four isolates from three patients (two isolates from patient 5, one isolate from patients 4, and one isolate from patient 6) were available for genotypic analysis and PCR amplification to detect cytotoxic necrotizing factor genes. For this purpose, two strains isolated from two patients with type I polymicrobial necrotizing fasciitis were selected for comparison (controls).
Bacterial DNA purification.
Total DNA was extracted from E. coli colonies using the AccuPrep genomic DNA extraction kit (Bioneer, South Korea).
RAPD.
Randomly amplified polymorphic DNA (RAPD) PCR was performed using primers 1290, 1254, and 1252 as described previously (15). Each sample was repeated twice to assess reproducibility. The amplified DNA fragments were separated on 2% (wt/vol) agarose gels, stained with ethidium bromide, and photographed under UV light. DNA fingerprints were compared by visual inspection. Similar isolates with the same banding pattern were assigned to the same RAPD type.
PCR amplification and sequencing of cnf1 and cnf2 genes.
PCR amplification was performed on bacterial DNA using the AccuPower PCR PreMix kit (Bioneer, South Korea) and sets of primers for detecting cytotoxic necrotizing factors 1 and 2 (cnf1 and cnf2) genes (7, 14). Amplification products were purified and sequenced on the ABI PRISM 3730x1 genetic DNA analyzer (Applied Biosystems Inc., CA). Obtained sequences were aligned and compared with archived NCBI sequences for gene identification.
RESULTS
Case 1.
In May 2010, a 52-year-old female with a history of liver cirrhosis was admitted to the emergency room complaining of severe pain and a petechial rash on her right leg. She appeared ill, her temperature was 38°C, and her right leg was edematous with severe tenderness. Deep vein thrombosis was ruled out. She received opiates, penicillin, and clindamycin on the presumptive diagnosis of cellulitis. On day 2, the patient developed septic shock and a diagnosis of necrotizing fasciitis was suspected. However, despite intensive therapy, the patient died due to multiorgan failure. E. coli was isolated from all her blood cultures. Anaerobic blood cultures were negative.
Case 2.
In November 2010, a 61-year-old male with cryoglobulinemia was admitted with worsening leg pain and fever. The patient was treated with steroids, cyclophosphamide and plasmapheresis. On examination, his left leg was swollen, red, and tender. Laboratory tests revealed a new onset of neutropenia, acute renal failure, and elevated creatine phosphokinase. A presumptive diagnosis of necrotizing fasciitis was made, and a fasciotomy was performed. During surgery, necrotic fascia was not observed macroscopically. E. coli was isolated from all blood and fasciotomy cultures. Anaerobic cultures from blood and soft tissue were negative. The patient died in the intensive care unit (ICU) on the 21st hospital day from organ failure and uncontrolled Acinetobacter and Clostridium difficile infections.
Case 3.
In March 2011, an 88-year-old male with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and chronic renal failure (CRF) was admitted with fever and redness of his left arm. He had been hospitalized 2 weeks prior to the current admission, suffering from C. difficile-associated diarrhea. At presentation, his temperature was 38.2°C and blood pressure was 118/67 mm Hg. His left arm was red, warm, and swollen at the site of a previous peripheral venous catheter insertion. His white blood cell count was 22,000 cells/mm3. The patient was admitted and diagnosed with cellulitis. Treatment with cefazolin was started. On the second day, he complained of excruciating pain in his left arm, left chest, and back. On examination, his blood pressure was 60/30 mm Hg. The redness had spread to the left back and chest, and there was severe tenderness to light touch. The presumptive diagnosis was changed to necrotizing fasciitis. The patient became stuporous, and his family refused surgery. A few hours later, severe necrosis of the left arm developed and the patient died. E. coli was isolated from all blood cultures. Anaerobic blood cultures were negative.
Case 4.
In July 2011, a 65-year-old male was admitted with acute functional deterioration and pancytopenia. His history included B-cell lymphoma and allogeneic bone marrow transplantation 4 months before his current admission. One month prior to admission, he had developed cellulitis of the right leg and E. coli was isolated from the blister fluid. On the night of his current admission, he developed severe pain in the right leg, acute respiratory failure, and septic shock. There was severe tenderness to touch with no redness, crepitus, or necrosis of the leg. He was transferred to the ICU and died 48 h following admission. Blood culture showed the same E. coli previously isolated 1 month before. Anaerobic cultures from blood and soft tissue were negative.
Case 5.
In September 2011, a 75-year-old female with diffuse large B-cell lymphoma was admitted with fever and a painful lesion on her left arm. The patient had been diagnosed with lymphoma a few months before and had received 4 courses of CHOP (cyclophosphamide, hydroxydaunorubicin, oncovine, and prednisone) the last 2 months prior to this admission. The patient also had received high-dose steroids. On examination, the patient appeared sick, her blood pressure was 93/64 mm Hg, and her temperature was 37.5°C. There was a large painful red lesion (no signs of trauma or skin puncture were recorded) on her left forearm which had rapidly spread. She was noted to have leukopenia but not neutropenia. Creatinine kinase was normal. Computed tomography (CT) of the hand showed severe edema and infiltration of the soft tissues into the posterior forearm with suspected muscle involvement. Local necrosis ensued, and in view of a presumptive diagnosis of necrotizing fasciitis, the patient was taken to the operating room. During surgery, the soft tissue was found to be necrotic and the fascia was opened (Fig. 1). Cultures from the fascia and soft tissue yielded E. coli. Anaerobic blood and tissue cultures were sterile. The patient died 1 month later from further complications.
Fig 1.
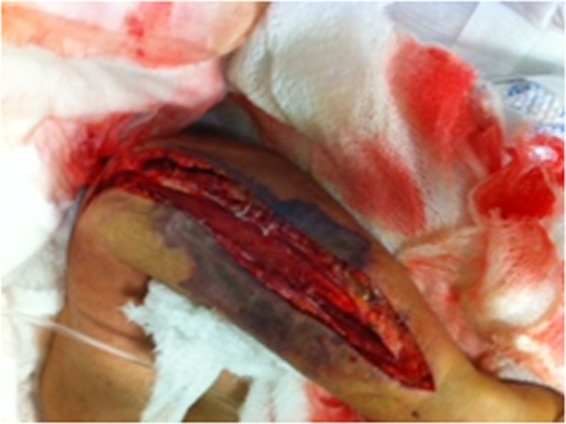
Left arm with necrotizing fasciitis after extensive debridement (patient 5).
Case 6.
In September 2011, a 65-year-old male with diffuse large B-cell lymphoma was admitted to the hospital due to neutropenic fever and pain in the neck area. CT of the neck showed severe edema and infiltration of the soft tissues and suspected muscle and fascia involvement. The next day, he developed septic shock with cellulitis and edema of the soft tissue in the neck. E. coli was isolated from all aerobic blood cultures. Anaerobic blood cultures were all negative. The patient died 1 week later.
Case 7.
In October 2011, a 91-year-old male with multiple myeloma was admitted with fever, redness, and blisters on his left hand. He was treated with cefazolin. E. coli was cultured from all blood cultures, and antibiotic therapy was changed accordingly. Anaerobic blood cultures were negative. The patient died 1 week later with no improvement in the physical findings of his left hand.
Case summary and microbiological analysis.
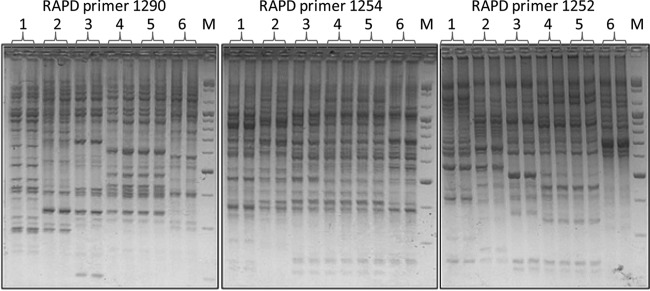
Table 1 summarizes patient characteristics and outcomes. All patients had some type of immune suppression. Their main comorbidities included cirrhosis, lymphoma, multiple myeloma, cryoglobulinemia, and hematopoietic stem cell transplantation. The involved organ was the limb, except for one patient, who presented with a soft tissue infection of the neck. E. coli was isolated in all cases as a monomicrobial pathogen taken from blood, fascia, or both. All patients died during hospitalization, three during the first 48 h. No epidemiological relationship was found between patients. The RAPD amplification assay showed a high degree of genetic diversity among the flesh-eating strains and between the control strains (Fig. 2). The cnf1 toxin gene was identified by PCR sequencing in two out of three of our patients (Table 2). In the two control patients, the cnf1 toxin gene was not present (Fig. 3, patients 2 and 6). cnf2 was not detected in any of the patients (not shown).
TABLE 1.
Patient characteristics and outcomes
| Patient | Age (yr) | Comorbidities | Neutropenia | Organ involved | ICU | Surgery | Death (days from hospitalization) | E. coli isolation |
|---|---|---|---|---|---|---|---|---|
| 1 | 52 | Cirrhosis | Yes (as part of sepsis) | Leg | No | No | 2 | Blood |
| 2 | 60 | Cryoglobulinemia | Yes (as part of sepsis) | Leg | Yes | Fasciotomy | 21 | Blood + fascia |
| 3 | 88 | CHF, CRF | No | Arm | No | No | 2 | Blood |
| 4 | 65 | Bone marrow transplantation | Yes (as part of sepsis) | Leg | Yes | No | 2 | Blood + bulla |
| 5 | 75 | Lymphoma | No | Hand | Yes | Yes | 30 | Fascia |
| 6 | 65 | Lymphoma | Yes | Neck | No | No | 7 | Blood |
| 7 | 91 | Multiple myeloma | No | Hand | No | No | 7 | Blood |
Fig 2.
Duplicates from 6 E. coli isolates. Duplicate 1 is from patient 4; duplicates 2 and 6 are from patients with a polymicrobial infection not included in this series (controls). Duplicate 3 is from patient 6; duplicates 4 and 5 are from patient 5. The entire figure shows the polyclonality of the bacteria. M, DNA size marker.
TABLE 2.
Antimicrobial susceptibility profiles and cnf1 presence of seven E. coli isolates from patients in our seriesa
| Patient | Specimen | ESBL | Amikacin | Ceftriaxone | Ciprofloxacin | Piperacillin-tazobactam | Co-trimoxazole | cnf1 |
|---|---|---|---|---|---|---|---|---|
| 1 | Blood | − | S | S | S | S | S | Not evaluated |
| 2 | Blood | − | S | S | R | S | R | Not evaluated |
| 2 | Fascia | − | S | S | R | S | R | Not evaluated |
| 3 | Blood | + | S | R | R | R | R | Not evaluated |
| 4 | Blood | − | S | S | R | S | R | Negative |
| 5 | Fascia | − | S | S | S | S | S | Positive |
| 6 | Blood | − | S | S | S | S | S | Positive |
| 7 | Blood | − | S | S | R | S | R | Not evaluated |
S, sensitive; R, resistant.
Fig 3.
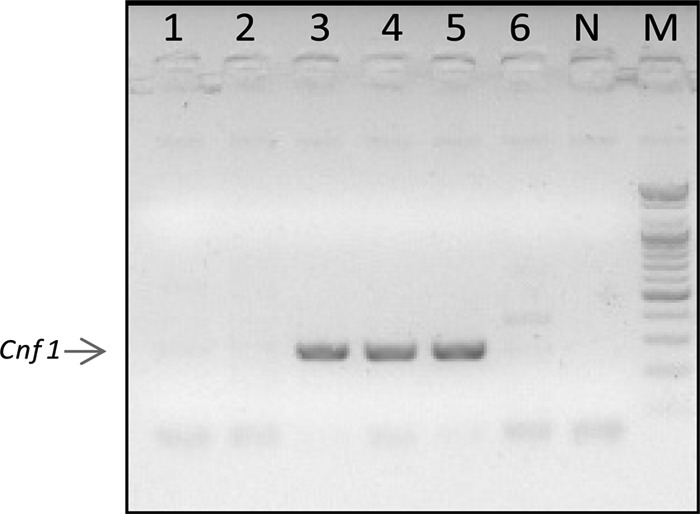
Lane 1, E. coli from patient 4 in our series; lanes 2 and 6, E. coli as part of the polymicrobial flora from 2 patients not included in our series; lane 3, E. coli from patient 6 in our series; lanes 4 and 5, E. coli from patient 5. N, negative control; M, DNA size marker.
DISCUSSION
To the best of our knowledge, this is the largest case series of monobacterial E. coli necrotizing fasciitis published in the literature. We were able to demonstrate the presence of the cnf1 toxin gene, which causes cell necrosis through activation of the Rho GTPases in three isolates from two patients (9). In vivo, cnf1 has been reported to induce dermal necrosis in rabbits. Strains harboring this virulence factor have been designated necrotoxic E. coli (3). This is the first time, in our institution, that E. coli monobacterial necrotizing fasciitis has been detected. In our institution, group A streptococcus is the most common agent causing monomicrobial necrotizing fasciitis. During the study period, we encountered 5 cases of necrotizing fasciitis due to group A streptococci (data not shown).
It should be emphasized that, in our center, anaerobic cultures are always taken from blood and tissue at the time of surgery, when soft tissue are debrided. We could not identify possible predisposing factors leading to the E. coli infection, i.e., receiving Gram-positive active antibiotics prior to the presentation.
The reported incidence of E. coli as a monomicrobial pathogen resulting in necrotizing fasciitis is very rare, ranging from 0 to 10% (1, 6, 11, 12). Chen et al. (4) reported on 126 patients with a single etiologic agent causing necrotizing fasciitis. The incidence of E. coli was 1.6% isolated from the fascia and 4.9% isolated from the blood.
E. coli as a cause of pyomyositis has been described in a retrospective series performed at the MD Anderson Cancer Center (16). Six cases of E. coli pyomyositis were identified between 2003 and 2007. All patients received chemotherapy for hematologic malignancy; five were severely neutropenic, and two (33%) died.
Grimaldi et al. (8) reported on a case of monomicrobial E. coli necrotizing fasciitis in an 83-year-old man with aplastic anemia. In this case, the E. coli strain was also found to carry the cnf1 gene. Another similar case of E. coli necrotizing fasciitis of the thigh, associated with E. coli bacteremia, was recently reported in Taiwan (13). However, the authors made no attempt to analyze the virulent factors of their isolates (13).
There are two distinct bacteriological entities of necrotizing fasciitis. In type I, the infection is polymicrobial, caused by at least one anaerobic species (most commonly Bacteroides or Peptostreptococcus) in combination with one or more facultative anaerobic species such as streptococci (other than group A) and/or Enterobacteriaceae (e.g., E. coli, Enterobacter, Klebsiella, and Proteus). In type II, group A streptococci are isolated alone or in combination with other species, most commonly S. aureus (5). The course and prognosis of the disease described herein are compatible with necrotizing fasciitis.
Etiology of Fournier's gangrene is polymicrobial, usually caused by facultative organisms (E. coli, Klebsiella, and enterococci) together with anaerobes (Bacteroides, Fusobacterium, Clostridium, and anaerobic or microaerophilic streptococci) (5).
The recommended empirical antibiotic regimen for group A streptococcal necrotizing fasciitis is penicillin or ampicillin plus clindamycin. In addition to prompt surgical debridement, some researchers have recommended intravenous immune globulin to treat the streptococcal toxic shock-like syndrome which accompanies necrotizing fasciitis (5).
During the past 2 years, we encountered a new phenomenon of necrotizing fasciitis and severe soft tissue infection with unusual/atypical/unpredictable bacteria. As a result of these cases, at present, the empirical coverage of type II-associated necrotizing fasciitis in our center includes prescribing an antibiotic with Gram-negative activity. We believe that this life-threatening syndrome is due to the interaction between pathogen virulence factors, probably the cnf1 toxin and host factors.
Physicians should be aware that E. coli may be responsible for necrotizing fasciitis. Following the current report and another recent report (8, 13), empirical coverage among immunocompromised patients should also comprise Gram-negative bacteria. Further research is needed to reveal relevant risk factors, reservoirs, and modes of transmission of cnf1 E. coli strains in order to develop effective measures to prevent further dissemination and emergence of this life-threatening, “new” pathogen.
ACKNOWLEDGMENTS
We declare no conflicts of interest and no funding support.
We thank Phyllis Curchack Kornspan for her editorial services.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Angoules AG, et al. 2007. Necrotising fasciitis of upper and lower limb: a systematic review. Injury 38(Suppl 5):S19–S26 [DOI] [PubMed] [Google Scholar]
- 2. Bisno AL, Stevens DL. 1996. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 334:240–245 [DOI] [PubMed] [Google Scholar]
- 3. Caprioli A, Falbo V, Roda LG, Ruggeri FM, Zona C. 1983. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 39:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen IC, et al. 2011. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit. Care 15:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donnenberg MS. 2010. Enterobacteriaceae, p 2815–2829 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas and Bennett's principles and practice of infectious diseases, 7th ed Churchill Livingstone, Elsevier, Philadelphia, PA [Google Scholar]
- 6. Espandar R, Sibdari SY, Rafiee E, Yazdanian S. 2011. Necrotizing fasciitis of the extremities: a prospective study. Strategies Trauma Limb Reconstr. 6:121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falbo V, Famiglietti M, Caprioli A. 1992. Gene block encoding production of cytotoxic necrotising factor 1 and hemolysin in Escherichia coli. J. Infect. Immun. 60:2182–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grimaldi D, et al. 2010. Unusual “flesh-eating” strain of Escherichia coli. J. Clin. Microbiol. 48:3794–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horiguchi Y. 2001. Escherichia coli cytotoxic necrotizing factors and Bordetella dermonecrotic toxin: the dermonecrosis-inducing toxins activating Rho small GTPases. Toxicon 39:1619–1627 [DOI] [PubMed] [Google Scholar]
- 10. Le Dantec L, et al. 1996. Peripheral pyogenic arthritis. A study of one hundred seventy-nine cases. Rev. Rhum. Engl. Ed. 63:103–110 [PubMed] [Google Scholar]
- 11. Lee CC, et al. 2008. Necrotizing fasciitis in patients with liver cirrhosis: predominance of monomicrobial Gram-negative bacillary infections. Diagn. Microbiol. Infect. Dis. 62:219–225 [DOI] [PubMed] [Google Scholar]
- 12. Lee CY, et al. 2011. Prognostic factors and monomicrobial necrotizing fasciitis: gram-positive versus gram-negative pathogens. BMC Infect. Dis. 11:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu CT, Chen YC, Chen TH, Ou TY. 2012. Necrotizing fasciitis of thigh associated with Escherichia coli bacteremia in a patient on chronic hemodialysis. Hemodial. Int. [Epub ahead of print.] doi:10.1111/j.1542 4758.2011.00658.x [DOI] [PubMed] [Google Scholar]
- 14. Pass MA, Odera R, Batt RM. 2000. Multiplex PCRs for identification of Escherichia coli virulence genes. J. Clin. Microbiol. 38:2001–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samra Z, Ofira O, Lishtzinsky Y, Madar-Shapiro L, Bishara J. 2007. Outbreak of carbapenem-resistant Klebsiella pneumoniae producing KPC-3 in a tertiary medical centre in Israel. Int. J. Antimicrob. Agents. 30:525–529 [DOI] [PubMed] [Google Scholar]
- 16. Vigil KJ, et al. 2010. Escherichia coli pyomyositis: an emerging infectious disease among patients with hematologic malignancies. Clin. Infect. Dis. 50:374–380 [DOI] [PubMed] [Google Scholar]