Abstract
A set of 104 isolates from human clinical samples from the United States, morphologically compatible with Bipolaris, were morphologically and molecularly identified through the sequence analysis of the internal transcribed space (ITS) region of the nuclear ribosomal DNA (rDNA). The predominant species was Bipolaris spicifera (67.3%), followed by B. hawaiiensis (18.2%), B. cynodontis (8.6%), B. micropus (2.9%), B. australiensis (2%), and B. setariae (1%). Bipolaris cynodontis, B. micropus, and B. setariae represent new records from clinical samples. The most common anatomical sites where isolates were recovered were the nasal region (30.7%), skin (19.2%), lungs (14.4%), and eyes (12.5%). The antifungal susceptibilities of 5 species of Bipolaris to 9 drugs are provided. With the exception of fluconazole and flucytosine, the antifungals tested showed good activity.
INTRODUCTION
Bipolaris is a large genus of dematiaceous hyphomycetes with more than 100 species, most of them being saprobes in soil and pathogens of plants, while some of the saprobic species are potentially able to infect humans and animals (27). They are anamorphs of the ascomycetous genera Cochliobolus and Pseudocochliobolus (family Pleosporaceae, order Pleosporales) (20).
The typical morphological features of Bipolaris species include rapidly growing dark colonies, geniculate conidiophores with sympodial conidiogenesis, and large conidia with transverse distosepta, usually without a protuberant hilum (a basal scar indicating the point of attachment in the conidiogenous cell) and with bipolar germination. Morphologically similar anamorphic genera are Drechslera, Curvularia, and Exserohilum (27).
Clinically relevant Bipolaris species are B. australiensis, B. hawaiiensis, B. spicifera, and, to a lesser extent, B. papendorfii (8). These fungi are able to infect both immunocompetent and immunosuppressed patients, mainly in tropical and subtropical areas. The most common clinical presentations are allergic sinusitis, keratitis, endophthalmitis, onychomycosis, peritoneal dialysis-associated peritonitis, lung and skin infections, and, less frequently, central nervous system (CNS) infections (2, 4, 5, 10, 16, 26, 28, 31).
The prevalence of the different species of Bipolaris in human infections is poorly known since only a few studies involving this genus have been published and the isolates were usually identified only by morphological criteria. Considering the similarity among the species of Bipolaris and the fact that the separation of species is based on subtle characters, some published identifications are doubtful or remain unresolved (10, 26). In the present study, we have identified a large number of isolates of clinical origin by molecular methods and by comparison of their sequences with those of type or reference strains in order to assess the spectrum of Bipolaris species in clinical samples in the United States.
MATERIALS AND METHODS
Fungal isolates.
A total of 104 isolates from human clinical samples, presumably belonging to the genus Bipolaris, were morphologically examined and sequenced in the present study (see the supplemental material). They were received at the Fungus Testing Laboratory in the Department of Pathology at the University of Texas Health Science Center at San Antonio, mostly over a period of 5 years (2006 to 2010), for identification or antifungal susceptibility determination. In addition, 14 ex-type or reference strains were also included in the study.
Morphological study.
All isolates were cultured on potato carrot agar (PCA; 20 g of potatoes, 20 g of carrots, 20 g of agar, 1 liter of distilled water) and oatmeal agar (OA; 30 g of filtered oat flakes, 20 g of agar, 1 liter of distilled water) media and incubated at 25°C for 10 to 21 days. The identification criteria were after Ellis (11, 12) and Sivanesan (27). Using light microscopy, microscopic features were examined by making direct wet mounts in 85% lactic acid from the different culture media.
Molecular study.
Isolates were grown on yeast extract sucrose (YES; yeast extract, 2%; sucrose, 15%; agar, 2%; water, 1 liter) for 3 days at 25°C, and DNA was extracted using a PrepMan Ultra sample preparation reagent (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Briefly, the fungal mycelium from a colony was gently mixed with 100 μl of the PrepMan Ultra sample reagent (Applied Biosystems, Foster City, CA). The mixture was then incubated at 100°C for 10 min, pelleted by centrifugation for 8 min at 13,000 rpm, and stored at 4°C. The DNA was quantified using GeneQuant pro (Amersham Pharmacia Biotech, Cambridge, England). The internal transcribed spacer (ITS) region of the nuclear ribosomal DNA (rDNA) was amplified and sequenced following the protocol described by Álvarez et al. (1). The specific primers used for the amplification of the ITS region were ITS 5, 5′ GGAAGTAAAAGTCGTAACAAGG 3′, and ITS 4, 5′ TCCTCCGCTTATTGATATGC 3′, and the expected size of the PCR products was 575 bp.
The program SeqMan (Lasergene, Madison, Wisconsin) was used to obtain consensus sequences from the complementary sequences of each isolate.
Phylogenetic analysis.
Nucleotide sequence alignments were performed with Clustal X version 1.81 (30), followed by manual adjustments with a text editor. Distance trees were constructed with the maximum likelihood (ML) method using the Kimura 2-parameter substitution model with a pairwise deletion of gaps, as implemented in the MEGA 5.0 computer program (29). The robustness of branches was assessed by a bootstrap analysis of 1,000 replicates. The sequences of Corynespora cassiicola (IMI 056007, U95173.1) and Alternaria alternata (CBS 916.96, FJ196306) were used as outgroups in the ITS analysis.
Antifungal susceptibility.
A total of 77 isolates with sufficient conidial production to standardize an inoculum were tested. Antifungal susceptibility testing was accomplished via methods outlined in CLSI document M38-A2 (7). This involves standardizing the inoculum by spectrophotometer to 0.4 × 104 to 5 × 104 CFU/ml (0.25 to 0.3 optical density at 530 nm [OD530]), use of RPMI 1640 with l-glutamine but without bicarbonate, and incubation at 35°C. The minimum effective concentration (MEC) was determined at 24 h for the candins, and the MIC was determined at 48 h for the remaining drugs. The MIC was defined as the lowest concentration exhibiting 100% visual inhibition of growth for amphotericin B, itraconazole, posaconazole, and voriconazole while at a 50% reduction in growth for fluconazole and flucytosine. In the event that sufficient growth was not observed at the prescribed reading times, the tests continued to be incubated until sufficient growth was observed, enabling accurate endpoint determination.
RESULTS
Of the 104 clinical isolates studied, 100 were morphologically identified as members of the genus Bipolaris. Four isolates did not sporulate (UTHSC 07-3454, UTHSC 08-1495, UTHSC 10-2300, and UTHSC 10-2807), but the analysis of their ITS sequences demonstrated that they were B. spicifera.
With the primers used, we were able to amplify and sequence 571 to 575 bp of the ITS region. The predominant species was Bipolaris spicifera (70 isolates, 67.3%), followed by B. hawaiiensis (19 isolates, 18.2%), B. cynodontis (9 isolates, 8.6%), B. micropus (3 isolates, 2.9%), B. australiensis (2 isolates, 2%), and B. setariae (1 isolate, 1%). The correlation between the morphological and molecular identification was 89.7%.
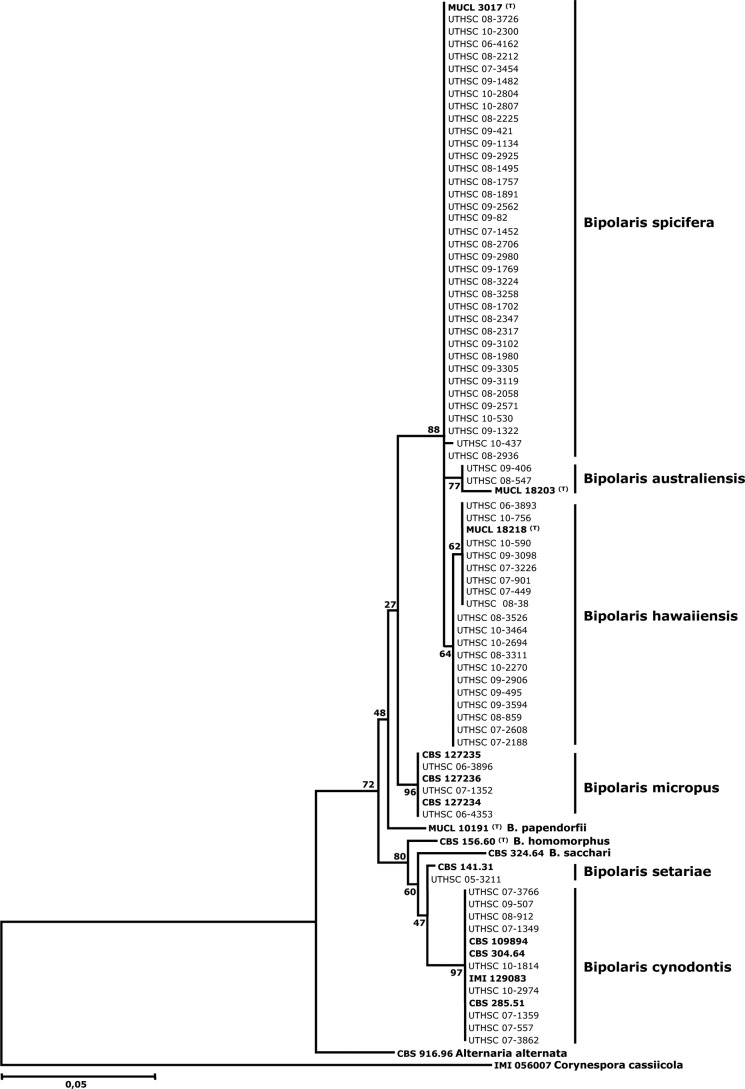
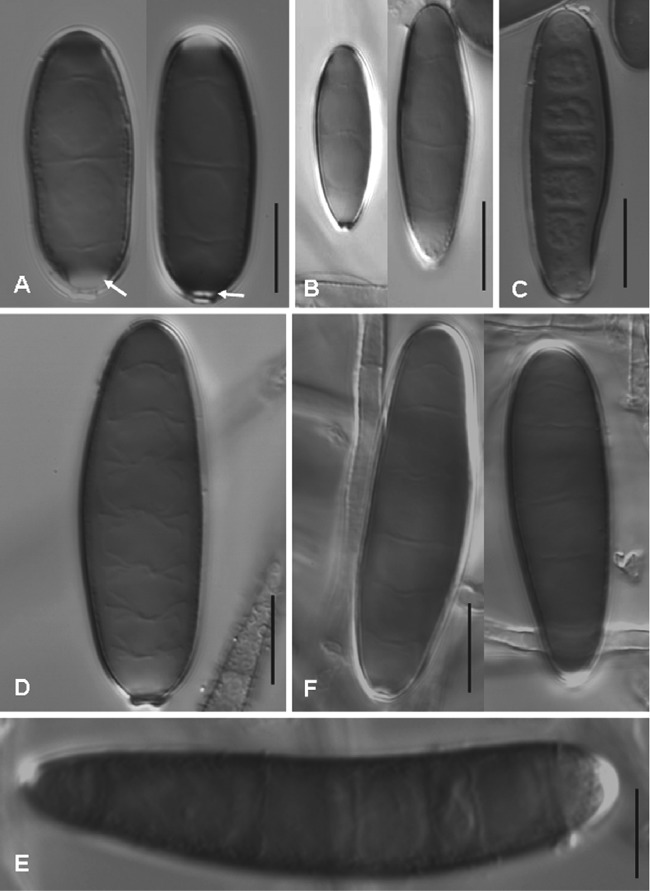
In addition to the 14 reference and ex-type strains, 70 of 104 clinical isolates were included in the phylogenetic analysis. Of the 70 isolates identified as B. spicifera, 36 were randomly chosen for the analysis since the sequences of the isolates of the mentioned species were very similar (99.4 to 100% similarity). Figure 1 shows the neighbor-joining (NJ) tree inferred from the analysis of the ITS region of a representative number of isolates treated in this study, including the type and reference strains. The isolates from clinical origin that were included in the analysis clustered in six well-supported clades, each of them representing a different phylogenetic species. The largest clade, comprising the ex-type strain of B. spicifera and 36 clinical isolates, received 88% of bootstrap support (BS). The sequences of the isolates of this clade deviated maximally by 0.6% from that of the ex-type strain of B. spicifera (MUCL 3017). Isolates were characterized by brown, gray, or black colonies and 3-distoseptate cylindrical conidia with rounded ends, measuring 13 to 41 by 7 to 14 μm, without a protuberant hilum that were medium brown, except for a narrow hyaline to subhyaline area just above the conidial scar (Fig. 2A).
Fig 1.
Maximum likelihood tree inferred from ITS sequences of Bipolaris listed in Table S1 in the supplemental material. Branch lengths are proportional to the distance. Type or reference strains are shown in bold.
Fig 2.
Conidia of Bipolaris species on OA at 25°C after 10 days. (A) B. spicifera, UTHSC 08-1706; (B) B. australiensis, UTHSC 09-406; (C) B. hawaiiensis, UTHSC 09-2906; (D) B. micropus, UTHSC 06-3896; (E) B. setariae, UTHSC05-3211; and (F) B. cynodontis, UTHSC 09-507. Scale bars, 10 μm.
The ex-type strain of B. australiensis and two clinical isolates, morphologically identified as this species, constituted another clade (BS, 77%). The similarity of the sequences of such isolates with the ex-type strain was 98.1%. They showed gray to blackish brown, velvety colonies and pale brown to medium reddish brown, 3-distoseptate, ellipsoidal or oblong, straight conidia with rounded ends, measuring 15 to 32 by 7 to 11 μm, with a nonprotuberant hilum and smooth to finely roughened walls (Fig. 2B).
The clade corresponding to B. hawaiiensis (BS, 64%) encompassed 19 clinical isolates in addition to the ex-type strain with which it showed 99.1 to 100% similarity. The species was characterized by pale to medium brown, 2- to 6-distoseptate, oblong, cylindrical, or ellipsoidal conidia, measuring 13 to 37 by 4 to 11 μm, with a nonprotuberant hilum (Fig. 2C).
Three reference strains of B. micropus were grouped with three clinical isolates that had been morphologically identified as this species (BS, 96%). These isolates produced conidia that were pale brown, 3- to 7-distoseptate, cylindrical, and straight, measuring 19 to 47 by 10 to 15 μm, with smooth walls and a short protuberant hilum (Fig. 2D).
Isolate UTHSC 05-3211 showed 99% similarity with a reference strain of B. setariae (CBS 141.31). The clinical isolate showed pale brown, 3- to 9-distoseptate conidia that were cylindrical and straight to slightly curved, measuring 37 to 92 by 11 to 16 μm, with a nonprotuberant hilum (Fig. 2E). These conidial features were similar to those described for B. sacchari and B. setariae (30). However, no type strains of those species are known to exist and, in addition, our isolate only showed a similarity of 95.5% with a reference strain of B. sacchari (CBS 324.64).
Nine clinical isolates were grouped with four reference strains of B. cynodontis (BS, 97%). The sequence similarity among the isolates of this clade was 99.4 to 100%. However, important morphological differences were observed among them. Most showed conidial features typical of B. cynodontis; i.e., conidia with 3 to 8 distosepta, narrowly ellipsoidal to oval, irregularly curved, without a protuberant hilum, pale brown, and smooth-walled, measuring 19 to 61 by 8 to 15 μm (Fig. 2E). In contrast, two isolates (UTHSC 07-557 and UTHSC 07-3862) showed considerably shorter (up to 41-μm-long) conidia with fewer septa (from 3 to 6).
The majority of the isolates included in this study were from the nasal region (30.7%), followed by various cutaneous presentations (19.2%) and the lungs (14.4%), eyes (12.5%), nails (7.6%), and blood (4.8%). The remaining 10.8% of the isolates were from other sites like the ear, the brain, bile, and the chest, among others.
With the exception of fluconazole and flucytosine, the antifungal drugs tested showed good activity against all the isolates tested (Table 1). The MICs of the three drugs most commonly tested in these types of infections, i.e., amphotericin B, voriconazole, and itraconazole, were <0.03 to 2, 0.05 to 2, and <0.03 to 4 μg/ml, respectively.
TABLE 1.
Results of in vitro antifungal susceptibility testing for Bipolaris species
| Species (no. of isolates) | Antifungal agent | MIC (μg/ml) |
||
|---|---|---|---|---|
| Range | GMa | 90% | ||
| B. australiensis (2) | Anidulafungin | <0.015–0.06 | 0.06 | |
| Amphotericin B | 0.06–0.125 | 0.08 | ||
| Caspofungin | 1 | 1 | ||
| Itraconazole | 0.25–0.5 | 0.35 | ||
| Fluconazole | 8–16 | 11.3 | ||
| Flucytosine | >64 | >64 | ||
| Micafungin | <0.015–0.06 | 0.06 | ||
| Posaconazole | 0.06 | 0.06 | ||
| Voriconazole | 0.05–1 | 0.70 | ||
| B. cynodontis (6) | Anidulafungin | 0.03–0.06 | 0.04 | |
| Amphotericin B | <0.03–1 | 0.25 | ||
| Caspofungin | <0.015–1 | 0.21 | ||
| Itraconazole | <0.03–1 | 0.24 | ||
| Fluconazole | 2–16 | 4 | ||
| Flucytosine | 32–>64 | >64 | ||
| Micafungin | <0.015–0.03 | <0.015 | ||
| Posaconazole | <0.03–0.125 | 0.08 | ||
| Voriconazole | 0.25–1 | 0.39 | ||
| B. hawaiiensis (14) | Anidulafungin | <0.015–>8 | 0.07 | 0.125 |
| Amphotericin B | 0.125–0.25 | 0.18 | 0.25 | |
| Caspofungin | 0.5–1 | 0.90 | 1 | |
| Itraconazole | <0.03–0.5 | 0.32 | 0.5 | |
| Fluconazole | 2–32 | 8 | 16 | |
| Flucytosine | >64 | >64 | >64 | |
| Micafungin | <0.015–0.06 | 0.04 | 0.06 | |
| Posaconazole | <0.03–0.5 | 0.14 | 0.25 | |
| Voriconazole | 0.25–2 | 0.67 | 1 | |
| B. micropus (3) | Anidulafungin | <0.015–0.125 | 0.015 | |
| Amphotericin B | 0.06–0.25 | 0.09 | ||
| Caspofungin | 0.25–0.5 | 0.39 | ||
| Itraconazole | <0.03–0.25 | 0.25 | ||
| Fluconazole | 0.125–1 | 0.5 | ||
| Flucytosine | >64 | >64 | ||
| Micafungin | <0.015–0.06 | <0.015 | ||
| Posaconazole | <0.03 | <0.03 | ||
| Voriconazole | 0.125–0.5 | 0.25 | ||
| B. spicifera (52) | Anidulafungin | <0.015–>8 | 0.06 | 0.25 |
| Amphotericin B | <0.03–2 | 0.21 | 1 | |
| Caspofungin | 0.25–2 | 0.89 | 1 | |
| Itraconazole | <0.03–4 | 0.63 | 1 | |
| Fluconazole | 4->64 | 38.7 | >64 | |
| Flucytosine | >64 | >64 | >64 | |
| Micafungin | <0.015–0.125 | 0.05 | 0.125 | |
| Posaconazole | <0.03–1 | 0.26 | 0.5 | |
| Voriconazole | 0.25–4 | 1.56 | 2 | |
GM, geometric mean.
DISCUSSION
This is the first study where a large panel of clinical isolates of Bipolaris has been identified down to the species level using both phenotypic and molecular methods. Although it seems that Bipolaris is an uncommon fungus involved in human infections, the fact that more than 250 clinical isolates belonging to that genus were received at the Fungus Testing Laboratory over a period of 5 years demonstrates that its low incidence is only relative. Close to 100 of such isolates were included in the present study. However, it should be mentioned that the role of these isolates as a cause of infection was not demonstrated.
A recent retrospective study, performed in Saudi Arabia (10), recorded a total of 23 clinical isolates of Bipolaris from different anatomical sites, collected over a period of 2 years. Although they were identified only morphologically, the most frequent species were B. spicifera, B. australiensis, and B. hawaiiensis, a finding which, in general, correlates with the prevalent etiologic agents in human infections reported in the literature (5, 15, 17, 19, 21, 23). In our study, B. spicifera and B. hawaiiensis were also the most commonly identified species, but, surprisingly, B. australiensis was represented only by two isolates. The fact that B. spicifera and B. australiensis are morphologically similar, especially when they grow in vitro, may have been a cause of misidentification in many cases. Bipolaris spicifera differs from B. australiensis by its 3-distoseptate conidia, which show a pale area at the base, just above the scar, while those of B. australiensis are usually 3- to 4(−5)-distoseptate, and the pale basal area is absent (27). The ITS sequences of the type strains of both species showed a similarity of 97.8%. Bipolaris hawaiiensis is morphologically distinguished from B. spicifera and B. australiensis by its 5(−7)-distoseptate narrow conidia (up to 7 μm wide), while those of B. australiensis and B. spicifera are 6 to 11 μm and 9 to 14 μm wide, respectively (27). The ITS sequence of the type strain of B. hawaiiensis showed similarities of 93.7 and 94.8% with those of the type strains of B. australiensis and B. spicifera, respectively. A BLAST search comparing ITS sequences is the procedure usually used in recent years to confirm Bipolaris species identification (3, 6, 9, 16, 18). However, this procedure may not be useful when comparisons are made with inaccurate sequences or when sequences of authentic strains of the species to which the problem isolate belongs have not been deposited.
Interestingly, we identified three species, B. cynodontis, B. micropus, and B. setariae, which, up to now, never had been found in clinical samples. In contrast, Bipolaris papendorfii, reported in three clinical cases (8), was not found in this study.
In our study, the predominant anatomic sites where strains were isolated were the nasal region, the eye, and the skin, a finding which agrees with the clinical cases of Bipolaris infections reported in the literature (5, 10, 15, 17, 19, 21, 23, 26).
The remarkably high in vitro activity of most of the antifungal drugs tested against Bipolaris species agrees with previous studies (13, 14, 24, 25). Owing to the infrequent occurrence of Bipolaris infections, clinical experience on their treatment is very scarce. In the reported clinical case studies, different antifungal drugs, such as amphotericin B, itraconazole, and voriconazole, have been used, with variable results (6, 15, 17, 22, 26, 31).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the curators of the Centraalbureau voor Schimmelcultures (Utrecht, the Netherlands), Mycothèque de L'Université Catholique de Louvain (Louvain-la-Neuve, Belgium, Belgium), and International Mycological Institute (Surrey, England) for providing fungal strains.
This study was supported by the Spanish Ministerio de Ciencia e Innovación, grants CGL 2009-08698/BOS and CGL 2011-27185/BOS, and by Coordenaçåo de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil), grant BEX 065311208.
Footnotes
Published ahead of print 10 October 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Álvarez E, et al. 2010. Molecular phylogenetic diversity of the emerging mucoralean fungus Apophysomyces: proposal of three new species. Rev. Iberoam. Micol. 27:80–89 [DOI] [PubMed] [Google Scholar]
- 2. Aribandi M, Bazan C. 2007. CT and MRI features in Bipolaris fungal sinusitis. Australas. Radiol. 51:127–132 [DOI] [PubMed] [Google Scholar]
- 3. Bagyalakshmi R, Therese KL, Prasanna S, Madhavan HN. 2008. Newer emerging pathogens of ocular nonsporulating molds (NSM) identified by polymerase chain reaction (PCR)-based DNA sequencing technique targeting internal transcribed spacer (ITS) region. Curr. Eye Res. 33:139–147 [DOI] [PubMed] [Google Scholar]
- 4. Buzina W, Braun H, Schimpl K, Stammberger H. 2003. Bipolaris spicifera causes fungus balls of the sinuses and triggers polypoid chronic rhinosinusitis in an immunocompetent patient. J. Clin. Microbiol. 4:4885–4887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castelnuovo P, et al. 2004. Invasive fungal sinusitis due to Bipolaris hawaiiensis. Mycoses 47:76–81 [DOI] [PubMed] [Google Scholar]
- 6. Chowdhary A, et al. 2011. Bipolaris hawaiiensis as etiologic agent of allergic bronchopulmonary mycosis: first case in a pediatric patient. Med. Mycol. 49:760–765 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard—second edition. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. de Hoog GS, Guarro J, Gené J, Figueras MJ. 2000. Atlas of clinical fungi, 2nd ed Centraalbureau voor Schimmelcultures, Baarn, the Netherlands [Google Scholar]
- 9. Dyer ZA, Wright RS, Rong IH, Jacobs A. 2008. Back pain associated with endobronchial mucus impaction due to Bipolaris australiensis colonization representing atypical allergic bronchopulmonary mycosis. Med. Mycol. 46:589–594 [DOI] [PubMed] [Google Scholar]
- 10. El Khizzi N, Bakheshwain S, Parvez S. 2010. Bipolaris: a plant pathogen causing human infections: an emerging problem in Saudi Arabia. Res. J. Microbiol. 5:212–217 [Google Scholar]
- 11. Ellis MB. 1971. Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, United Kingdom [Google Scholar]
- 12. Ellis MB. 1976. More dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew, United Kingdom [Google Scholar]
- 13. Espinel-Ingroff A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Espinel-Ingroff A. 2001. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J. Clin. Microbiol. 39:954–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flanagan KL, Bryceson AD. 1997. Disseminated infection due to Bipolaris australiensis in a young immunocompetent man: case report and review. Clin. Infect.Dis. 25:311–313 [DOI] [PubMed] [Google Scholar]
- 16. Fryen A, et al. 1999. Allergic fungal sinusitis caused by Bipolaris (Drechslera) hawaiiensis. Eur. Arch. Otorhinolaryngol. 256:330–334 [DOI] [PubMed] [Google Scholar]
- 17. Kobayashi H, et al. 2008. Disseminated infection by Bipolaris spicifera in an immunocompetent subject. Med. Mycol. 346:361–365 [DOI] [PubMed] [Google Scholar]
- 18. Kumar M, Mishra NK, Shukla PK. 2005. Sensitive and rapid polymerase chain reaction based diagnosis of mycotic keratitis through single stranded conformation polymorphism. Am. J. Ophthalmol. 140:851–857 [DOI] [PubMed] [Google Scholar]
- 19. Latham RH. 2000. Bipolaris spicifera meningitis complicating a neurosurgical procedure. Scand. J. Infect. Dis. 32:102–103 [DOI] [PubMed] [Google Scholar]
- 20. Manamgoda DS, Cai L, Bahkali AH, Chukeatirote E, Hyde KD. 2011. Cochliobolus: an overview and current status of species. Fungal Divers. 51:3–42 [Google Scholar]
- 21. McGinnis MR, Rinaldi MG, Winn RE. 1986. Emerging agents of phaeohyphomycosis: pathogenic species of Bipolaris and Exserohilum. J. Clin. Microbiol. 24:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moore ML, Collins GR, Hawk BJ, Russell TS. 2001. Disseminated Bipolaris spicifera in a neonate. J. Perinatol. 21:399–401 [DOI] [PubMed] [Google Scholar]
- 23. Pauzner R, et al. 1997. Phaeohyphomycosis following cardiac surgery: case report and review of serious infection due to Bipolaris and Exserohilum species. Clin. Infect. Dis. 25:921–923 [DOI] [PubMed] [Google Scholar]
- 24. Pfaller MA, Messer SA, Hollis RJ, Jones RN, SENTRY Participants Group 2002. Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob. Agents Chemother. 46:1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Revankar SG. 2005. Therapy of infections caused by dematiaceous fungi. Expert Rev. Anti Infect. Ther. 3:601–612 [DOI] [PubMed] [Google Scholar]
- 26. Revankar SG, Sutton DA. 2010. Melanized fungi in human disease. Clin. Microbiol. Rev. 23:884–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sivanesan A. 1987. Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum, and their teleomorphs. Mycol. Pap. 158:1–261 [Google Scholar]
- 28. Taguchi K, et al. 2007. Allergic fungal sinusitis caused by Bipolaris spicifera and Schizophyllum commune. Med. Mycol. 45:559–564 [DOI] [PubMed] [Google Scholar]
- 29. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods molecular biology and evolution. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viola GM, Sutton R. 2010. Allergic fungal sinusitis complicated by fungal brain mass. Int. J. Infect. Dis. 14:299–301 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.