Abstract
Hepatitis C virus genotype 4 (HCV-4) is the cause of approximately 20% of the 180 million cases of chronic hepatitis C in the world. HCV-4 infection is common in the Middle East and Africa, with an extraordinarily high prevalence in Egypt. Viral genetic polymorphisms, especially within core and NS5A regions, have been implicated in influencing the response to pegylated-interferon and ribavirin (PEG-IFN/RBV) combination therapy in HCV-1 infection. However, this has not been confirmed in HCV-4 infection. Here, we investigated the impact of heterogeneity of NS5A and core proteins of HCV-4, mostly subtype HCV-4a, on the clinical outcomes of 43 Egyptian patients treated with PEG-IFN/RBV. Sliding window analysis over the carboxy terminus of NS5A protein identified the IFN/RBV resistance-determining region (IRRDR) as the most prominent region associated with sustained virological response (SVR). Indeed, 21 (84%) of 25 patients with SVR, but only 5 (28%) of 18 patients with non-SVR, were infected with HCV having IRRDR with 4 or more mutations (IRRDR ≥ 4) (P = 0.0004). Multivariate analysis identified IRRDR ≥ 4 as an independent SVR predictor. The positive predictive value of IRRDR ≥ 4 for SVR was 81% (21/26; P = 0.002), while its negative predictive value for non-SVR was 76% (13/17; P = 0.02). On the other hand, there was no significant correlation between core protein polymorphisms, either at residue 70 or at residue 91, and treatment outcome. In conclusion, the present results demonstrate for the first time that IRRDR ≥ 4, a viral genetic heterogeneity, would be a useful predictive marker for SVR in HCV-4 infection when treated with PEG-IFN/RBV.
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of chronic liver disease, hepatocellular carcinoma, and deaths from liver disease and is the most common indication for liver transplantation (7, 26–28, 38). HCV has been classified into seven major genotypes and a series of subtypes (35, 36). In general, HCV genotype 4 (HCV-4) is common in the Middle East and Africa, where it is responsible for more than 80% of HCV infections (23). Although HCV-4 is the cause of approximately 20% of the 180 million cases of chronic hepatitis C in the world, it has not been a major subject of research.
Egypt has the highest prevalence of HCV worldwide (15%) and the highest prevalence of HCV-4, which is responsible for 90% of the total HCV infections, with a predominance of the subtype 4a (HCV-4a) (1, 32). This extraordinarily high prevalence results in an increasing incidence of hepatocellular carcinoma in Egypt, which is now the second most frequent cause of cancer and cancer mortality among men (17, 21). More than 2 decades have passed since the discovery of HCV, and yet therapeutic options remain limited. Up to 2011, the standard treatment for chronic hepatitis C consisted of pegylated alpha interferon (PEG-IFN) and ribavirin (RBV) (19); however, by May 2011 two protease inhibitors (telaprevir and boceprevir) were approved by the Food and Drug Administration (FDA) for use in combination with PEG-IFN/RBV for adult chronic hepatitis C patients with HCV genotype 1 (24, 34). Since the approval of these new protease inhibitors for treatment of HCV-1 infection, the response of HCV-4 to the standard regimen of treatment (PEG-IFN/RBV) has lagged behind other genotypes and HCV-4 has become the most resistant genotype to treat. As PEG-IFN/RBV still remains to be used to treat HCV-4-infected patients, exploring the factors that predict the outcome of PEG-IFN/RBV treatment, such as sustained virological response (SVR), for HCV-4 infections is needed to assess more accurately the likelihood of SVR and thus to make more informed treatment decisions.
While the SVR rate for PEG-IFN/RBV treatment hovers at 50 to 60% in HCV-1 and -4 infection, it is up to 80% in HCV-2 and -3 infections (19, 33). This difference in responses among patients infected with different HCV genotypes suggests that viral genetic heterogeneity could affect, at least to some extent, the sensitivity to IFN-based therapy. In this context, the correlation between IFN-based therapy outcome and sequence polymorphisms within the viral core and NS5A proteins has been widely discussed, in particular in regard to Japanese patients with HCV-1b infection. Initially, in the era of IFN monotherapy, it was proposed that sequence variations within a region in NS5A of HCV-1b, called the IFN sensitivity-determining region (ISDR), were correlated with IFN responsiveness (18). Subsequently, in the era of PEG-IFN/RBV combination therapy, we identified a new region near the C terminus of NS5A, referred to as the IFN/RBV resistance-determining region (IRRDR) (13). Recently, we also demonstrated the correlation between IRRDR polymorphism and PEG-IFN/RBV treatment outcome in HCV-2a and -2b infections (15). In addition, HCV core protein polymorphism, in particular at positions 70 and 91, was also proposed as a pretreatment predictor of poor virological response in patients infected with HCV-1b (4–6). To the best of our knowledge, there is no information regarding the correlation between sequence heterogeneity in the NS5A and core proteins of HCV-4 and PEG-IFN/RBV treatment outcome. In the present study, we aimed to investigate this issue in Egyptian patients infected with HCV-4.
MATERIALS AND METHODS
Ethics statement.
The study protocol, which conforms to the provisions of the Declaration of Helsinki, was approved beforehand by the Ethic Committees in Cairo University Hospital and in Kobe University, and written informed consent was obtained from each patient prior to the treatment.
Patients.
A total of 43 previously untreated patients who were chronically infected with HCV-4a (34 patients), HCV-4m (3 patients), HCV-4n (3 patients), or HCV-4o (3 patients) were consecutively evaluated for antiviral treatment at Cairo University Hospital, Cairo, Egypt, between January 2008 and September 2010. The HCV subtype was determined according to the method of Okamoto et al. (31). The patients were treated with PEG-IFN α-2a (180 μg/week, subcutaneously) and RBV (1,000 to 1,200 mg daily, per os) for 48 weeks. The quantification of serum HCV RNA titers was performed as previously reported (14). To minimize the therapeutic burdens, including the high cost and possible side effects, therapy was discontinued if HCV RNA titers at week 12 did not drop by 2 log compared with baseline values or if HCV RNA was still detectable at week 24. These were considered a null response (see Results).
Sequence analysis of the NS5A and core regions of the HCV genome.
Blood samples were collected using Vacutainer tubes. The sera were separated within 2 h of blood collection, transferred to sterile cryovials, and kept frozen at −80°C until use. HCV RNA was extracted from 140 μl of serum using a commercially available kit (QIAmp viral RNA kit; Qiagen, Tokyo, Japan). The extracted RNA was reverse transcribed and amplified for the HCV genome encoding a carboxy terminus of NS5A (amino acids [aa] 2193 to 2417) and the core protein (aa 1 to 191) using SuperScript III one-step RT-PCR Platinum Taq HiFi (Invitrogen, Tokyo, Japan). The resultant reverse transcription (RT)-PCR product was subjected to a second-round PCR by using Platinum Taq DNA polymerase high fidelity III (Invitrogen). Primers used for amplification of the 3′ half of the NS5A region of HCV-4 were as follows: NS5A-4/F1 (5′-CTCAAYTCGTTCGTRGTGGGATC-3′; sense) and NS5A-4/R1 (5′-CGAAGGTCACCTTCTTCTGCCG-3′; antisense) for one-step RT-PCR; and NS5A-4/F2 (5′-ATGCGAGCCYGAGCCGGACGT-3′; sense) and NS5A-4/R2 (5′-GCTCAGGGGGYTRATTGGCAGCT-3′; antisense) for the second-round PCR. Primers for amplification of the core region of HCV-4 were 249-F (5′-GCTAGCCGAGTAGTGTTG-3′; sense) and 984-R (5′-GATGTGRTGRTCGGCCTC-3′; antisense) (40) for one-step RT-PCR; and 319-F (5′-GGAGGTCTCGTAGACCGTGC-3′; sense) (40) and primer-186 (5′-ATGTACCCCATGAGGTCGGC-3′; antisense) (2) for the second-round PCR. RT was performed at 45°C for 30 min and terminated at 94°C for 2 min, followed by the first-round PCR over 35 cycles, with each cycle consisting of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 68°C for 90 s. The second-round PCR was performed under the same conditions. The sequences of the amplified fragments were determined by direct sequencing without subcloning. The amino acid sequences were deduced and aligned using Genetyx Win software version 7.0 (Genetyx Corp., Tokyo, Japan). The numbering of amino acid residues for HCV-4 isolates is according to the polyprotein of ED43 isolate (accession no. Y11604) (10). Consensus sequences of the carboxy terminus of NS5A of a given HCV-4 subtype were inferred by alignment of all sequences obtained in this study as well as all available NS5A sequences of HCV-4a (accession no. Y11604, DQ418782 to DQ418789, DQ516084, and DQ988073 to DQ988079), HCV-4m (FJ462433), HCV-4n (FJ462441), and HCV-4o (FJ462440) from the databases.
Statistical analysis.
Numerical data were analyzed by Student's t test and categorical data by Fisher's exact probability test. To evaluate the optimal threshold of the number of amino acid mutations in IRRDR for prediction of treatment outcomes, the receiver operating characteristic (ROC) curve was constructed. Univariate and multivariate logistic regression analyses were performed to identify independent predictors for treatment outcomes. All statistical analyses were performed using the SPSS version 16 software (SPSS Inc., Chicago, IL). Unless otherwise stated, a P value of <0.05 was considered statistically significant.
Nucleotide sequence accession numbers.
The sequence data reported in this paper have been deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers AB725987 through AB726066.
RESULTS
Patients' responses to PEG-IFN/RBV combination therapy.
Among 43 patients enrolled in this study, 30 (70%) patients completed the entire course of PEG-IFN/RBV treatment for 48 weeks and follow-up for 24 weeks. On the other hand, the treatment was discontinued for 13 (30%) patients due to poor virological responses at 12 or 24 weeks after initiation of the therapy. Overall, 25 (58%) patients achieved SVR while 18 (42%) patients had non-SVR (Table 1). When analyzed on the basis of the subtype classification, SVR was achieved by 56% (19/34), 100% (3/3), 33% (1/3), and 67% (2/3) of patients infected with HCV-4a, -4m, -4n, and -4o, respectively.
TABLE 1.
Virological responses of HCV-4-infected patients treated with PEG-IFN/RBV
| Virological response | Proportion (%) of patients with indicated response (no. of patients/total no.) |
||||
|---|---|---|---|---|---|
| HCV-4a | HCV-4a | HCV-4 m | HCV-4n | HCV-4o | |
| SVR | 58 (25/43) | 56 (19/34) | 100 (3/3) | 33 (1/3) | 67 (2/3) |
| Non-SVR | 42 (18/43) | 44 (15/34) | 0 (0/3) | 67 (2/3) | 33 (1/3) |
| Null response | 30 (13/43) | 32 (11/34) | 0 (0/3) | 67 (2/3) | 0 (0/3) |
| Relapse | 12 (5/43) | 12 (4/34) | 0 (0/3) | 0 (0/3) | 33 (1/3) |
Includes all 43 cases with HCV-4 infection (34 cases with HCV-4a and 3 cases each with HCV-4m, -4n, and -4o).
Non-SVR patients are classified into two groups: (i) patients with null response, who did not achieve >2-log reduction of the initial viral load at week 12 or who had detectable viremia at week 24 of the treatment period; and (ii) patients with relapse, who were negative for HCV-RNA at the end of the treatment period (week 48) followed by a rebound viremia at a certain time point during the follow-up period of 24 weeks. Patients with null response represented 30% (13/43) of all the HCV-4-infected subjects analyzed, while those with relapse represented 12% (5/43). A similar tendency was observed for subtype HCV-4a.
Among various patients' demographic characteristics, SVR patients had a significantly lower average age than that of non-SVR patients (Table 2). Furthermore, a tendency for SVR patients to have a lower average titer of initial viral load than that of non-SVR was noted, although the difference was not statistically significant, due possibly to the small number of patients analyzed (P = 0.07).
TABLE 2.
Demographic characteristics of HCV-4-infected patients with SVR and non-SVRa
| Factor | SVR | Non-SVR | P value |
|---|---|---|---|
| Age | 38.47 ± 9.51 | 45.80 ± 5.65 | 0.014 |
| Sex (male/female) | 18/7 | 15/3 | 0.48 |
| BMI | 27.36 ± 3.65 | 27.67 ± 5.28 | 0.85 |
| Platelets (× 103/μl) | 204.4 ± 40.63 | 216.7 ± 87.25 | 0.59 |
| Hemoglobin (g/dl) | 14.54 ± 1.38 | 15.08 ± 1.39 | 0.25 |
| WBC count | 7,041 ± 1,876 | 7,078 ± 2,977 | 0.96 |
| Albumin (g/dl) | 4.12 ± 0.36 | 4.328 ± 0.41 | 0.11 |
| ALT (IU/liter) | 78.72 ± 59.68 | 82.39 ± 41.80 | 0.83 |
| AST (IU/liter) | 64.94 ± 27.63 | 58.17 ± 23.98 | 0.44 |
| HCV-RNA (IU/ml) | 84,290 ± 186,300 | 501,800 ± 816,700 | 0.07 |
Values are means ± standard deviations. SVR, sustained virological response; BMI, body mass index; WBC, white blood cell; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Correlation between NS5A sequence heterogeneity and SVR in HCV-4 infection.
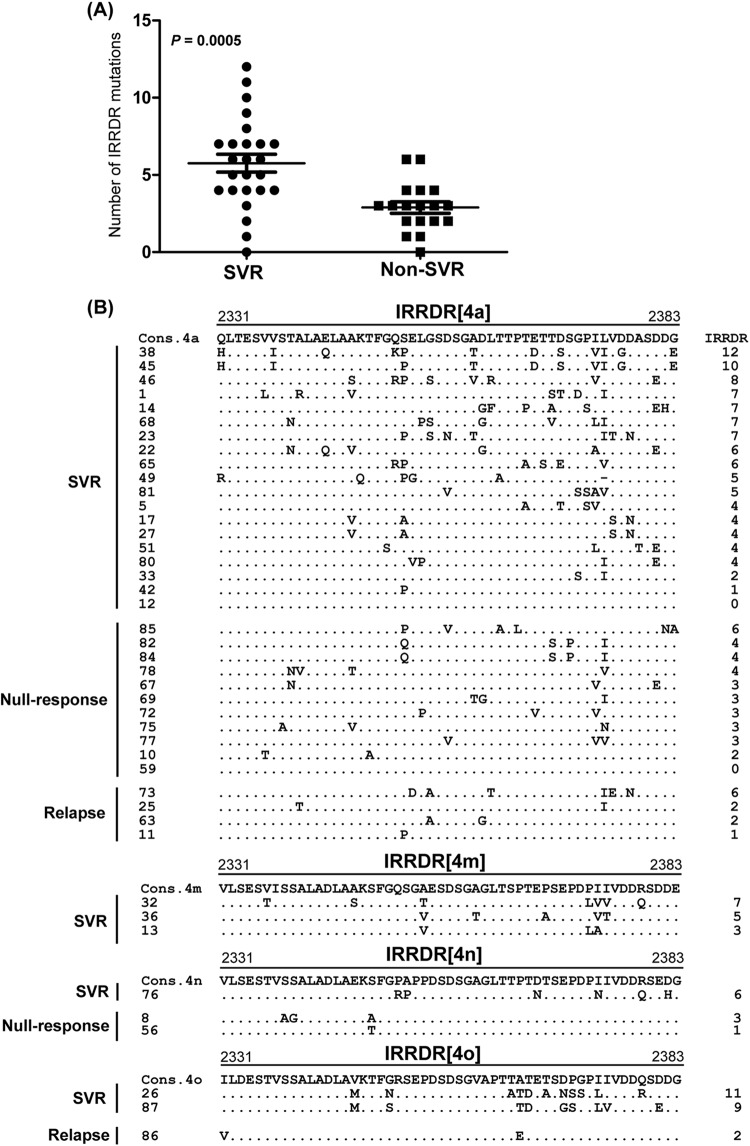
We and other researchers reported significant correlation between sequence polymorphisms within the C-terminal half of NS5A, including that in ISDR and IRRDR, and PEG-IFN/RBV treatment outcome in HCV-1 and HCV-2 infections (13, 15, 18, 30). However, this information is quite limited in HCV-4 infection. To clarify this issue, part of the HCV-4 genome encoding a carboxy terminus (aa 2193 to 2417) of NS5A in pretreatment sera was amplified and sequenced, and amino acid sequences were deduced. The sequences obtained as well as all available NS5A sequences of HCV-4a, -4m, -4n, and -4o from the databases were aligned, and the consensus sequences for a desired HCV-4 subtype were inferred (see Materials and Methods). Next, to identify an NS5A region(s) that would be significantly correlated with treatment outcome, we carried out a sliding window analysis with a window size of 30 residues over the C-terminal half (aa 2193 to 2417) of NS5A sequences obtained from all SVR (n = 25) and non-SVR (n = 18) patients along with corresponding consensus sequences of each HCV-4 subtype as described previously (30). This analysis revealed that the difference in the overall number of amino acid mutations between SVR and non-SVR isolates exceeded the significant threshold only in a region corresponding to IRRDR of HCV-1b (13), ranging from aa 2331 to 2383, thus being referred to as IRRDR[HCV-4] (Fig. 1). Indeed, the average number of amino acid mutations in IRRDR[HCV-4] was significantly larger in SVR than in non-SVR (P = 0.0005) isolates (Fig. 2A). Sequences of IRRDR of HCV-4a, -4m, -4n, and -4o obtained from SVR and non-SVR patients along with the number of IRRDR mutations of each isolate are shown in Fig. 2B.
Fig 1.
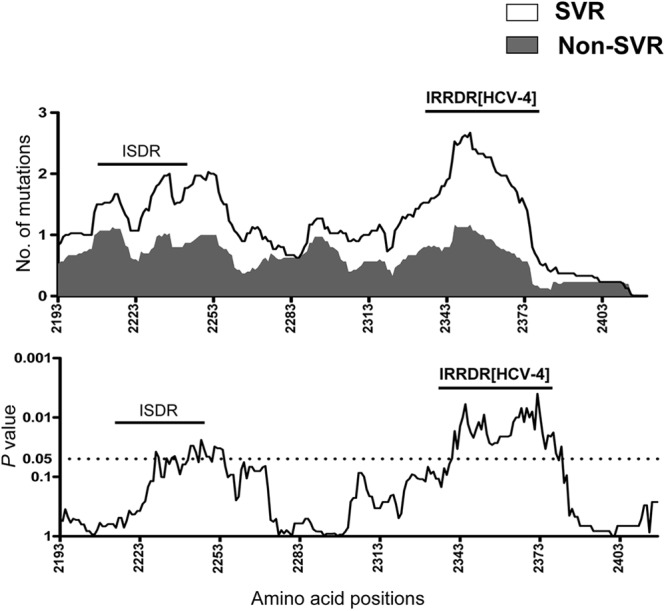
Sliding window analysis over the carboxy terminus (aa 2193 to 2417) of NS5A of HCV-4 obtained from SVR and non-SVR patients.
Fig 2.
Correlation between IRRDR[HCV-4] sequence variations and treatment outcome. (A) Average number of amino acid mutations in IRRDR[HCV-4] obtained from SVR and non-SVR patients. (B) Alignment of IRRDR[HCV-4] sequences obtained from SVR and non-SVR patients with HCV-4a, -4m, -4n, and -4o. The consensus sequence (Cons) of each subtype is shown on the top. The numbers along the sequence indicate the amino acid positions. Dots indicate residues identical to those of the Cons sequence. The numbers of the mutations in each IRRDR (4a, 4m, 4n, or 4o) are shown on the right.
Next, we performed ROC curve analysis to estimate the optimal cutoff number of IRRDR[HCV-4] mutations for SVR prediction. This analysis estimated 4 mutations as the optimal number of IRRDR[HCV-4] mutations to predict SVR, since it achieved the highest sensitivity (84%; sensitivity refers to the proportion of SVR patients who were infected with HCV isolates of IRRDR[HCV-4] with 4 or more mutations) and specificity (72%; specificity refers to the proportion of non-SVR patients who were infected with HCV isolates of IRRDR[HCV-4] with 3 or fewer mutations) with an area under the curve (AUC) of 0.82 (Fig. 3). Accordingly, 21 (84%) of 25 patients with SVR, in contrast to only 5 (28%) of 18 patients with non-SVR, had IRRDR[HCV-4] with 4 or more mutations (referred to as IRRDR[HCV-4]≥4), with the difference between the two groups being statistically significant (P = 0.0004) (Table 3). It should be noted that 4 (31%) of 13 patients with null response and only 1 (20%) of 5 patients with relapse had HCV with IRRDR[HCV-4]≥4. These results collectively suggest that IRRDR[HCV-4]≥4 is significantly associated with SVR. In this connection, we also tested the impact of a higher (≥5) and a lower (≥3) degree of IRRDR mutations on treatment outcome. IRRDR[HCV-4]≥5 was significantly associated with SVR, though with a relatively lower sensitivity (64%) than that of IRRDR[HCV-4]≥4 (Table 3). On the other hand, there was no significant correlation between IRRDR[HCV-4]≥3 and SVR.
Fig 3.
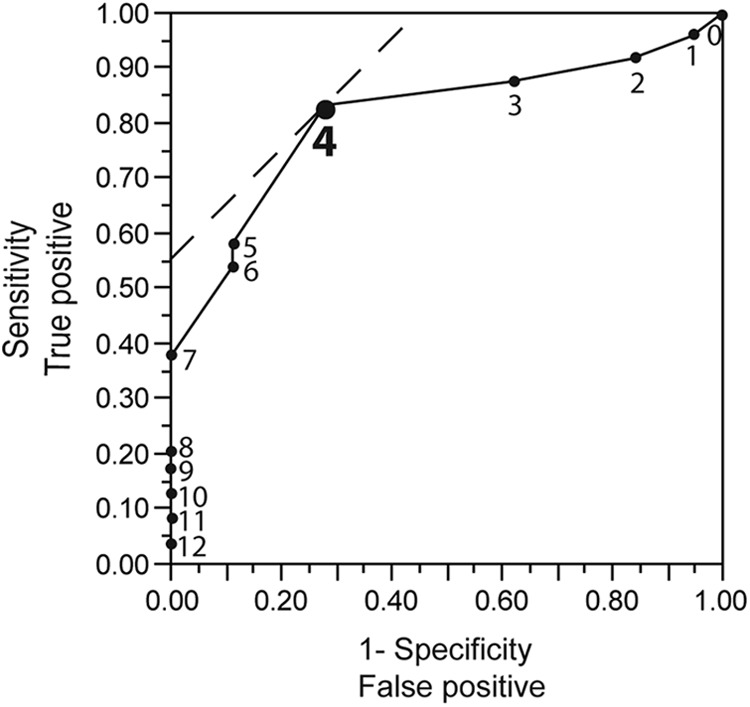
ROC curve analysis of IRRDR[HCV-4] sequence heterogeneity for SVR prediction. The solid line curve shows the AUC. Solid circles with numerals plotted on the curve represent different numbers of IRRDR mutations analyzed. The dashed line in the upper left corner indicates the optimal number of IRRDR[HCV-4] mutations for SVR prediction, which yields the highest sensitivity (84%) and the highest specificity (72%).
TABLE 3.
Correlation between NS5A sequence heterogeneity and virological responses in HCV-4 infection
| Factor | No. of isolates/total no. (%) |
P value for SVR versus: |
|||||
|---|---|---|---|---|---|---|---|
| SVR | Non-SVR | Null response | Relapse | Non-SVR | Null response | Relapse | |
| IRRDR ≥ 4 | 21/25 (84)a | 5/18 (28) | 4/13 (31) | 1/5 (20) | 0.0004 | 0.003 | 0.01 |
| IRRDR ≤ 3 | 4/25 (16) | 13/18 (72)b | 9/13 (69) | 4/5 (80) | |||
| IRRDR ≥ 5 | 16/25 (64)a | 2/18 (11) | 1/13 (8) | 1/5 (20) | 0.0006 | 0.002 | 0.14 |
| IRRDR ≤ 4 | 9/25 (36) | 16/18 (89)b | 12/13 (92) | 4/5 (80) | |||
| IRRDR ≥ 3 | 22/25 (88)a | 11/18 (61) | 10/13 (77) | 1/5 (20) | 0.066 | 0.39 | 0.006 |
| IRRDR ≤ 2 | 3/25 (12) | 7/18 (39)b | 3/13 (23) | 4/5 (80) | |||
Sensitivity (proportion of SVR patients with the favorable factor).
Specificity (proportion of non-SVR patients with the unfavorable factor).
Correlation between core protein sequence heterogeneity and SVR in HCV-4 infection.
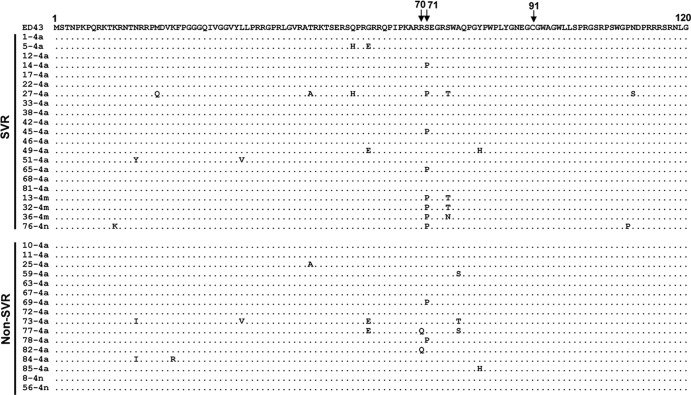
A close correlation between core protein sequence patterns at positions 70 and 91 and treatment outcome has been proposed, especially in Japanese patients with HCV-1b infection (4–6). To examine this hypothesis in Egyptian patients infected with HCV-4, core sequences of the viral genome were amplified from the pretreated sera, and the amino acid sequences were deduced. Due to a high degree of sequence homology among core sequences of various HCV-4 subtypes, all sequences obtained were aligned with the prototype sequence, ED43 (10). The residues at positions 70 and 91 were both well conserved among the sequences analyzed, and therefore, no correlation with treatment outcome was observed for these residues (Fig. 4). All but two isolates had arginine at position 70 (Arg70), the residue that has been associated with an IFN-sensitive phenotype as far as the core protein of HCV-1b is concerned (4–6). On the other hand, Pro at position 71 showed a tendency to be more frequent in SVR than in non-SVR patients; however, the frequency was not statistically different between the two groups.
Fig 4.
Sequence alignment of the core protein of HCV-4 isolates. Core protein sequences (aa 1 to 120) of HCV-4 obtained from SVR and non-SVR patients are aligned. The prototype sequence of ED43 (10) is shown on the top. The numbers along the sequence indicate the amino acid positions. Dots indicate residues identical to those of the prototype sequence.
Identification of independent predictive factors for SVR in HCV-4 infection.
In order to identify significant independent predictive factors of SVR for PEG-IFN/RBV treatment outcome in HCV-4 infection, first, all available data of baseline patients' parameters and IRRDR[HCV-4] polymorphism were entered in a univariate logistic analysis. This analysis yielded 3 factors that were correlated or nearly correlated with SVR: IRRDR[HCV-4]≥4 (P = 0.0004), patient's age (<42 years; P = 0.03), and HCV RNA titer (<5,200 IU/ml; P = 0.08). Subsequently, these 3 factors were entered in multivariate logistic regression analysis. This analysis revealed that the IRRDR[HCV-4]≥4 was the only independent predictive factor for SVR in HCV-4 infection (Table 4). We then assessed SVR predictability by means of IRRDR[HCV-4]≥4. As shown in Table 5, IRRDR[HCV-4]≥4 would predict SVR with a positive predictive value (PPV) of 81% (P = 0.002) and sensitivity of 84%. On the other hand, IRRDR[HCV-4]≤3 would predict non-SVR with a negative predictive value (NPV) of 76% (P = 0.02) and specificity of 72%. Thus, the degree of sequence variation in IRRDR[HCV-4] would yield useful positive and negative predictive markers for PEG-IFN/RBV therapy outcome in HCV-4-infected patients.
TABLE 4.
Univariate and multivariate analyses for identification of independent predictive factors for SVR in HCV-4-infected patients treated with PEG-IFN/RBV therapy
| Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|
| Variable | P value | Odds ratio (95% CI) | P value |
| IRRDR mutations (IRRDR ≥ 4 versus IRRDR ≤ 3) | 0.0004 | 10.5 (1.12–98.91) | 0.04 |
| Age (<42 years) | 0.03 | ||
| HCV-RNA (<5,200 IU/ml) | 0.08 | ||
TABLE 5.
PPV, NPV, sensitivity, and specificity of IRRDR sequence heterogeneity on the likelihood of achieving SVR and non-SVR in HCV-4 infection
| Factor | PPV | NPV | Sensitivityc | Specificityd |
|---|---|---|---|---|
| IRRDR ≥ 4 | 81% (21/26)a | 84% (21/25) | ||
| IRRDR ≤ 3 | 76% (13/17)b | 72% (13/18) |
P = 0.002.
P = 0.02.
Proportion of SVR patients who were infected with HCV isolates with IRRDR of ≥4.
Proportion of non-SVR patients who were infected with HCV isolates with IRRDR of ≤3.
DISCUSSION
Both host and viral genetic factors have been implicated in influencing the clinical response to PEG-IFN/RBV therapy for HCV infection (22). It has recently been reported that host genetic polymorphisms near or within the IL28B gene on chromosome 19 show a critical impact on the treatment outcome of patients infected with HCV-1 (20, 37, 39). As for the viral factor(s), polymorphisms of NS5A and core regions of a given HCV genotype have been linked to a difference in SVR rates (3, 4, 13, 18, 30). This hypothesis was mostly inferred from studies carried out with Asian populations, in particular Japanese, with HCV-1b infection. However, whether it can be applied to non-Asian populations infected with non-HCV-1 is still unknown. To the best of our knowledge, this is the first study that specifically examines the relationship between HCV genome heterogeneity, in particular in NS5A and core regions, and PEG-IFN/RBV treatment outcome in Egyptian patients infected with HCV-4. In analogy with our previous studies that identified IRRDR as a significant determinant for PEG-IFN/RBV treatment outcome in Japanese patients infected with HCV-1b, -2a, and -2b (12–16), we have demonstrated in the present study that sequence heterogeneity within IRRDR is closely associated with the ultimate treatment outcome in Egyptian patients infected with HCV-4. A high degree of sequence variation in IRRDR[HCV-4], i.e., more than 4 (IRRDR ≥ 4), significantly correlated with SVR, while a low degree of sequence variation in this region (IRRDR ≤ 3) correlated with non-SVR, null response, and relapse. The majority of patients with SVR (84%) had HCV with IRRDR of ≥4. In contrast, nearly two-thirds (72%) of the patients with non-SVR had HCV with IRRDR ≤ 3 (P = 0.0004) (Table 3). Notably, 21 of the 26 patients infected with HCV with IRRDR[HCV-4]≥4 achieved SVR. Accordingly, the PPV and NPV of IRRDR[HCV-4]≥4 for SVR and non-SVR patients were 81% (P = 0.002) and 76% (P = 0.02), respectively (Table 5). Our present results thus strongly suggest that the degree of sequence heterogeneity within IRRDR[HCV-4] would be a useful marker for prediction of treatment outcome in HCV-4 infection.
The molecular mechanism underlying the possible involvement of this region in IFN responsiveness of the virus is still unknown. The significant difference among IRRDR sequence patterns may suggest genetic flexibility of this region. Indeed, the C-terminal portion of NS5A was shown to tolerate sequence insertions and deletions (29). This flexibility might play an important role in modulating the interaction with various host systems, including IFN-induced antiviral machineries. It is also possible that the genetic flexibility of IRRDR is accompanied by compensatory changes elsewhere in the viral genome and that these compensatory changes affect overall viral fitness and responses to IFN-based therapy (8, 29, 41). Also, it is worth noting that IRRDR is among the most variable sequences across the different genotypes and subtypes of HCV (25) whereas its upstream and downstream sequences show a higher degree of sequence conservation (15). This may suggest that whereas the upstream and downstream sequences have a conserved function(s) across all the HCV genotypes, IRRDR sequences have a genotype-dependent or even a strain-dependent function(s).
A mutation at position 70 of the core protein of HCV-1b has been reported to be correlated with PEG-IFN/RBV treatment outcome (4, 12). In the present study, however, we found no significant correlation between core protein polymorphism and treatment outcome in HCV-4 infection. The residue at position 70 of the core protein of all but two HCV-4 isolates analyzed in this study was Arg (Fig. 4), which is known to be associated with SVR in HCV-1b infection (4, 12). This high degree of sequence conservation at position 70 might be the reason for the lack of significant correlation between core protein polymorphism and treatment outcome in HCV-4 infection.
Single nucleotide polymorphisms (SNPs) near the IL28B region have been identified as the strongest baseline predictors of SVR to PEG-IFN/RBV in patients with HCV-1 infection. More recently, in two major studies that were carried out exclusively with HCV-4-infected patients (9, 11), the CC genotype of rs12979860 IL28B SNP was also strongly associated with SVR. It is worth noting that although the SVR rate was more than 80% among the patients with the CC genotype, these patients represented only around 40% of total SVR cases in both studies. Furthermore, the CC genotype was found in only 34% of all Egyptian patients analyzed (9). Taken together, those observations support the idea that in addition to IL28B polymorphism, there should be an additional factor(s) that influences SVR. In this context, an interplay between IRRDR and IL28B polymorphisms might explain why some patients with undesirable IL28B genotype achieve SVR and why some patients infected with HCV isolates with IRRDR[HCV-4]≥4 do not achieve SVR. Further comprehensive study is needed to validate the importance of IRRDR and IL28B polymorphisms in predicting the treatment outcome of HCV-4-infected patients.
In conclusion, the present study emphasizes the importance of IRRDR sequence heterogeneity in the prediction of PEG-IFN/RBV treatment outcome for different HCV genotype infections in different ethnic groups, including Egyptian patients infected with HCV-4.
ACKNOWLEDGMENTS
This study was supported in part by Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan, and a SATREPS Grant from Japan Science and Technology Agency (JST) and Japan International Cooperation Agency (JICA).
This study was also carried out as part of Japan Initiative for Global Research Network on Infectious Diseases (J-GRID), Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Global Center of Excellence (G-COE) Program at Kobe University Graduate School of Medicine.
No conflicts of interest exist.
Footnotes
Published ahead of print 19 September 2012
REFERENCES
- 1. Abdel-Aziz F, et al. 2000. Hepatitis C virus (HCV) infection in a community in the Nile Delta: population description and HCV prevalence. Hepatology 32:111–115 [DOI] [PubMed] [Google Scholar]
- 2. Abdel-Hamid M, et al. 2007. Genetic diversity in hepatitis C virus in Egypt and possible association with hepatocellular carcinoma. J. Gen. Virol. 88:1526–1531 [DOI] [PubMed] [Google Scholar]
- 3. Akuta N, et al. 2009. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 2a high viral load and virological response to interferon-ribavirin combination therapy. Intervirology 52:301–309 [DOI] [PubMed] [Google Scholar]
- 4. Akuta N, et al. 2007. Predictive factors of early and sustained responses to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b: amino acid substitutions in the core region and low-density lipoprotein cholesterol levels. J. Hepatol. 46:403–410 [DOI] [PubMed] [Google Scholar]
- 5. Akuta N, et al. 2007. Prediction of response to pegylated interferon and ribavirin in hepatitis C by polymorphisms in the viral core protein and very early dynamics of viremia. Intervirology 50:361–368 [DOI] [PubMed] [Google Scholar]
- 6. Akuta N, et al. 2005. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non-virological response to interferon-ribavirin combination therapy. Intervirology 48:372–380 [DOI] [PubMed] [Google Scholar]
- 7. Amoroso P, et al. 1998. Correlation between virus genotype and chronicity rate in acute hepatitis C. J. Hepatol. 28:939–944 [DOI] [PubMed] [Google Scholar]
- 8. Appel N, Pietschmann T, Bartenschlager R. 2005. Mutational analysis of hepatitis C virus nonstructural protein 5A: potential role of differential phosphorylation in RNA replication and identification of a genetically flexible domain. J. Virol. 79:3187–3194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asselah T, et al. 2012. IL28B polymorphism is associated with treatment response in patients with genotype 4 chronic hepatitis C. J. Hepatol. 56:527–532 [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain RW, Adams N, Saeed AA, Simmonds P, Elliott RM. 1997. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J. Gen. Virol. 78(Pt 6):1341–1347 [DOI] [PubMed] [Google Scholar]
- 11. De Nicola S, et al. 2012. Interleukin 28B polymorphism predicts pegylated interferon plus ribavirin treatment outcome in chronic hepatitis C genotype 4. Hepatology 55:336–342 [DOI] [PubMed] [Google Scholar]
- 12. El-Shamy A, et al. 2012. Polymorphisms of hepatitis C virus non-structural protein 5A and core protein and clinical outcome of pegylated-interferon/ribavirin combination therapy. Intervirology 55:1–11 [DOI] [PubMed] [Google Scholar]
- 13. El-Shamy A, et al. 2008. Sequence variation in hepatitis C virus nonstructural protein 5A predicts clinical outcome of pegylated interferon/ribavirin combination therapy. Hepatology 48:38–47 [DOI] [PubMed] [Google Scholar]
- 14. El-Shamy A, et al. 2007. Prediction of efficient virological response to pegylated interferon/ribavirin combination therapy by NS5A sequences of hepatitis C virus and anti-NS5A antibodies in pre-treatment sera. Microbiol. Immunol. 51:471–482 [DOI] [PubMed] [Google Scholar]
- 15. El-Shamy A, et al. 2012. Sequence heterogeneity in NS5A of hepatitis C virus genotypes 2a and 2b and clinical outcome of pegylated-interferon/ribavirin therapy. PLoS One 7:e30513 doi:10.1371/journal.pone.0030513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El-Shamy A, et al. 2011. Sequence heterogeneity of NS5A and core proteins of hepatitis C virus and virological responses to pegylated-interferon/ribavirin combination therapy. Microbiol. Immunol. 55:418–426 [DOI] [PubMed] [Google Scholar]
- 17. el-Zayadi AR, et al. 2005. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J. Gastroenterol. 11:5193–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Enomoto N, et al. 1996. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 334:77–81 [DOI] [PubMed] [Google Scholar]
- 19. Fried MW, et al. 2002. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347:975–982 [DOI] [PubMed] [Google Scholar]
- 20. Ge D, et al. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399–401 [DOI] [PubMed] [Google Scholar]
- 21. Hassan MM, et al. 2001. The role of hepatitis C in hepatocellular carcinoma: a case control study among Egyptian patients. J. Clin. Gastroenterol. 33:123–126 [DOI] [PubMed] [Google Scholar]
- 22. Kau A, Vermehren J, Sarrazin C. 2008. Treatment predictors of a sustained virologic response in hepatitis B and C. J. Hepatol. 49:634–651 [DOI] [PubMed] [Google Scholar]
- 23. Khattab MA, et al. 2011. Management of hepatitis C virus genotype 4: recommendations of an international expert panel. J. Hepatol. 54:1250–1262 [DOI] [PubMed] [Google Scholar]
- 24. Limaye AR, Draganov PV, Cabrera R. 2011. Boceprevir for chronic HCV genotype 1 infection. N. Engl. J. Med. 365:176, 177–178 [DOI] [PubMed] [Google Scholar]
- 25. Macdonald A, Harris M. 2004. Hepatitis C virus NS5A: tales of a promiscuous protein. J. Gen. Virol. 85:2485–2502 [DOI] [PubMed] [Google Scholar]
- 26. Maekawa S, Enomoto N. 2009. Viral factors influencing the response to the combination therapy of peginterferon plus ribavirin in chronic hepatitis C. J. Gastroenterol. 44:1009–1015 [DOI] [PubMed] [Google Scholar]
- 27. Mattsson L, Sonnerborg A, Weiland O. 1993. Outcome of acute symptomatic non-A, non-B hepatitis: a 13-year follow-up study of hepatitis C virus markers. Liver 13:274–278 [DOI] [PubMed] [Google Scholar]
- 28. Micallef JM, Kaldor JM, Dore GJ. 2006. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J. Viral Hepat. 13:34–41 [DOI] [PubMed] [Google Scholar]
- 29. Moradpour D, et al. 2004. Insertion of green fluorescent protein into nonstructural protein 5A allows direct visualization of functional hepatitis C virus replication complexes. J. Virol. 78:7400–7409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murakami T, et al. 1999. Mutations in nonstructural protein 5A gene and response to interferon in hepatitis C virus genotype 2 infection. Hepatology 30:1045–1053 [DOI] [PubMed] [Google Scholar]
- 31. Okamoto H, et al. 1992. Typing hepatitis C virus by polymerase chain reaction with type-specific primers: application to clinical surveys and tracing infectious sources. J. Gen. Virol. 73(Pt 3):673–679 [DOI] [PubMed] [Google Scholar]
- 32. Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. 2000. Genetic epidemiology of hepatitis C virus throughout Egypt. J. Infect. Dis. 182:698–707 [DOI] [PubMed] [Google Scholar]
- 33. Sarasin-Filipowicz M. 2010. Interferon therapy of hepatitis C: molecular insights into success and failure. Swiss Med. Wkly. 140:3–11 [DOI] [PubMed] [Google Scholar]
- 34. Sherman KE, et al. 2011. Response-guided telaprevir combination treatment for hepatitis C virus infection. N. Engl. J. Med. 365:1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Simmonds P, et al. 2005. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42:962–973 [DOI] [PubMed] [Google Scholar]
- 36. Simmonds P, et al. 1993. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J. Gen. Virol. 74(Pt 11):2391–2399 [DOI] [PubMed] [Google Scholar]
- 37. Suppiah V, et al. 2009. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 41:1100–1104 [DOI] [PubMed] [Google Scholar]
- 38. Tanaka E, Kiyosawa K. 2000. Natural history of acute hepatitis C. J. Gastroenterol. Hepatol. 15(Suppl):E97–E104 [DOI] [PubMed] [Google Scholar]
- 39. Tanaka Y, et al. 2009. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41:1105–1109 [DOI] [PubMed] [Google Scholar]
- 40. Timm J, et al. 2007. Characterization of full-length hepatitis C virus genotype 4 sequences. J. Viral Hepat. 14:330–337 [DOI] [PubMed] [Google Scholar]
- 41. Yuan HJ, Jain M, Snow KK, Gale M, Jr, Lee WM. 2010. Evolution of hepatitis C virus NS5A region in breakthrough patients during pegylated interferon and ribavirin therapy. J. Viral Hepat. 17:208–216 [DOI] [PMC free article] [PubMed] [Google Scholar]