Abstract
We describe the utility of PCR and electrospray ionization with mass spectrometry (PCR/ESI-MS) of culture-negative cerebrospinal fluid (CSF) in order to identify Gram-positive cocci noted on a Gram stain of CSF from a previously healthy 26-year-old man with community-acquired pneumonia (CAP) and multiple brain abscesses. CSF samples were obtained 2 weeks apart, first by lumbar puncture and 2 weeks later from an external ventricular drain that was inserted into the right ventricle. Both CSF cultures were negative. A Gram stain of bronchoalveolar lavage (BAL) fluid was notable for many Gram-positive cocci (GPC), but cultures of BAL fluid and subcarinal lymph node biopsy tissue were negative. PCR/ESI-MS detected Streptococcus intermedius, a common cause of brain abscesses, in both CSF samples as well as in the fixed tissue from the biopsy. This unique case confirms S. intermedius pulmonary infection as the source of metastatic CNS infection and reveals the potential of PCR/ESI-MS to detect a streptococcal pathogen not captured by conventional cultures.
CASE REPORT
Apreviously healthy 26-year-old male was transferred to a tertiary care medical center with a 5-day history of cough, high-grade fever, and 1-day history of delirium, combativeness, and complaints of photophobia. In the emergency department (ED) at the outlying hospital, he was alert and oriented. His temperature on presentation was 39.2°C. His white blood cell (WBC) count was 27,200 cells/mm3 (with 85% neutrophils). A computed tomography (CT) scan of the brain noted areas of hypodensity that were suggestive of emboli and encephalitis. A lumbar puncture (LP) was performed, revealing a cerebrospinal fluid (CSF) WBC count of 6,200 cells/mm3 with 74% neutrophils. The red blood cell (RBC) count, total protein, and glucose were 700 cells/mm3, 472 mg/dl, and 56 mg/dl, respectively. Organisms were not seen on a Gram stain. The patient was stabilized in the outlying ED and then transferred to the medical intensive care unit (MICU), where treatment with intravenous (i.v.) vancomycin and ceftriaxone was initiated. One day following admission, the patient was transferred to the MICU at our facility.
Upon presentation to our facility, he was afebrile and lucid. The physical exam was unremarkable, and the initial neurologic exam was normal. A second head CT without intravenous contrast at our hospital following transfer was notable only for mucosal thickening of the sphenoid sinuses. Intracranial abnormalities were not appreciated. CT examination of the chest, abdomen, and pelvis revealed pneumonia and mild prominence of right hilar and subcarinal lymph nodes. The patient was monitored closely in the ICU. Acyclovir and dexamethasone were both added to i.v. vancomycin and ceftriaxone treatment, which was continued following transfer. Blood and CSF cultures obtained on admission proved to be negative, and a two-dimensional (2D) transthoracic echocardiogram was unremarkable.
In the next four hospital days, our patient began complaining of increased headache, with fluctuating alertness and combative behavior. On hospital day four, he developed new left arm weakness and a possible Kernig's sign. Pupils were 2 to 3 mm with equal bilateral light reaction, and hyperreflexia and pronator drift were noted in the left upper extremity, with sustained clonus in both lower extremities. The Babinski sign was absent.
A magnetic resonance imaging (MRI) of the brain with contrast demonstrated multiple supratentorial, infratentorial, and deep nuclear 8- to 12-mm-thin, smooth-walled, ring-enhancing lesions with restricted diffusion throughout the brain (Fig. 1). These lesions were located predominantly at the gray-white junctions and the deep nuclear structures and were suggestive of thromboembolic septic emboli with multiple intra-axial abscesses. A transesophageal echocardiogram was negative for valvular vegetations or atrial mural thrombi. Intravenous metronidazole was substituted for acyclovir in the antimicrobial regimen. The patient experienced only mild improvement in symptoms with persistent headaches. Serologic testing, including a rapid plasma reagin (RPR), HIV-1/2 antibodies, hepatitis A, B, and C antibodies, and hepatitis B antigen (HBsAg), was all negative. A whole-body positron emission tomography (PET) scan was negative for malignancy.
Fig 1.
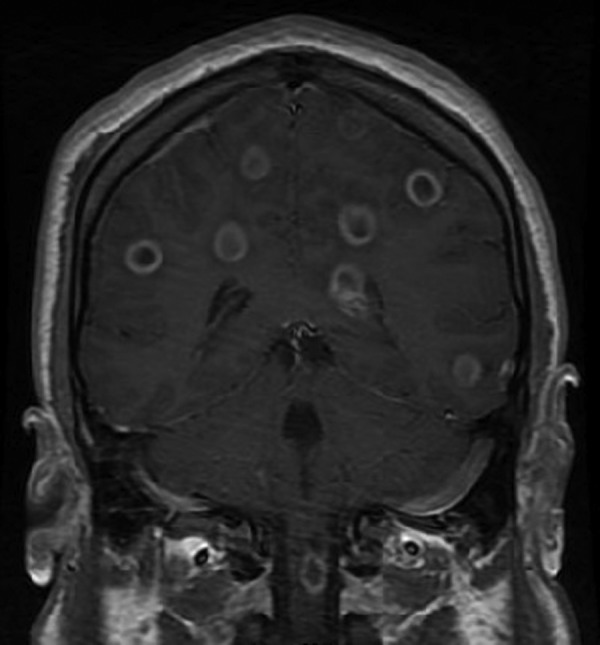
MRI of the patient's brain with contrast. Coronal view demonstrating intracranial and cervical spinal cord abscesses.
Concern for a pulmonary infection as the source of a disseminated central nervous system (CNS) infection led to a fiberoptic bronchoscopic exam. The visual inspection of the larynx, trachea, carina, and right and left bronchial trees was completely normal. Bronchoalveolar lavage (BAL) fluid was obtained. A Gram stain of BAL fluid was notable for many Gram-positive cocci (GPC), but cultures of BAL fluid and subcarinal lymph node biopsy tissue were negative. Abscess-like collections of neutrophils and necrotic cellular debris were appreciated in the microscopic exam of the subcarinal lymph node tissue.
The patient's headache worsened, elevated intracranial pressure was suspected, 12 mg dexamethasone i.v. every 6 h (q6h) was resumed, and a one-time i.v. dose of 50 g mannitol was administered. A repeat MRI demonstrated an increase in the size of the lesions, including a particularly concerning lesion within the spinal cord at the level of the first cervical vertebral body. To relieve intracranial pressure, a twist drill craniostomy was performed, the dura was opened, and a Bactiseal ventricular catheter was placed into the right frontal horn. The ependymal surface was firmer than normal, and turbid CSF was returned. A Gram stain of the fluid was notable for intracellular Gram-positive structures resembling cocci but no identifiable organisms. The CSF WBC count was 2,248 cells/mm3 with 90% granulocytes. The RBC count was 280 cells/mm3. CSF cryptococcal antigen (Meridian Bioscience, Inc., Cincinnati, OH), a microscopic exam of Ziehl-Neelsen acid fast, and Gomori methenamine silver stains of CSF were all negative. No growth after 48 h was appreciated on either blood or chocolate agar inoculated with CSF and incubated at 37°C in 5% CO2.
Treatment with intrathecal (i.t.) vancomycin was initiated, and CSF vancomycin levels were monitored daily. A repeat 20-mg vancomycin dose was administered i.t. 2 days later. Our patient ultimately completed a 10-day course of i.t. vancomycin, after which the external ventricular drain (EVD) was removed. He also completed a tapering course of i.v. dexamethasone. After 25 days of inpatient treatment, our patient was discharged to home. When he was seen as an outpatient at the completion of a 3-month course of treatment with intravenous ceftriaxone, he was completely asymptomatic and had returned to work. A neurologic exam and repeat MRI of the brain were also normal.
PCR and electrospray ionization with mass spectrometry (PCR/ESI-MS) is a diagnostic modality that has demonstrated the capacity to detect pathogenic organisms in both environmental and clinical samples (3, 5, 9). Although cultures of CSF from our patient were negative, intracellular Gram-positive structures resembling cocci were noted in CSF from the EVD, suggesting that nonviable organisms may have been present. PCR/ESI-MS on the PLEX-ID platform (Abbott Molecular, Des Plaines, IL) was performed on two samples of CSF and fixed subcarinal lymph node tissue. GPC were also noted on a Gram stain of BAL fluid; unfortunately, the BAL fluid was not available for PCR/ESI-MS testing. However, fixed tissue from the subcarinal lymph node sample was extracted for PCR/ESI-MS.
We followed the protocol previously described and validated by Kaleta et al. (6). The protocol is part of a kit (BAC Detection Assay; Abbott Molecular). The preparation of the sample and DNA extraction protocol, primer pairs, and ribosomal regions were previously described (6). Compared to clinical samples, the assay performs with 98.7% and 96.6% concordance at the genus and species levels, respectively.
PCR/ESI-MS detected Streptococcus intermedius, a common cause of CNS abscesses (7) in both CSF samples as well as the subcarinal lymph node (Q score = 0.99) (Table 1). The level of detection (LOD) from the CSF specimens, reported as numbers of genome equivalents per PCR, was 204 in CSF obtained by LP in the ED and 229 in CSF obtained via the EVD. The LOD in the lymph node tissue was 178. This identification was confirmed by partial 16S rRNA gene sequencing using established primers. The aligned, trimmed sequence (with primers removed; AGGACGAACGCTGGCGGCGTGCCTAATACATGCAAGTAGAACGCACAGGATACACCGTAGTTTACTACACCGTATTCTGTGAGTTGCGAACGGGTGAGTAACGCGTAGGTAACCTGCCTGGTAGCGGGGGATAACTATTGGAAACGATAGCTAATACCGCATAAGAACATTTACTGCATGGTAGATGTTTAAAAGGTGCAAATGCATCACTACCAGATGGACCTGCGTTGTATTAGCTAGTAGGTGAGGTAACGGCTCACCTAGGCGACGATACATAGCCGACCTGAGAGGGTGATCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCGGCAATGGGGGGAACCCTGACCGAGCAACGCCGCGTGAGTGAAGAAGGTTTTCGGATCGTAAAGCTCTGTTGTTAAGGAAGAACGAGTGTGAGAATGGAAAGTTCATACTGTGACGGTACTTAACCAGAAAGGGACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTCCCGAGCGTTGTCCGGATTTATTGGGCGTAAAGCGAGCGCAGGCGGTTAGATAAGTCTGAAGTTAAAGGCAGTGGCTCAACCATTGTAGGCTTTGGAAACTGTTTAACTTGAGTGCAGAAGGGGAGAGTGGAATTCCATGTGTAGCGGTGAAATGCGTAGATATATGGAGGAACACCGGTGGCGAAAGCGGCTCTCTGGTCTGTAACTGACGCTGAGGCTCGAAAGCGTGGGAGCGAACAGGAT) matched S. intermedius 16S rRNA gene sequences in NCBI Blast, leBIBI, and SeptiTest-Blast. The sequence result is summarized in Table 2.
TABLE 1.
Specimen, culture result, PCR/ESI-MS result, and level of detection
| Specimen | Vol | Culture result | Gram stain findings | PCR/ESI-MS result (GE/well)a |
|---|---|---|---|---|
| CSF from LP performed in ED | 2 ml | No growth | Many segments; no organisms | S. intermedius (204) |
| CSF from fluid obtained when the EVD was placed | 2 ml | No growth | Many segments with intracellular Gram-positive structures resembling cocci | S. intermedius (229) |
| Fixed tissue from a transbronchial biopsy of the subcarinal lymph node | NAb | No growth | Many neutrophils with necrotic cellular debris; no organisms | S. intermedius (178) |
GE/well, number of genome equivalents per PCR.
NA, not applicable.
TABLE 2.
First 5 NCBI Blast results
| GenBank accession no. | Descriptiona | Maximum score | Total score | Query coverage (%) | E value | Maximum identity (%) |
|---|---|---|---|---|---|---|
| AB236926.1 | Streptococcus intermedius genes for NanA-like sialidase, putative sigma factor, complete CDS | 1,423 | 1,423 | 100 | 0.0 | 99 |
| HM596275.1 | Streptococcus intermedius strain F0413 16S rRNA gene, partial sequence | 1,423 | 1,423 | 100 | 0.0 | 99 |
| FJ558133.1 | Uncultured bacterium clone ET_A_2b12 16S rRNA gene, partial sequence | 1,423 | 1,423 | 100 | 0.0 | 99 |
| NR_028736.1 | Streptococcus intermedius strain 1877 16S rRNA, partial sequence | 1,423 | 1,423 | 100 | 0.0 | 99 |
| AM420148.1 | Uncultured Streptococcus sp. partial 16S rRNA gene, clone 402F02 (oral) | 1,423 | 1,423 | 100 | 0.0 | 99 |
| AF104671.1 | Streptococcus intermedius strain ATCC 27335 16S rRNA gene, partial sequence | 1,423 | 1,423 | 100 | 0.0 | 99 |
CDS, coding sequence.
In conclusion, our case is the first application of molecular microbiology to confirm S. intermedius pulmonary infection as the source of metastatic CNS infection (1, 2, 8). More generally, this case demonstrates that PCR/ESI-MS may be an important adjunct to conventional diagnostic microbiologic methods in cases of culture-negative CNS infections. Frequently, empirical antimicrobial treatment is initiated when patients present with signs and symptoms of CNS infection before CSF is obtained by LP. When CSF cultures are negative because of administration of antimicrobial treatment before CSF samples are collected for culture, patients who respond to complex empirical treatment regimens may be exposed to unnecessary and prolonged courses of antibiotics for a variety of potential pathogens. PCR/ESI-MS offers an alternative to conventional culture for identification of invasive pathogens in culture-negative CNS infections.
Future investigations conducted prospectively to analyze the diagnostic accuracy and utility of PCR/ESI-MS must be conducted before empirical antimicrobial treatment is replaced by specific antimicrobial treatment based on PCR/ESI-MS results.
ACKNOWLEDGMENTS
The VA Merit Review Program, VISN 10 GRECC, and the National Institutes of Health supported R.A.B.
R. Sampath, R. Ranken, M. A. Rounds, and D. J. Ecker are salaried employees of Ibis Biosciences, a division of Abbott.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Carpenter J, Stapleton S, Holliman R. 2007. Retrospective analysis of 49 cases of brain abscess and review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 26:1–11 [DOI] [PubMed] [Google Scholar]
- 2. Chun CH, Johnson JD, Hofstetter M, Raff MJ. 1986. Brain abscess. A study of 45 consecutive cases. Medicine 65:415–431 [PubMed] [Google Scholar]
- 3. Ecker DJ, et al. 2008. Ibis T5000: a universal biosensor approach for microbiology. Nat. Rev. Microbiol. 6:553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reference deleted.
- 5. Hofstadler SA, Sampath R, Blyn LB, Eshoo MW, Hall TA. 2005. TIGER: the universal biosensor. Int. J. Mass Spectrom. 242:23–41 [Google Scholar]
- 6. Kaleta EJ, et al. 2011. Use of PCR coupled with electrospray ionization mass spectrometry for rapid identification of bacterial and yeast bloodstream pathogens from blood culture bottles. J. Clin. Microbiol. 49:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kao PT, Tseng HK, Liu CP, Su SC, Lee CM. 2003. Brain abscess: clinical analysis of 53 cases. J. Microbiol. Immunol. Infect. 36:129–136 [PubMed] [Google Scholar]
- 8. Kowlessar PI, O'Connell NH, Mitchell RD, Elliott S, Elliott TS. 2006. Management of patients with Streptococcus milleri brain abscesses. J. Infect. 52:443–450 [DOI] [PubMed] [Google Scholar]
- 9. Modi DA, et al. 2012. Rapid identification of Aspergillus terreus from bronchoalveolar lavage fluid by PCR and electrospray ionization with mass spectrometry. J. Clin. Microbiol. 50:2529–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]


