Abstract
Under stress conditions such as high light intensity or nutrient starvation, cells of the unicellular alga Dunaliella bardawil overproduce β-carotene, which is accumulated in the plastids in newly formed triacylglycerol droplets. We report here that the formation of these sequestering structures and β-carotene are interdependent. When the synthesis of triacylglycerol is blocked, the overproduction of β-carotene is also inhibited. During overproduction of β-carotene no up-regulation of phytoene synthase or phytoene desaturase is observed on the transcriptional or translational level, whereas at the same time acetyl-CoA carboxylase, the key regulatory enzyme of acyl lipid biosynthesis, is increased, at least in its enzymatic activity. We conclude that under normal conditions the carotenogenic pathway is not maximally active and may be appreciably stimulated in the presence of sequestering structures, creating a plastid-localized sink for the end product of the carotenoid biosynthetic pathway.
Carotenoids are vitally important in all photosynthetic membranes because of their ability to prevent photooxidative damage and to harvest light (for a recent review, see Frank and Cogdell, 1996). Underscoring their importance, chlorophylls and carotenoids are synthesized in a quantitatively and qualitatively coordinated manner in chloroplasts. Whenever this balance is strongly changed in favor of carotenoids, the plastid ultrastructure is also changed and, concomitantly, chlorophylls are degraded. The resulting chromoplasts are photosynthetically inactive, yellow to red in color, and differentiate in specialized plant organs such as petals, roots, and fruits. Chromoplasts are morphologically characterized by the absence of thylakoids and by the presence of newly formed structures in which the overproduced carotenoids are sequestered (for a recent review of chromoplast development, see Camara et al., 1995). These carotenoid-bearing structures may be plastoglobules (lipid droplets in most carotenoid-bearing flower petals), crystals (e.g. in Lycopersicon esculentum fruits), fibrils/tubules (e.g. in Capsicum annuum fruits), or membranes (e.g. in Narcissus pseudonarcissus petals). These structures probably prevent products from overloading the chromoplast membranes, the site of carotenoid formation.
To investigate the apparently strict interdependence of carotenoid overproduction and the formation of sequestering structures, we exploited the unicellular alga Dunaliella bardawil as a laboratory system inducible for enhanced carotenoid formation. When exposed to stress conditions such as high light intensity or nutrient starvation, two stereoisomers of β-carotene, all-trans and 9-cis β-carotene, accumulated, reaching up to 10% of the cell's dry weight, with the pigment being deposited into plastid lipid globules as the sequestering structure (Ben Amotz et al., 1982, 1988; Ben Amotz and Avron, 1983; Jeminez and Pick, 1994). These lipid structures are stabilized and maintained in size by a peripherally associated 38-kD protein (Carotene globule protein, Cpg), its formation induced in parallel with β-carotene accumulation (Katz et al., 1995). Although these carotene-rich algal plastids still possess thylakoids and perform photosynthetic reactions (Vorst et al., 1994) and thus do not represent true chromoplasts, the underlying structural basis for carotene accumulation is well preserved. Therefore, we considered D. bardawil to be a suitable model system for investigating the above-mentioned interdependence.
With this system we wished to address the causality question, i.e. whether β-carotene accumulation is a consequence of enhanced lipid production. Providing a sequestering structure could, for instance, remove the β-carotene end product from the biosynthetic machinery in the membrane, leading to enhanced reaction velocities and thus contributing to enhanced carotene formation. The results of the molecular analyses presented here substantiate the validity of such a sequestering hypothesis.
MATERIALS AND METHODS
Growth and Induction Conditions
Dunaliella bardawil strain 30861 (kindly provided by U. Pick, Rehovot, Israel) was cultivated in an artificial hypersaline medium (Ben Amotz, 1975). The algae were grown at 25°C in 0.5-L Erlenmeyer flasks containing 200 mL of medium under continuous shaking and illumination (46 μmol m−2 s−1, 400–700 nm). For induction of β-carotene synthesis, cells can be grown either in a sulfate-free medium (MgCl2 instead of MgSO4) or by applying high light intensities (690 μmol m−2 s−1). High-light induction led to a more rapid induction compared with sulfate starvation and was therefore used in our experiments here. Cells were harvested by centrifugation at 500g for 10 min and either frozen at −70°C or used directly.
Inhibition of Fatty Acid Biosynthesis in Vivo
For inhibiting the biosynthesis of acyl lipids during the induction of β-carotene synthesis, we used the herbicide sethoxydim (2-[1-{ethoxyimino}butyl]-5-[2-{ethylthio}propyl]-3-hydroxy-2-cyclohexen-1-one; Riedel-de Haen, Seelze, Germany), which was added to cells at the onset of induction at concentrations ranging from 10 to 50 μm. Alternatively, the antibiotic cerulenin (2,3-epoxy-4-oxo-7,10-dodecadienamide; Sigma) was used at final concentrations of 0.5 to 8 μm.
Incubation Assays and Analysis of Reaction Products
Lipids were radioactively labeled in vivo by adding 138.73 kBq NaH14CO3 (20.4 MBq mmol−1, Amersham) to cultures at the onset of high-light treatment. After 48 h the cells were pelleted (500g, 10 min) and lipids were extracted by the addition of 2 mL of chloroform:methanol (2:1, v/v). The lipids were then separated by two-dimensional TLC (first dimension, chloroform:methanol:water, 65:25:4 [v/v/v]; second dimension, chloroform:methanol:ammonium hydroxide [25% solution]:isopropylamine, 65:35:5:0.5 [v/v/v/v]) and visualized by spraying the plates with rhodamine-tinopal (Kleinig and Lempert, 1970). The identification of individual lipids was based on co-chromatography with known standards (Kleinig and Liedvogel, 1978). The separation between chlorophylls and carotenoids was achieved with a second system consisting of silica gel plates developed with the solvent system hexane:diethylether:formic acid, 80:20:2 (v/v/v). Aliquots of individual lipids scraped off from the plates were mixed with 5 mL of scintillation fluid (Rotiszint-Ecoplus, Roth, Karlsruhe, Germany) and 0.2 mL of 1 n HCl to prevent the phosphorescence caused by the rhodamine reagent.
For [1-14C]acetate labeling, cells were resuspended in buffer containing 100 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 1 mm dithioerythritol, 0.5 m sorbitol, and 2 mm ATP. The standard assay contained (in 0.5 mL) 1.7 mg of protein and 37 kBq [1-14C]acetate (2.07 GBq mmol−1, Amersham). Incubation was carried out at 25°C for 2 h. The reaction was stopped by adding 2 mL of chloroform:methanol, 2:1 (v/v). After the sample was mixed and phase separated by brief centrifugation, the acetate incorporation into lipids was quantified by liquid-scintillation counting.
To determine acylation activities during induction, cells were lysed in buffer containing 0.25 mm Mops-NaOH, pH 7.4, and incubated in 0.5 mL of 1.5 mg of protein in the presence of 0.4 mm oleoyl-CoA, 0.4 mm palmitoyl-CoA, and 9.25 kBq [U-14C]glycerol-3-phosphate (5.81 GBq mmol−1; Amersham). After incubation for 1 h at 26°C, 1 mL of a solution containing 1 m KCl in 0.2 m phosphoric acid (Hajra, 1974) was added. Lipids were extracted with 2 mL of chloroform:methanol, 2:1 (v/v), and separated on silica gel plates (Kieselgel GF254, Merck, Darmstadt, Germany) using petroleum ether:chloroform:acetone, 50:20:2 (v/v/v). Individual bands were scraped off and quantified by liquid-scintillation counting, as above.
For in vitro formation of isoprenoids, cells were lysed in buffer (as for acetate labeling) by short ultrasonication and incubated for 6 h at 27°C in 1 mL in the presence of 3 mm ATP, 10 mm MgCl2, 1 mm MnCl2, and 28 kBq [1-14C]IPP (1.96 GBq mmol−1; Amersham). The reaction products were extracted with chloroform:methanol, 2:1 (v/v), and analyzed by HPLC as described by Beyer and Kleinig (1992).
For measuring acetyl-CoA carboxylase activity, cells were resuspended in 30 mL of buffer containing 0.1 m Tris-HCl, pH 8.0, 20 mm mercaptoethanol, 1 mm EDTA, 0.5% Triton X-100, 1 mm benzamidine, 10 mm MgCl2, and 20% glycerol. The suspension was sonicated and centrifuged at 27,500g for 30 min at 8°C. The supernatant was precipitated with (NH4)2SO4 between 35 and 40% saturation. The pellet was finally resuspended in a small volume of buffer containing 0.1 m Tricine-KOH, pH 8.0, 2.5 mm MgCl2, 50 mm KCl, and 1 mm DTT. Acetyl-CoA carboxylase activity was assayed according to the method of Nikolau et al. (1981) in 0.5 mL of buffer containing additionally 0.5 mm ATP, 138.75 kBq NaH14CO3 (20.4 MBq mmol−1, Amersham), and 0.3 mm acetyl-CoA. The sample was preincubated at 30°C for 3 min and the reaction started by adding acetyl-CoA. The incubation was carried out at 30°C for 20 min. The reaction was stopped by adding 50 mL of 6 n HCl and vigorously mixing. Aliquots of 100 mL were dried on 2- × 2-cm squares of Whatman 3M paper. The radioactivity representing the incorporation into acid-stable products was determined by scintillation counting. Inhibition experiments were carried out using sethoxydim or cerulenin at 50 μm and 8 mm, respectively.
[35S]Met Labeling of Inhibited Cells
Uninhibited and (sethoxydim- or cerulenin-) inhibited cells were pelleted as above, washed twice with medium, and resuspended in Met assay medium (Difco, Detroit, MI). The cultures were labeled with 37 kBq [35S]Met (111,000 GBq mmol−1; Amersham) for 1 h. The proteins were precipitated (Chua, 1980) and separated by SDS-PAGE (see below). The dried gel was then exposed to film for autoradiography. An aliquot of each sample was quantified by scintillation counting.
Analytical Methods
Carotenes and Chlorophylls
Chlorophyll was assayed photometrically according to the method of Arnon (1949). β-Carotene was quantified photometrically using as a molar extinction coefficient ε450 nm = 134,500 L mol−1 cm−1.
Lipids and Fatty Acids
Fatty acid residues were estimated photometrically after saponification (1 mL of 10% KOH in ethanol for 2 h) according to the method described by Duncombe (1963). Individual fatty acids were identified after methylation using diazomethane by GLC (Kleinig et al., 1986).
Proteins
Soluble and membrane-bound proteins were precipitated with 10% TCA in 80% acetone (Chua, 1980) and quantified (Schacterle and Pollack, 1973). SDS-PAGE was performed on 10 to 12% polyacrylamide gels according to the method of Laemmli (1970). For western analysis the proteins were electrotransferred semidry onto nitrocellulose membranes using a LKB-Multiphor apparatus (LKB/Pharmacia, Freiburg, Germany). Blots were examined by staining reversibly with 0.02% (w/v) Ponceau S in 3% (v/v) TCA. For immunostaining and detection we used the enhanced chemiluminescence detection system (Amersham) according to the manufacturer's instructions. The primary antibodies anti-phytoene synthase and anti-phytoene desaturase raised against the overexpressed proteins (Al-Babili et al., 1996; Schledz et al., 1996), both from Narcissus pseudonarcissus, were used.
Prenylphosphates
Prenylphosphates were converted to the corresponding alcohols by the addition of 50 mL of alkaline phosphatase (1400 units mL−1) in 1 mL of lysed cells resuspended in buffer using a closed-loop device (Grob and Zürcher, 1976). The incubation took place for 12 h at room temperature with vigorous mixing. The resulting products were analyzed by GLC (Mettal et al., 1988).
DNA/RNA Techniques
Standard molecular biological techniques were carried out according to the method of Sambrook et al. (1989). RNA was isolated from frozen cells (100 mg) harvested at different stages of induction and isolated according to the method of Chirgwin et al. (1979). The RT-PCR assay was performed in an incubation buffer containing 5 mm MgCl2, 50 mm KCl, 50 mm Tris-HCl, pH 8.3, 2 mm deoxyribonucleotide triphosphates, 10 mm DTT, 100 ng of the respective downstream primer, and 20 units of Superscript II (GIBCO-BRL) at 42°C for 30 min in a total volume of 10 mL. The reaction was stopped by heating the samples to 94°C for 5 min. The tubes were transferred to ice and 40 mL of precooled PCR master mixture containing 50 mm KCl, 1.5 mm MgCl2, 10 mm Tris-HCl, pH 9.0, 100 ng of upstream primer, and 0.5 units of Taq polymerase (Pharmacia) was added. PCR was carried out for 28 cycles of the following program: 1 min at 93°C, 1 min at 55°C, and 1 min at 72°C. For quantitation of relative transcript levels, different amounts of RNA (100, 33, and 11 ng/μL) for each induction time were subjected to the RT-PCR protocol described above. Quantitation was as described by Giuliano et al. (1993).
RESULTS
Effect of High Light Intensity on β-Carotene Overproduction and Lipid Deposition
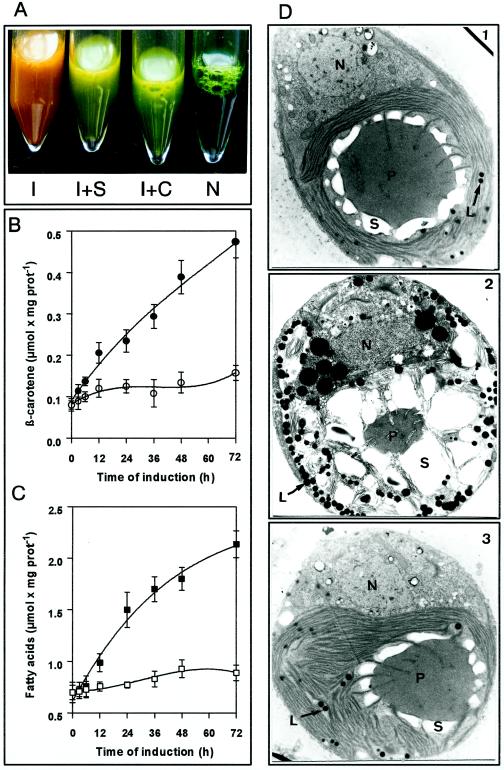
D. bardawil cells turn orange due to massive β-carotene formation when cultivated under high light intensities or under sulfate or nitrate starvation (Ben Amotz et al., 1982). Figure 1 shows the response of the cells to a high-light stress situation for 72 h. On the ultrastructural level (Fig. 1D, 1 and 2), a reduction of thylakoid membranes, an increase in starch granules, and a large accumulation of lipid droplets can be observed. These droplets, when isolated by flotation, contained triacylglycerol as the main lipid constituent (triacylglycerols, 63%; polar lipids, 12%; carotene, 35%; Fried et al., 1982) and proved to be red due to the presence of β-carotene.
Figure 1.
Light induction of D. bardawil cells. A, N, noninduced cells; I, cells induced by high-light treatment; I + S, induced cells in the presence of 50 μm sethoxydim; I + C, induced cells in the presence of 8 μm cerulenin. All samples shown represent a concentrated cell culture harvested 48 h after the onset of the induction. B, Time course of β-carotene formation. ○, Control cells; •, induced cells. The data are the mean of two replicates. Vertical bars indicate ± se. prot, Protein. C, Time course of lipid formation (measured as total fatty acids upon saponification). □, Control cells; ▪, induced cells. The data are the means of two replicates. Vertical bars indicate ± se. prot, Protein. D, Electron micrographs of noninduced cells (1), induced cells after 72 h (2), and induced cells in the presence of 8 μm cerulenin after 72 h (3). N, Nucleus; P, pyrenoid; S, starch; L, lipid globules.
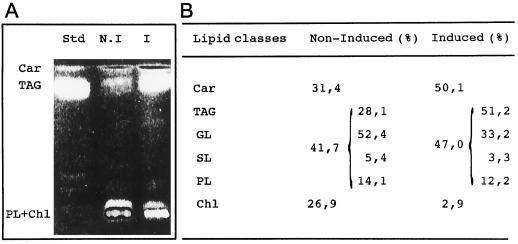
We also noted the parallel accumulation of β-carotene and acyl lipids (as total fatty acids liberated upon saponification; Fig. 1, B and C); both increase by a factor of 3 to 4 relative to protein. HPLC analysis showed that β-carotene was the major carotenoid, approximately 42% being all-trans and 48% 9-cis β-carotene; lutein was also present to a significant extent (data not shown). It turned out that the only lipid class that increased during the 72 h of high-light-stress conditions was the triacylglycerols (Fig. 2A), which increased from 0.196 μmol mg−1 protein in noninduced cells at the beginning of the experiment to 0.882 μmol mg−1 protein in induced cells after 72 h. This specific increase was further confirmed by in vivo incubation of induced and noninduced cells with NaH14CO3 for 48 h and subsequent analysis of the distribution of radioactivity in the lipid classes by two-dimensional TLC (Fig. 2B). The labeling of triacylglycerol dramatically increased relative to the other lipids, whereas the labeling of the plastid membrane galactolipids decreased relatively, as expected.
Figure 2.
Analysis of lipid classes formed after 48 h of high-light treatment. A, TLC results. Std, Standard (tripalmitoylglycerol); N.I, noninduced cells; I, induced cells. Car, β-carotene; TAG, triacylglycerol; PL + Chl, phospholipids + galactolipids + chlorophyll. B, Distribution of radioactivity from NaH14CO3 (incubation time, 48 h) into different lipid classes in control cells and in induced cells. Car, β-Carotene; GL, galactolipid; SL, sulfolipid; PL, phospholipid; and Chl, chlorophyll.
Inhibition of Fatty Acid Biosynthesis Reduces β-Carotene Accumulation
As discussed above, sequestering β-carotene as the final product of the membrane-bound carotene biosynthetic pathway into a spatially separated site may provide a mechanism to accelerate its biosynthesis. To test for this possibility, cells were induced in the presence of two differentially acting inhibitors of the lipid biosynthetic pathway: sethoxydim, an inhibitor of the enzyme acetyl-CoA carboxylase (Burton et al., 1987; Lichtenthaler, 1990), and cerulenin, which inhibits β-ketoacyl-(acyl carrier protein) synthases (Von Wettstein-Knowles, 1995).
To avoid interference with cell viability, these compounds were added to the medium at minimal inhibitory concentrations (50 mm sethoxydim or 8 mm cerulenin). At these concentrations the cells were motile and appeared vital by microscopic inspection during the time course of the experiments. As judged by the incorporation of l-[35S]Met, the inhibitors also did not significantly interfere with protein biosynthesis (not shown); similar results for cerulenin have been described elsewhere (Nomura et al., 1972).
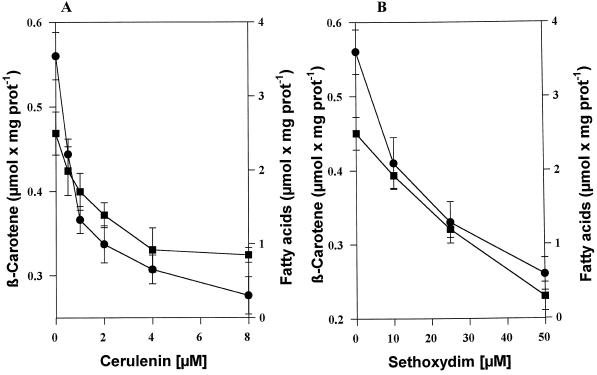
The first effect of both inhibitors was that the treated cells failed to turn orange upon stress (Fig. 1A). Electron microscopic examination revealed that osmiophilic globules were nearly absent in induced inhibitor-treated cells (Fig. 1D3); in fact, they were almost indistinguishable from noninduced cells (Fig. 1D1). Only the increase in size and the more rounded cell shape were maintained, indicating the induction process. The chemical analysis of the induced inhibitor-treated cells again revealed a perfect parallelism between triacylglycerol deposition and the capacity for β-carotene accumulation (Fig. 3); both rapidly decreased with increasing inhibitor concentrations.
Figure 3.
Cerulenin (A) and sethoxydim (B) inhibit high-light-induced β-carotene accumulation. Cells were induced by high light and cultured in the absence (control cells) or presence of different concentrations of inhibitors for 48 h and then analyzed. ▪, β-Carotene; •, total fatty acids. The data represent the means of three values ± se. prot, Protein.
These results show that the inhibition of the formation of sequestering structures strongly affects β-carotene overproduction. In control experiments performed with D. bardawil and N. pseudonarcissus chromoplasts no inhibitory effect on the carotene biosynthetic pathway was noted for either compound in vitro (Table I). The carotene pathway as a whole seemed to be arrested, since in vivo there was no accumulation of intermediates such as upstream carotenes or medium- and short-chain isoprenoid diphosphates or alcohols, as analyzed using a combined closed-loop stripping/GC analysis we described previously (Mettal et al., 1988).
Table I.
The inhibitors of lipid biosynthesis, sethoxydim and cerulenin, do not interfere with carotene biosynthesis in vitro
| Assay | Incorporation into Carotenes | Lycopene | ζ-Carotene | Phytoene |
|---|---|---|---|---|
| % | ||||
| IPP | 57.5 | 35.5 | 30.3 | 34.2 |
| IPP + S | 54 | 32.6 | 31.7 | 35.7 |
| IPP + C | 53.9 | 34 | 33.2 | 32.8 |
Cell lysates (1.6 mg protein mL−1) from high-light-treated D. bardawil cells were incubated for 4 h at 27°C with 18 kBq [1-14C]IPP in the presence of sethoxydim (50 μm) or cerulenin (8 μm). Radioactive carotenes were isolated by TLC and individual carotenes were collected from HPLC and quantified by liquid scintillation counting. S, Sethoxydim; C, cerulenin.
From these findings the conclusion must be drawn that lipid accumulation and enhanced β-carotene biosynthesis are linked to each other in a causal relationship. In molecular terms, this could mean that a genetic induction of the carotene biosynthetic pathway is not necessarily required to stimulate β-carotene overproduction. Induction of the triacylglycerol biosynthetic pathway alone and the formation of a sink consisting of sequestering lipid globules may be sufficient to cause enhanced β-carotene formation. To test this hypothesis more closely, we monitored enzyme activities and investigated protein and mRNA abundance during the induction process.
Protein Abundance and Enzyme Activities
As was hypothesized above, the activities of carotenoid biosynthetic enzymes should be increased, even though their protein levels remained constant, during light treatment. To test this expectation, western analysis and incubation assays with radiolabeled precursors for triacylglycerols and carotenoids were performed in parallel. Affinity-purified antibodies raised against heterologous phytoene synthase (Schledz et al., 1996) and phytoene desaturase (Al-Babili et al., 1996) from N. pseudonarcissus cross-reacted with the corresponding proteins in D. bardawil.
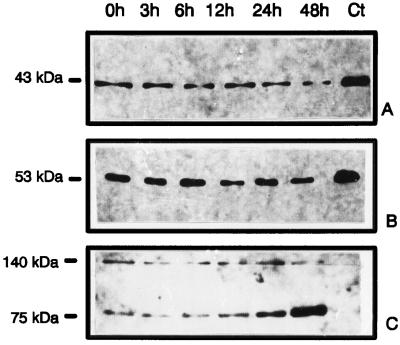
Western analysis performed with cells harvested at selected times during high-light treatment revealed that there was indeed no significant up-regulation in the protein amount of either phytoene synthase (Fig. 4A) or phytoene desaturase (Fig. 4B). In contrast, an unidentified, 75-kD, biotin-containing band, which we used as an internal marker, clearly showed an increase (Fig. 4C). For the detection of biotin-containing bands we used a streptavidin/alkaline phosphatase-detection assay. With this method, we also tried repeatedly using 7% SDS-polyacrylamide gels to demonstrate an up-regulation of the eukaryotic (sethoxydim-sensitive) form of ACCase in high-light-treated cells. This was unsuccessful with D. bardawil. Thus, two important enzymes of the carotenoid biosynthetic pathway remained at the same low levels; therefore, it must be concluded that the strong increase in carotene synthetic capacity was indeed due to enhanced reaction velocities upon induction of the cells.
Figure 4.
Western analysis showing the abundance of phytoene synthase and phytoene desaturase during high-light treatment. Equal amounts of protein (30 μg per lane) were separated and electro-transferred as described in the experimental procedures. Primary antibodies were anti-phytoene synthase antibodies (A) and anti-phytoene desaturase antibodies (B). Secondary antibodies were linked to horseradish peroxidase. Detection was performed using the enhanced chemiluminescence system. C, The biotin-containing bands showing an increase during high-light treatment represent an internal control and were detected using alkaline phosphatase-conjugated streptavidin. Ct, Control (N. pseudonarcissus chromoplast extract).
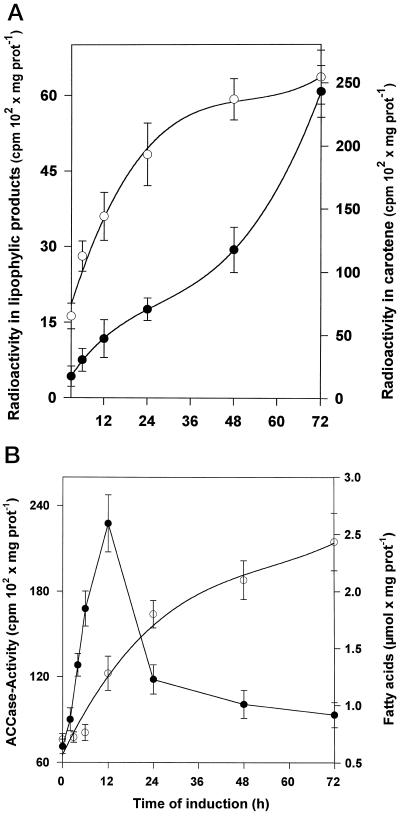
To correlate the results from western analysis to enzyme activities, incubation experiments were carried out with lysed cells in the presence of [1-14C]IPP. Additionally, incubation experiments with [1-14C]acetate were performed to investigate the lipid biosynthetic pathway in parallel (Fig. 5). In contrast to the constitutive expression of the two carotenoid biosynthetic enzymes, strong increases in specific activities of the carotene but also of the acyl lipid biosynthetic pathway were noted during 72 h of induction. Typically, in a time course of induction, the increase of acetate incorporation into acyl lipids preceded the increase of IPP incorporation into isoprenoids. A detailed analysis of the isoprenoids formed in vitro revealed that geranylgeranyl diphosphate, geranylgeraniol (liberated artificially by contaminating phosphatase action), and carotenes (from phytoene to mainly lycopene) were the main products. Only phytoene was formed in the presence of the desaturase inhibitor norflurazone (10 nm; data not shown).
Figure 5.
Time course of the in vitro synthesis of acyl lipids and prenyl lipids in D. bardawil cell lysates at various times after onset of high-light induction of cells. A, The time course of the incorporation of radioactivity into acyl lipids (○; right scale) and prenyl lipids (•; left scale) from [1-14C]acetate and [1-14C]IPP, respectively. At the times indicated, cells were lysed and incubated in the presence of 37 kBq [1-14C]acetate or 28 kBq [1-14C]IPP for 2 and 6 h, respectively. Data are the means of three values. Vertical bars indicate ± se. B, ACCase activity (•) and lipid accumulation (○; measured as total fatty acids) during high-light treatment of the cells. Values are means ± se for three repetitions. prot, Protein.
The activity profile for ACCase was investigated in more detail in incubation experiments in vitro in the presence of NaH14CO3 (Fig. 6), since this biotin-containing enzyme catalyzes a key regulatory step in lipid biosynthesis (Post-Beittenmiller et al., 1991, 1992; Page et al., 1994). A remarkably strong increase in its specific activity was noted during the first 12 h after induction, but it then declined rapidly. Similar characteristics of ACCase activation have been reported previously, e.g. during rapeseed formation (Turnham and Northcote, 1983). The ACCase activity measured in vitro was found to be 45% inhibited by 50 mm sethoxydim (data not shown). Since, as was shown above, triacylglycerols represent the main lipid constituent of lipid globules, the specific activity of glycerol-3-phosphate acyltransferases in vitro was measured using [U-14C]glycerol-3-phosphate and oleoyl-CoA as substrates. Indeed, 48 h after induction the specific activity of this enzyme had concomitantly increased about 8-fold compared with uninduced cells (not shown).
Figure 6.
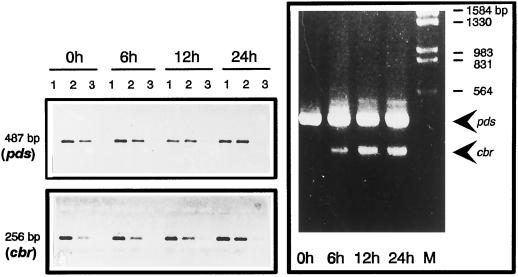
RT-PCR analysis of phytoene desaturase (pds) and CBR (cbr) mRNA expression in D. bardawil during high-light induction. Total RNA was isolated at different times after the onset of high-light treatment. Left, RT-PCR reactions were performed with 100 (lanes 1), 33 (lanes 2), and 11 ng (lanes 3) of total RNA. The 487-bp band is derived from phytoene desaturase mRNA; 256-bp bands represent the CBR mRNA. Right, The two transcripts were co-amplified using all four specific primers. M, Marker (λ-DNA digested using EcoRI/HindIII).
The data presented here show that the carotene pathway is enzymatically activated during induction, since the increase in enzymatic activity is not accompanied by an increase in abundance of either phytoene synthase or phytoene desaturase. This activation is due to triacylglycerol deposition, as was shown by the effect of two differentially acting inhibitors in the lipid biosynthetic pathway interfering with β-carotene accumulation.
Transcript Levels for Phytoene Desaturase and an Early Light-Induced-Like Protein during High-Light Treatment
To further substantiate the data on protein abundance, the changes of mRNA levels were investigated. Northern analysis using the cDNAs coding for phytoene synthase and phytoene desaturase from N. pseudonarcissus as heterologous probes were unsuccessful, probably because of the very low abundance of transcripts, although insufficient homology cannot be excluded. Therefore, as a more sensitive method to detect low transcript levels, we then used RT-PCR with primers from the sequence for phytoene desaturase, the only currently available sequence for a carotenoid biosynthetic enzyme from D. bardawil (DNA sequence kindly provided by J. Hirschberg, Jerusalem, Israel). As a control we also used specific primers for transcripts of the D. bardawil CBR mRNA; the latter mRNA codes for an early light-induced-like protein (Carotene Biosynthesis Related) and is known to be induced during high-light treatment (Lers et al., 1991). The phytoene desaturase transcript levels (Fig. 6) remained constant during high-light treatment, as was expected, whereas the CBR transcripts increased steadily. This was also evident in an RT-PCR co-amplification assay using all four primers; the bands, however, cannot be directly compared in quantitative terms. In conclusion, although β-carotene synthesis is massively induced, both the mRNA and protein steady-state concentrations for the biosynthetic enzymes remain constant.
DISCUSSION
In the present paper we addressed the question of whether, during overproduction of carotenoids in the chloroplasts of the unicellular alga D. bardawil, the formation of specialized lipophilic carotenoid sequestering structures represents a mere parallelism or whether these structures exert a positive effect on carotenoid biosynthesis by pulling the pathway toward completion. On the basis of the data presented here, we favor such a causal interdependence of structure (lipid globule) formation and enhanced carotenoid biosynthesis. Sethoxydim and cerulenin, two inhibitors targeting different enzymes (ACCase and β-ketoacyl-[acyl carrier protein] synthase, respectively) in the lipid biosynthetic pathway, both inhibited β-carotene accumulation. The β-carotene biosynthetic pathway itself proved to be insensitive toward these compounds. The underlying chemical mechanisms of such an indirect acceleration of β-carotene synthesis are still unclear and may involve favorable chemical disequilibria created by such a lipophilic sink.
Alternatively, more specific mechanisms may be involved, such as avoidance of enzymatic end-product inhibition (Bejarano et al., 1988). Thus, we propose that sequestering mechanisms contribute to the regulation of carotenoid biosynthesis, at least in D. bardawil. And, since carotenoid overproduction is generally accompanied by the development of specialized lipophilic structures, it seems realistic to assume a more general role of such a structure/activity relationship.
This must be distinguished from a second, more physical role of carotene sequestering to provide a site into which lipophilic carotenes are deposited, thus allowing their nondeleterious massive accumulation. Such an enrichment, yielding bright colors, is common in petals and fruits of higher plants displaying an ecological distribution function. The Capsicum annuum fruit represents a well-known example in which the function of globules is replaced by complex lipoprotein structures termed fibrils (Deruère et al., 1994). It must be noted that these isolated fibrils, like the D. bardawil globules, did not show any carotenogenic activity in vitro; all carotenoids bound here represented “dead-end products.” It is currently unknown what mechanism permits carotenoids to reach these structures (globules, fibrils, or crystals), which are spatially distinct from their site of formation in membranes. In this regard the simplest case seems to be present in the so-called membranous type chromoplast in N. pseudonarcissus petals, in which the plastid membranes are proliferated so that the two sites (formation and deposition) are identical.
The question is whether an up-regulation of carotenoid biosynthetic enzymes alone is sufficient to promote carotenoid accumulation or whether this is even necessary. The abundance of the two carotenoid biosynthetic enzymes examined here, phytoene synthase and phytoene desaturase, remained constant throughout high-light treatment, as did the mRNA coding for phytoene desaturase, even though incubations in vitro showed that the enzymatic activities of the pathway increased considerably. The latter assays were performed knowing that for these two enzymes the protein abundance is not necessarily correlated with enzymatic activity (Al-Babili et al., 1996; Schledz et al., 1996). The enzyme ACCase, generally regarded as a key regulatory enzyme in acyl lipid biosynthesis (for review, see Ohlrogge and Browse, 1995), was induced in its activity. Additionally, the incorporation of acetate into lipid products, the activities that drive globule formation, typically preceded β-carotene accumulation.
Taken together, these results indicate that in D. bardawil β-carotene accumulation is at least to some degree a consequence of triacylglycerol deposition. Similarly, during flower development (from closed green bulb to open flower) in N. pseudonarcissus, no strong increase of mRNA or of protein was noted for phytoene synthase or phytoene desaturase, whereas carotenoids increased and membranes proliferated, pointing to a similar regulatory mechanism (Al-Babili et al., 1996; Schledz et al., 1996). However, there is a notable up-regulation in N. pseudonarcissus flowers compared with the first green leaves. This is paralleled by findings with C. annuum chromoplasts, in which phytoene synthase and phytoene desaturase mRNAs (Hugueney et al., 1992; Römer et al., 1993) increase between the mature green fruit and the first defined ripening stage but then remain constant thereafter, not increasing subsequently in parallel to the massive increase of carotenoids occurring during later developmental stages (B. Camara, personal communication). In contrast, the formation of fibrillin, the protein constituent of fibrils, as well as the steady-state concentration of fibrillin mRNA, increases strongly during the later stages of C. annuum fruit ripening (Deruère et al., 1994). Geranylgeranyl-diphosphate synthase behaves somewhat differently, being induced in later stages (Kuntz et al., 1992). However, this enzyme is not solely devoted to carotenoid biosynthesis and can therefore not be readily considered so simply in this discussion.
Although RT-PCR data for phytoene synthase and phytoene desaturase mRNA in ripening tomato (Giuliano et al., 1993) indicate that mRNA abundance follows more closely color development, it must be taken into account that a different strategy for carotenoid accumulation is used, namely the formation of crystals. On thermodynamic grounds (no lipophilic containment), this may be the most difficult mechanism; therefore, the underlying genetic regulation may also be different.
In N. pseudonarcissus and, clearly, also in C. annuum chromoplasts, some up-regulation takes place for both phytoene synthase and phytoene desaturase in an early, green stage; thereafter, both the mRNA levels and the corresponding proteins remain more or less constant. However, as long as the valve is closed, no sequestering structures are formed as a sink, and carotenoids do not accumulate. The mechanism for carotenoid accumulation may thus be biphasic, consisting of an inductive developmental program in the first phase, in coordination with a second “pulling” and sequestering phase. In D. bardawil, the first phase is not pronounced and the second phase plays a major role, detected here as inhibited acyl lipid formation, which leads to strongly reduced β-carotene accumulation.
It must be noted that this sequestering mechanism for carotenoid accumulation represents only one of several possibilities. For N. pseudonarcissus chromoplasts, we pointed out earlier the importance of the redox state of membranes for the phytoene desaturation velocity (Nievelstein et al., 1995); moreover, the biochemical pathway for supplying the isopentenyl diphosphate substrate, which may be crucial, is still obscure.
In D. bardawil, the lipid biosynthetic pathway as a whole is increased in activity during high-light treatment, as we show here. However, ACCase is known to be tightly regulated and may be crucial. This enzyme exists either as a multifunctional, “eukaryotic” polypeptide or as multi-meric, “prokaryotic” enzymes with dissociable activities (for review, see Ohlrogge and Browse, 1995). Dicot plants are known to possess the prokaryotic type in their plastids (the site of fatty acid biosynthesis), whereas the eukaryotic type is present in this compartment in monocot plants (Sasaki et al., 1995). The two ACCase types are also distinguished by their differential susceptibility to the herbicide sethoxydim, which affects selectively the eukaryotic form (for review, see Harwood, 1988). This latter form of ACCase seems to be involved in the formation of plastid lipid globules in D. bardawil during high-light treatment. To substantiate this result, we are currently investigating ACCase molecularly.
From the inhibitor-based and molecular data presented here, and taking published data from other groups into account, we believe that there is now good evidence to support a sequestering hypothesis. In investigations using plant transformation, we are now seeking to obtain further validation by, for example, demonstrating that the overexpression of carotenoid-sequestering structures rather than the enhanced formation of carotenoid biosynthetic enzymes leads to enhanced color.
ACKNOWLEDGMENTS
We are indebted to J. Ohlrogge for helpful discussions. We also thank J. Hirschberg for providing D. bardawil phytoene desaturase DNA-sequence information. Electron microscopy was performed by V. Speth, whom we gratefully acknowledge. We would also like to thank R. Cassada, who corrected the English version of the manuscript.
Abbreviations:
- IPP
isopentenyl diphosphate
- RT
reverse transcriptase
Footnotes
This study was supported in part by the Deutscher Akademischer Austauschdienst (S.R.), by a European Molecular Biology Organization long-term fellowship (P.H.), by the German-Israeli Foundation (no. 214-240.12/91), and by the European Community BIOTECH Program.
LITERATURE CITED
- Al-Babili S, v Lintig J, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9: 601–612 [DOI] [PubMed]
- Arnon DJ (1949) Copper enzymes in isolated chloroplasts. Polyphenyoxydase in Beta vulgaris. Plant Physiol 42: 1–15 [DOI] [PMC free article] [PubMed]
- Bejarano ER, Parra F, Murillo FJ, Cerdá-Olmedo E. End-product regulation of carotenogenesis in Phycomyces. Arch Microbiol. 1988;150:209–214. [Google Scholar]
- Ben Amotz A. Adaptation of the unicellular alga Dunaliella parva to saline environment. J Phycol. 1975;11:50–54. [Google Scholar]
- Ben Amotz A, Avron M (1983) On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72: 593–597 [DOI] [PMC free article] [PubMed]
- Ben Amotz A, Katz A, Avron M. Accumulation of β-carotene in halotolerant algae: purification and characterization of β-carotene rich globules from Dunaliella bardawil (Chlorophyceae) J Phycol. 1982;18:529–537. [Google Scholar]
- Ben Amotz A, Lers A, Avron M (1988) Stereoisomers of β-carotene and phytoene in the alga Dunaliella bardawil. Plant Physiol 86: 1286–1291 [DOI] [PMC free article] [PubMed]
- Beyer P, Kleinig H. Analysis of the formation of carotene chromophore and ionone rings in Narcissus pseudonarcissus L. chromoplast membranes. Methods Enzymol. 1992;213:62–74. [Google Scholar]
- Burton JD, Gronwald JW, Somer DA, Connely JA, Gengenbach BG, Wyse DL (1987) Inhibition of plant acetyl-CoA carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun 148: 1039–1044 [DOI] [PubMed]
- Camara B, Hugueney P, Bouvier F, Kuntz M, Monéger R. Biochemistry and molecular biology of chromoplast development. Int Rev Cytol. 1995;163:175–247. doi: 10.1016/s0074-7696(08)62211-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chua NH (1980) Electrophoretic analysis of chloroplast proteins. Methods Enzymol 69: 434–436
- Deruère J, Römer S, d'Harlingue A, Backhaus RA, Kuntz M, Camara B. Fibril assembly and carotenoid overaccumulation in chromoplasts: a model for supramolecular lipoprotein structures. Plant Cell. 1994;6:119–133. doi: 10.1105/tpc.6.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncombe WG. The colorometric micro-determination of long-chain fatty acids. Biochem J. 1963;88:7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank HA, Cogdell RJ (1996) Carotenoids in photosynthesis. Photochem Photobiol 63: 257–264 [DOI] [PubMed]
- Fried A, Tietz A, Ben-Amotz A, Eichenberger W (1982) Lipid composition of the halotolerant alga Dunaliella bardawil. Biochim Biophys Acta 713: 419–426
- Giuliano G, Bartley GE, Scolnik P. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grob K, Zürcher F. Stripping of trace organic substances from water; equipment and procedure. J Chromatogr. 1976;177:285–294. [Google Scholar]
- Hajra AK. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974;9:502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Harwood JL. The site of action of some selective graminaceous herbicides is identified as acetyl-CoA carboxylase. Trends Biochem Sci. 1988;13:330–331. doi: 10.1016/0968-0004(88)90100-4. [DOI] [PubMed] [Google Scholar]
- Hugueney P, Römer S, Kuntz M, Camara B (1992) Characterization and molecular cloning of a bifunctional flavoprotein catalyzing the synthesis of phytofluene and ζ-carotene in Capsicum chromoplasts. Eur J Biochem 209: 399–407 [DOI] [PubMed]
- Jeminez C, Pick U (1994) Differential stereoisomer composition of β,β-carotene in thylakoids and in pigment globules in Dunaliella. J Plant Physiol 143: 257–263
- Katz A, Jeminez C, Pick U (1995) Isolation and characterization of a protein associated with carotene globules in the alga Dunaliella bardawil. Plant Physiol 108: 1657–1664 [DOI] [PMC free article] [PubMed]
- Kleinig H, Beyer P, Schubert C, Liedvogel B (1986) Cyanophora paradoxa: fatty acids and fatty acid synthesis in vitro. Z Natur-forsch 41c: 169–171
- Kleinig H, Lempert U. Phospholipid analysis on a micro scale. J Chromatogr. 1970;53:595–597. doi: 10.1016/s0021-9673(01)98523-1. [DOI] [PubMed] [Google Scholar]
- Kleinig H, Liedvogel B. Fatty acid synthesis by isolated chromoplasts from the daffodil. [14C]Acetate incorporation and distribution of labeled acids. Eur J Biochem. 1978;83:499–505. doi: 10.1111/j.1432-1033.1978.tb12116.x. [DOI] [PubMed] [Google Scholar]
- Kuntz M, Römer S, Suire C, Hugueney P, Weil JH, Schantz R, Camara B. Identification of a cDNA for the plastid-located geranylgeranyl pyrophosphate synthase from Capsicum annuum: correlative increase in enzyme activity and transcript level during fruit ripening. Plant J. 1992;2:25–34. doi: 10.1111/j.1365-313x.1992.00025.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lers A, Levy H, Zamir A (1991) Co-regulation of a gene homologous to early light-induced genes in higher plants and β-carotene biosynthesis in the alga Dunaliella bardawil. J Biol Chem 266: 13698–13705 [PubMed]
- Lichtenthaler HK. Mode of action of herbicides affecting acetyl-CoA carboxylase and fatty acid biosynthesis. Z Naturforsch. 1990;45c:521–528. [Google Scholar]
- Mettal U, Boland W, Beyer P, Kleinig H. Biosynthesis of monoterpene hydrocarbons by isolated chromoplasts from daffodil flowers. Eur J Biochem. 1988;170:613–616. doi: 10.1111/j.1432-1033.1988.tb13741.x. [DOI] [PubMed] [Google Scholar]
- Nievelstein V, Vandekerckhove J, Tadros M, von Lintig J, Nitschke W, Beyer P. Carotene desaturation is linked to a respiratory redox pathway in Narcissus pseudonarcissus chromoplast membranes: involvement of an OEC23-like protein. Eur J Biochem. 1995;233:864–872. doi: 10.1111/j.1432-1033.1995.864_3.x. [DOI] [PubMed] [Google Scholar]
- Nikolau BJ, Hawke JC, Slack CR. Acetyl-coenzyme A carboxylase in maize. Arch Biochem Biophys. 1981;211:605–612. doi: 10.1016/0003-9861(81)90495-1. [DOI] [PubMed] [Google Scholar]
- Nomura S, Horiuchi T, Omura S, Hata T. The action mechanism of cerulenin. J Biochem. 1972;71:783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RA, Okada S, Harwood JL. Biochim Biophys Acta. 1994;1210:369–372. doi: 10.1016/0005-2760(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski JG, Ohlrogge JB (1991) In vivo pools of free and acylated acyl carrier proteins in spinach: evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem 266: 1858–1865 [PubMed]
- Post-Beittenmiller D, Roughan PG, Ohlrogge JB. Regulation of plant fatty acid biosynthesis: analysis of acyl-CoA and acyl-ACP substrate pools in spinach and pea chloroplasts. Plant Physiol. 1992;100:923–930. doi: 10.1104/pp.100.2.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer S, Huegeney P, Bouvier F, Camara B, Kuntz M (1993) Expresiion of the genes encoding the early carotenoid biosynthetic enzymes in Capsicum annuum. Biochem Biophys Res Commun 196: 1414–1421 [DOI] [PubMed]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sasaki Y, Konishi T, Yukio N. The compartmentation of acetyl-coenzyme A carboxylase in plants. Plant Physiol. 1995;108:445–449. doi: 10.1104/pp.108.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle GR, Pollack RC. A simplified method for the quantitative assay of small amounts of protein in biological material. Anal Biochem. 1973;51:654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schledz M, Al-Babili S, v Lintig J, Haubruck H, Rabbani S, Kleinig H, Beyer P (1996) Phytoene synthase from Narcissuspseudonarcissus: functional expression, galactolipid requirement, topological distribution in chromoplasts and induction during flower development. Plant J 10: 781–792 [DOI] [PubMed]
- Turnham T, Northcote DH. Changes in the activity of acetyl-CoA carboxylase during rape-seed formation. Biochem J. 1983;212:223–229. doi: 10.1042/bj2120223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Wettstein-Knowles P. The secretive family of β-ketoacyl-ACP synthases. In: Kader JC, Mazliac P, editors. Plant Lipid Metabolism. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 61–71. [Google Scholar]
- Vorst P, Baard RL, Mur LR, Korthals HJ, van der Ende H (1994) Effect of growth arrest on carotene accumulation and photosynthesis in Dunaliella. Microbiology 140: 1411-1417