Abstract
Recent-infection testing assays/algorithms (RITAs) have been developed to exploit the titer and avidity of HIV antibody evolution following seroconversion for incidence estimation. The Vitros Anti-HIV 1+2 assay (Ortho-Clinical Diagnostics) was approved by the FDA to detect HIV-1 and HIV-2 infections. We developed a less-sensitive (LS) and an avidity-modified version of this assay to detect recent HIV infection. Seroconversion panels (80 subjects, 416 samples) were tested to calculate the mean duration of recent infection (MDR) for these assays. A panel from known long-term (2+ years) HIV-infected subjects on highly active antiretroviral therapy (HAART) (n = 134) and subjects with low CD4 counts (AIDS patients [n = 140]) was used to measure the false-recent rate (FRR) of the assays. Using a signal-to-cutoff ratio of 20 and the LS-Vitros assay gave a RITA MDR of 215 days (95% confidence interval [95% CI], ±65 days) and using an avidity index (AI) of 0.6 gave an MDR of 170 days (±44 days), while a combination of the two assays yielded a MDR of 146 days (±38.6) and an FRR of 8%. Misclassifying subjects with known long-term infection as recently infected occurred in 14% of AIDS patients and 29% (95% CI, 22, 38) of HAART subjects and 3% (95% CI, 0.8, 7.2) and 42% (95% CI, 33, 51), respectively, for the LS- and avidity-modified Vitros assays, with a misclassification rate of 15% (95% CI, 11, 20) overall using a dual-assay algorithm. Both modified Vitros assays can be used to estimate the length of time since seroconversion and in calculations for HIV incidence. Like other RITAs, they are subject to high FRR in subjects on HAART or with AIDS.
INTRODUCTION
While the detection of recent HIV infections is useful for individual patient counseling and management, it is essential for calculating the HIV incidence in a population. Monitoring incidence is important for tracking the epidemic and essential in evaluating the need for and effectiveness of HIV prevention programs (2). Incidence calculations can be done through longitudinal studies of cohorts or serial cross-sectional population serological surveys; these can be difficult and expensive to conduct and are prone to biases (1, 4, 15). To overcome this limitation, cross-sectional incidence testing was devised to capture individuals who were acutely infected; however, the short mean duration of recent infection (MDR) of detecting infection limited the effectiveness of this method (3). By diluting plasma samples from HIV-infected individuals and lengthening the MDR of detection of the assay, a larger number of recently infected individuals could be identified in HIV antibody detection assays (18). HIV investigators and public health laboratories developed the serological testing algorithm for recent HIV seroconversion (STARHS) to differentiate recent from established HIV infection in cross-sectional cohorts (17). The early approaches of the STARHS algorithm used a sensitive HIV antibody test to accurately identify individuals who were infected within weeks of infection, followed by a less-sensitive (LS) anti-HIV test to identify individuals who were still early in infection with evidence of evolving seroconversion (18, 25). The quantitative nature of the LS assay enables calculation of a mean duration of recent infection using seroconversion panels to determine the duration of time between HIV seroconversion and the time at which a given threshold of the assay is attained (2). The rate of detection of persons with recent infection in a screened population can then be used to interpolate population incidence rates (18). Currently, there are no tests approved by the FDA for detecting recent HIV infection.
One of the main problems with using serological assays for identifying recent infection is the detection of “false-recent” infections as a result of low-titer or low-affinity binding antibodies (13, 22). For individuals who are on highly active antiretroviral therapy (HAART) or who are naturally able to control the virus, such as “elite controllers” (ECs), there is a loss of circulating antigen or virus to boost antibody production (8). Additionally, low-titer or low-affinity binding antibodies can occur following waning immunity in individuals with low CD4 count or clinical AIDS (12). To try to exclude these individuals from incident infection calculations, other parameters such as antibody avidity have been assessed to detect individuals who are truly recently infected. These parameters can be combined into algorithms known as recent-infection testing algorithms (RITAs) (4). Laboratory methods for incorporation into accurate RITAs are now the focus of intensive study. Performance metrics include (i) the mean duration of recent infection intervals at various thresholds of detection, and (ii) the ability of these assays/algorithms to correctly discriminate individuals who are truly recently infected from false-recent misclassifications (“false-recent rates” or FRR) (9).
One problem with establishing an algorithm for recent HIV acquisition is the life span of the test used. Manufactured products for HIV detection have been in constant flux; whether due to manufacturer discontinuation or changes in availability, the assays used for recency testing need constant updating. Our primary test for recent HIV detection was the LS or “detuned” Vironostika assay; however, the production of this assay was discontinued early in 2008. Other research groups used the BED capture enzyme immunoassay (CEIA) for testing recent infection; however, after comparison of longitudinal incidence results to cross-sectional BED CEIA calculated incidence, it was determined that the assay was misclassifying recent infection and required analytical correction for this (14, 16). Since then, we and other researchers have been faced with making the transition to another assay for the detection of recent infection and the need to calibrate and validate new RITA assays.
In March 2008, Ortho-Clinical Diagnostics announced that the U.S. Food and Drug Administration (FDA) approved the Vitros Anti-HIV 1+2 assay (a registered trademark of Ortho-Clinical Diagnostics) as a new diagnostic assay for the detection of antibodies to HIV types 1 and/or 2 (anti-HIV-1 and/or anti-HIV-2). The Vitros platform is fully automated, with assay completion in less than 50 min. The system uses proprietary enhanced chemiluminescence (ECi) detection technology to provide excellent assay performance characteristics, including high sensitivity for early seroconversion. We present an evaluation of adapting the Vitros HIV assay to identify individuals with recent infection. These adaptations included a titer using diluted samples and an antibody avidity test using a guanidine-incubated sample to dissociate weakly bound antibodies. We reasoned that it may be possible that the LS- and avidity-modified Vitros methods could be used in concert in order to improve detection of individuals with recent HIV infection and reduce the misdiagnosis of recent infection in AIDS patients or HAART-treated patients with low antibody titers.
HIV-positive specimens, previously tested with other sensitive and less-sensitive assays, were used to calibrate the results of the modified Vitros assays, the LS- and avidity-modified Vitros assays, relative to the results from the Vironostika LS-EIA and BED capture enzyme immunoassay (BED CEIA) assays. Using specimens from individuals with recent and long-term HIV infection, we performed sample titrations and identified the optimal dilution that gave the best correlation with Vironostika LS-EIA and BED assays. By comparing diluted-sample results to the results of previous less-sensitive HIV-1 testing methods, we determined a threshold corresponding to the cutoffs for recent HIV as determined by the Vironostika LS-EIA and BED assays. This comparative method has been previously used during conversion to newer incidence assays (25). Once the assay conditions were established, we were able to calculate the MDR for these assays at various cutoffs using panels from a cohort of individuals sampled during seroconversion. This series of samples consisted of 80 individuals with 3 or more serial samples taken during the seroconversion period. These samples were tested by both less-sensitive and avidity-modified Vitros assays to calculate the MDR for these assays. A statistical analysis was performed, and the MDR was calculated for a number of cutoffs. We also evaluated the frequency of false-recent misclassifications based on analysis of samples from patients with long-standing HIV infections who were on suppressive HAART therapy and patients with clinical AIDS and low CD4 levels.
MATERIALS AND METHODS
Clinical samples.
All specimens evaluated in the current study were confirmed seropositive plasma samples that had been stored at −80°C and then thawed overnight at 4°C or at room temperature prior to analysis. Samples from HIV-positive repeat blood donors from the American Red Cross (n = 86) with documented seroconversion within the past year were used to calibrate the dilution and assay cutoff conditions for the LS-Vitros assay. The dilution and cutoffs were then used to test a larger group of first-time and repeat HIV-positive blood donors (n = 710) who had been previously tested by the BED and LS-Vironostika RITA assays. To quantify the recent infection MDR of these assays, we tested 359 serial blood samples from 71 subjects enrolled in the HIVNET study (5). To supplement these samples, we tested serial samples from an additional nine subjects (57 samples) identified at well-defined stages of very early infection according to the Fiebig algorithm and monitored with monthly and quarterly blood sample collections for 15 months. These subjects were a subset of the individuals in a cohort of 19 sexually transmitted disease (STD) clinic attendees collected between 2009 and 2011 for the Centers of Disease Control and Prevention (CDC). To investigate the ability of the assay to correctly discriminate recent from long-term infections, we tested samples from patients who were chronically infected with HIV (21). These patients had well-documented long-term HIV infections and were either on HAART (n = 134) or had low CD4 counts consistent with an AIDS diagnosis (n = 140); these samples were tested to discern whether the assay could correctly identify long-term infection. All samples were preexisting and were tested without linkage to specific subject identifiers (Table 1). This project was approved by the University of California, San Francisco (UCSF) Committee for Human Research.
TABLE 1.
Demographic and clinical characteristics of study subjects
| Group or panel and characteristic | Value for characteristic |
|---|---|
| Blood donorsa | |
| Total no. | 710 |
| No. of first-time donors | 624 |
| No. of repeat blood donors with known interdonation interval | 86 |
| Seroconversion panelb | |
| No. of individuals | |
| Total | 80 |
| HIVNET study | 71 |
| SIPPc | 9 |
| Treated SIPPd | 9 |
| No. of samples | |
| Total | 416 |
| HIVNET study | 359 |
| SIPP | 59 |
| Treated SIPP | 90 |
| Median no. of days followed postinfection (IQRe) | |
| HIVNET study | 499 (350–782) |
| SIPP | 328 (259–366) |
| Treated SIPP | 478 |
| Challenge panelf | |
| No. of total individuals with long-term infection | 274 |
| HAART-treated individuals | |
| No. of individuals | 134 |
| CD4 count, median no. (IQR) | 628 (560–737) |
| Viral load, median no. (IQR) | <50 (NAg) |
| AIDS patients | |
| No. of individuals | 140 |
| CD4 count, median no. (IQR) | 18 (7–33) |
| Viral load, median no. (IQR) | 83,817 (31,972–261,939) |
Used for calibration to other RITA assays.
Longitudinal panels of early HIV infection used for calculation of MDR and to calculate the false-recent rate (5).
SIPP, SeroIncidence Panel Project at UCSF.
Not included in MDR calculations.
IQR, interquartile range.
Long-term infection cohort samples used to challenge assays in false-recent rate detection (21).
NA, not available.
Ortho Vitros HIV 1+2 assay.
The Vitros Anti-HIV 1+2 assay (a registered trademark of Ortho-Clinical Diagnostics, Rochester, NY) contains four recombinant antigens (HIV-1 Env 13, HIV-1 Env 10, HIV-1 p24, and HIV-2 Env AL) derived from HIV-1 core, HIV-1 envelope, and HIV-2 envelope proteins. The assay uses a two-stage reaction: HIV antibodies present in the sample bind with HIV recombinant antigens coated on the wells, and after unbound antibodies in the sample were removed by washing, horseradish peroxidase (HRP)-labeled recombinant HIV antigens are added in the conjugate reagent. The conjugate binds specifically to any human anti-HIV-1 or anti-HIV-2 (IgG and IgM) captured on the well in the first stage. The bound HRP conjugate was measured by a luminescence reaction, the intensity of which is indicative of the level of anti-HIV-1 and anti-HIV-2 present in the clinical sample.
Optimization assays.
To optimize the best dilution required for the LS-modified application, a titration was performed that included undiluted and diluted plasma samples. The diluted plasma samples were diluted 1:100, 1:400, 1:1,000, and 1:4,000 and had been previously tested by BED and Vironostika LS-EIA. Using a regression analysis, the LS-Vitros was calibrated to the BED and LS-EIA assays at each dilution. The optimal dilution that gave the highest correlation coefficient for both LS-EIA and BED was selected for further study.
LS-Vitros assay.
The optimized version of the LS-Vitros assay uses a 1:400 dilution of HIV-positive sample in normal human plasma, with the assay run in duplicate. In the early stages of LS-modified assay development, the dilution of the HIV-positive plasma sample in normal, nonreactive human plasma was prepared using a two-step (diluted 1:20 twice) manual dilution. However, automation of this step is possible by transferring this dilution step to the Vitros platform after further development of system programming. Diluent plasma was purchased from Seracare (Milford, MA) or obtained from Blood Centers of the Pacific (San Francisco, CA) and prepared by filtration and testing against prior lots of plasma to test for consistency based on results of testing calibrators using the LS-Vitros assay. After the samples were diluted, the fully automated Vitros ECi robot took 49 min to run each sample in the assay and to report the results as signal-to-cutoff (S/C) values. The Vitros ECi robot has the capacity to run 70 samples per hour, while newer models have much higher throughput. Using samples previously tested by Vironostika, we identified potential S/C values for optimal discrimination of recent infection.
Avidity-modified Vitros assay.
The avidity-modified Vitros assay was performed using methods similar to those previously published for an HIV avidity assay developed using the AxSYM automated system (6, 20). In brief, 1 M guanidine (aminomethanamidine HCl) was prepared. Samples were diluted in duplicate in 200-μl aliquots at 1:10 in phosphate-buffered saline (PBS) or 1 M guanidine, using 20 μl of plasma to 180 μl of PBS or guanidine. The samples were mixed by gentle pipetting, taking care not to create bubbles, and incubated at room temperature for 10 min. Both guanidine- and PBS-treated duplicates were tested using the manufacturer's recommended procedure for the Vitros HIV 1+2 testing. The readings were determined with the Vitros software and reported as S/C values. The avidity results were reported as an avidity index (AI), which was calculated as a ratio of the S/C of the sample incubated in guanidine to the S/C of the sample incubated in PBS.
BED assay.
The HIV-1 BED CEIA (Calypte Biomedical Corporation, Portland, OR) was performed according to the manufacturer's instructions as previously published (24). Briefly, 100-μl plasma samples or control samples, diluted 1:101, were added to the wells on microwell plates. The wells had previously been coated with goat anti-human IgG antibody. After incubation and washing, biotinylated HIV-1 branched BED peptide (gp41) was added, and after a subsequent streptavidin-horseradish peroxidase conjugate incubation, the plates were developed with tetramethylbenzidine (TMB), and the optical density (OD) was determined. Interpretation was conducted using the Calypte Incidence Excel spreadsheet, and results were reported as normalized OD (OD-n).
Vironostika less-sensitive enzyme immunoassay.
The Vironostika HIV-1 MicroElisa kit (bioMérieux, l'Etoile, France) LS-EIA was performed as previously described (25). In brief, samples were diluted 1:20,000 using a two-step specimen dilution. Each well on the microtiter plate was coated with HIV-1 viral lysate proteins, envelope proteins (native gp160), and a synthetic peptide with HIV-1 group O (ANT 70) (7). Reconstituted peroxidase-conjugated goat anti-human (Enzabody) antibody was added to each well, followed by incubation at 37°C for 30 min. After incubation and washing, plates were developed and read with a spectrophotometer. Results were reported as optical density. Standardized OD (SOD) values were calculated by subtracting the median negative-control (NC) OD from the median specimen OD and dividing by the median OD of an HIV-positive calibrator (CAL) in the respective assay runs.
MDR analysis.
Longitudinal observations (n = 416) from 80 subjects who were documented to seroconvert to HIV were used in the estimation of mean duration of recent infection (MDR) (23). Using Kaplan-Meier methods, the mean survival was calculated from the time between the estimated seroconversion date to the estimated date the subject reached the cutoff value or the earliest cutoff value for the assay combination algorithms. For cases with donation intervals of more than 90 days between their last HIV-negative and first HIV-positive blood donation day, the number of days since seroconversion were multiply imputed (10 times) and were conditionally no longer than the number of days since the last negative HIV test and no shorter than the number of days since the first positive HIV test. The number of days since seroconversion was assumed to be the midpoint between the last negative and first positive test dates when the interval was ≤90 days. The slope and intercept from a random-effect regression, and LS S/C or avidity index were the variables in the multiple imputation regression models. The day the subject reached the cutoff value was linearly interpolated from the last known day below the assay cutoff value and the first known day above the assay cutoff value. If the subject did not reach the cutoff value, the data were censored at the earliest day of the highest measurement value. Specimens from HAART-naïve subjects collected 1 to 3.5 years after seroconversion were used to calculate the combined assay false-recent rate. One specimen per subject was randomly selected. Those subjects (n = 38) with evidence of HIV infection greater than 1 year and with both assay measurements below their respective threshold values were considered to have a false-recent infection.
RESULTS
Performance characteristics.
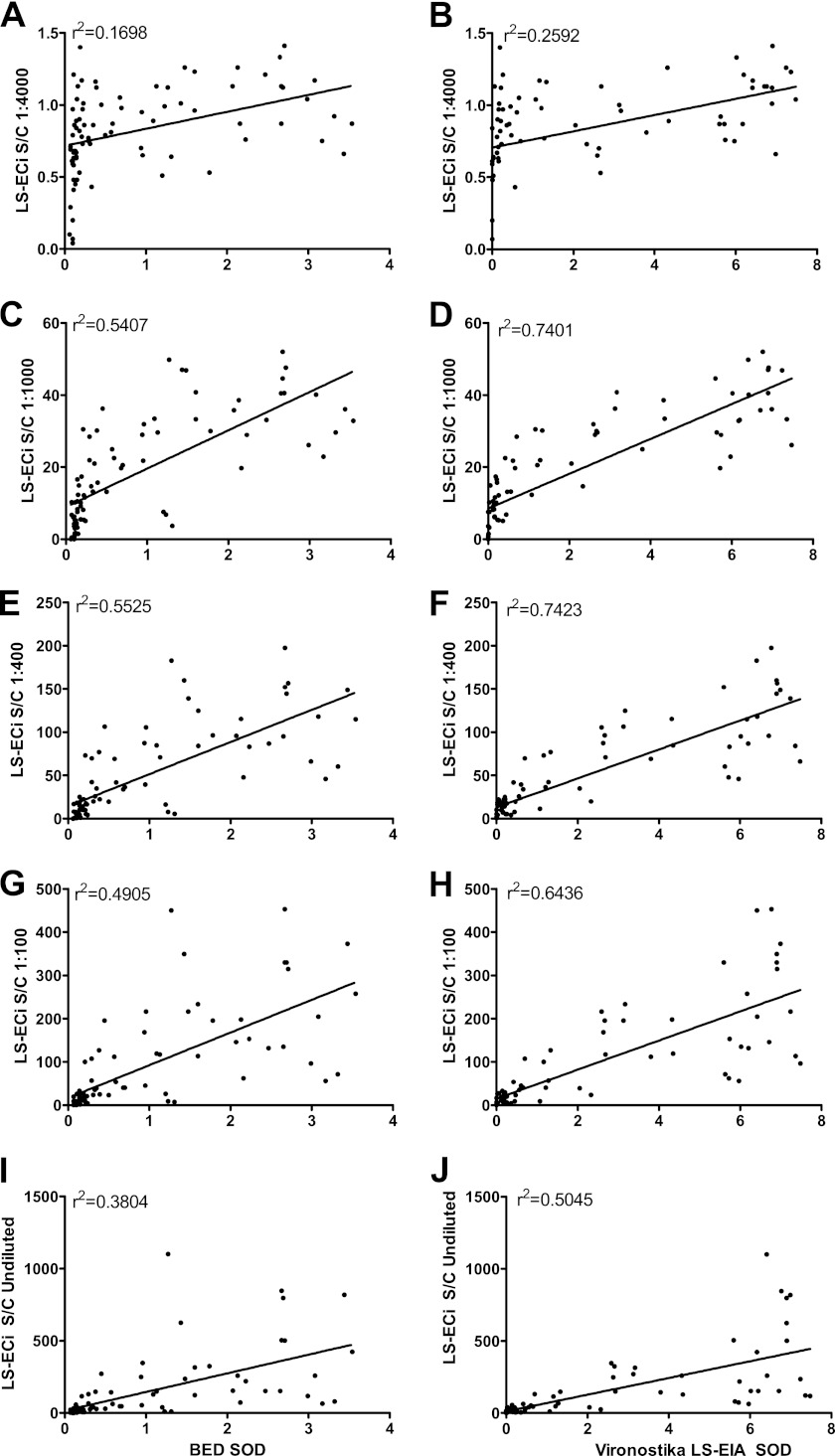
A series of titrations (undiluted and diluted 1:100, 1:400, 1:1,000, and 1:4,000) were run with samples from 80 repeat blood donors with documented seroconversion within the past year. This was done to determine the dilutions that best correlated with the Vironostika LS-EIA and BED assays. Figure 1 compares LS-Vitros results from dilutions of samples with Vironostika and BED results. Standard protocols for low-sensitivity EIA by Vironostika (1:20,000) and Calypte HIV-1 BED CEIA (1:100) were used. After comparing the LS-Vitros to the Vironostika and BED (Fig. 1E and F), an LS-Vitros dilution of 1:400 was determined to give the highest correlation (r2 = 0.74) between LS-Vironostika and LS-Vitros. The same dilution yielded the highest correlation between the BED and LS-Vitros (r2 = 0.55), though the correlation coefficient was somewhat lower with the BED assay. On the basis of these data, we used a 1:400 dilution as optimal for the LS-Vitros assay.
Fig 1.
Correlation of LS-Vitros S/C values with BED and Vironostika LS-EIA SOD values by assay dilution. Various dilutions of plasma from repeat blood donors were used to determine which dilutions yielded the highest correlations. Correlations for plasma samples that were diluted 1:4,000 (A and B), 1:1,000 (C and D), 1:400 (E and F), and 1:100 (G and H) and not diluted (I and J) are shown. Standard dilutions for Vironostika (1:20,000) and BED (1:100) were used. The 1:400 dilution yielded the highest r2 value (0.74) for the Vironostika versus LS-Vitros correlation and for the BED versus LS-Vitros correlation (0.55).
Calibration of the LS-Vitros assay.
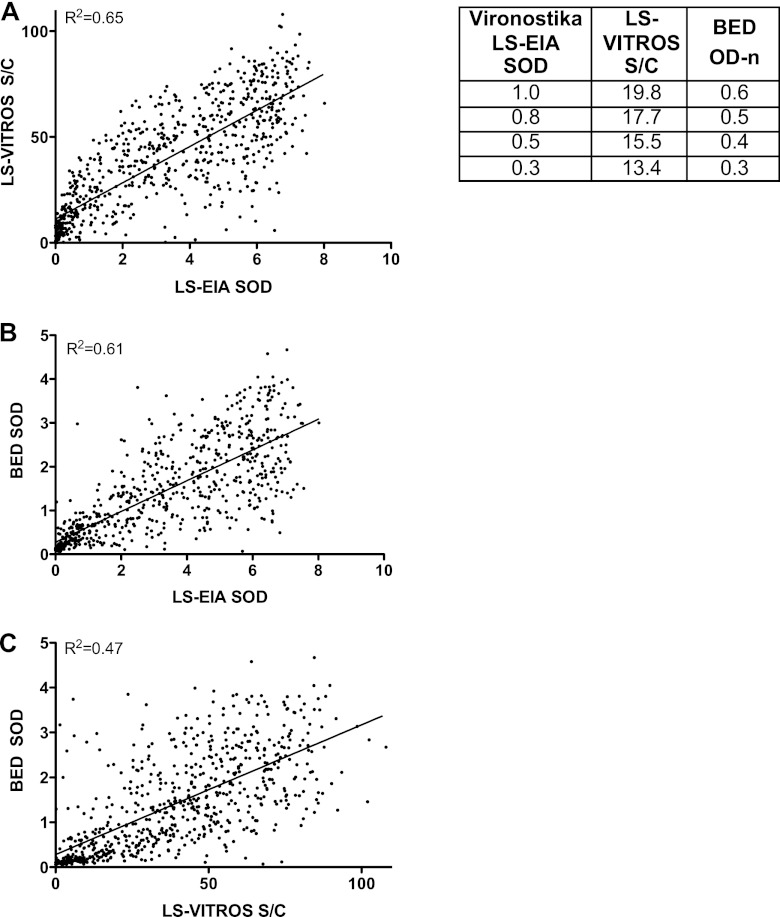
First-time and repeat HIV-positive blood donor samples (n = 710) were tested by Vironostika LS-EIA, LS-Vitros (1:400 dilution), and BED assays, and regression analyses were performed to estimate the predicted values for LS-Vitros corresponding to the selected and commonly used values for LS-EIA and BED (Fig. 2). The correlations of the Vironostika LS-EIA and LS-Vitros results (Fig. 2A; r2 = 0.65) and the Vironostika LS-EIA and BED results (Fig. 2B; r2 = 0.61) yielded the highest coefficients, with the LS-Vitros and BED correlation coefficient somewhat lower (Fig. 2C; r2 = 0.47). Using these trend line equations, the cutoff value of Vironostika LS-EIA, defined as an SOD of 1.0, was used to calibrate the corresponding cutoffs for the other assays. We found that the equivalent value for LS-Vitros was an S/C ratio of 20, and for the BED assay, it was 0.6 OD-n units, as listed in the table in Fig. 2. Further study with seroconversion panels enabled us to assess alternative cutoff values with corresponding MDR.
Fig 2.
(A to C) Predicted values for LS-Vitros and BED assays. For 710 samples from first-time and repeat blood donors, LS-Vironostika, LS-Vitros, and BED low-sensitivity assays were performed. Correlations between the assays were calculated. Vironostika versus Vitros comparison had an r2 value of 0.65 (A), Vironostika versus BED had an r2 value of 0.61 (B), and Vitros versus BED had an r2 value of 0.47 (C). The table lists cutoff values converted from the Vironostika LS-EIA to BED and LS-Vitros using the linear regression curves in panels A and B.
Period of recent infection.
There were 416 observations, collected at times prior to initiation of therapy or diagnosis of AIDS, from 80 subjects with three or more longitudinal samples following well-documented seroconversion, included in this analysis. Various cutoffs were explored for both the LS- and avidity-modified Vitros assays. The mean duration of recent infection and confidence limits are given for each cutoff in Tables 2 to 4. With an S/C ratio cutoff of 20, the LS-Vitros mean duration of recent infection was 215 days (95% confidence interval [95% CI], ±65 days) (Table 2). With a cutoff of 0.6 for AI for the avidity-modified Vitros assay, the mean duration of infection was 170 days (±44 days) (Table 3). The combination of these assays into an algorithm, whereby both assay measurements must remain below their respective cutoff values to be defined as recently infected, has a corresponding MDR of 146 days (±39 days) (Table 4). Using a small sample set from these subjects who were followed longer than 1 year after infection (n = 38), we calculated a combined algorithm false-recent rate of 8% (95% CI, 2, 21).
TABLE 2.
Mean recency period estimates for LS-Vitros assaya
| S/C ratio cutoff value | Recency period (days) |
False-recent rate | ||
|---|---|---|---|---|
| Mean | SD | 95% confidence limits | ||
| 10 | 105.8 | 16.5 | 73.5, 138.2 | 7.9 |
| 15 | 152.7 | 25 | 103.8, 201.7 | 13.5 |
| 20 | 215.4 | 33.3 | 150.1, 280.7 | 15.8 |
| 25 | 315.6 | 52.6 | 212.6, 418.6 | 18 |
Results mentioned in the text are shown in boldface type.
TABLE 4.
Mean recency period estimates for the algorithm combining the LS and avidity-modified Vitros assaysa
| S/C ratio cutoff value | Avidity index cutoff value | Recency period (days) |
False-recent rate | ||
|---|---|---|---|---|---|
| Mean | SD | 95% confidence limits | |||
| 15 | 0.7 | 162.7 | 23.4 | 116.8, 208.5 | 10.8 |
| 20 | 0.6 | 146 | 19.7 | 107.4, 184.7 | 7.9 |
| 20 | 0.65 | 174 | 21.5 | 131.8, 216.1 | 7.9 |
| 20 | 0.7 | 191.8 | 23.5 | 145.8, 237.9 | 10.5 |
| 25 | 0.6 | 162.2 | 23.9 | 115.4, 209.0 | 7.7 |
| 25 | 0.65 | 191.7 | 25.3 | 142.0, 241.3 | 7.7 |
Results mentioned in the text are shown in boldface type.
TABLE 3.
Mean recency period estimates for avidity-modified Vitros assaya
| Avidity index cutoff value | Recency period (days) |
False-recent rate | ||
|---|---|---|---|---|
| Mean | SD | 95% confidence limits | ||
| 0.5 | 123.9 | 19 | 86.7, 161.2 | 5.3 |
| 0.55 | 136.7 | 19 | 99.3, 174.0 | 5.3 |
| 0.6 | 169.7 | 22.4 | 125.9, 213.5 | 7.7 |
| 0.65 | 201.9 | 22.9 | 156.9, 246.9 | 7.9 |
| 0.7 | 223.9 | 25 | 175.0, 272.9 | 10.8 |
Results mentioned in the text are shown in boldface type.
A number of specimens (9 untreated and 9 treated) were followed from the date of HIV nucleic acid test positive (antibody negative) to more than 300 days after infection by both LS and avidity-modified Vitros assay (Fig. 3A). In individuals who were treated during the early days of infection, most individuals did not reach threshold cutoff values for these assays (Fig. 3B). This demonstrates the confounding effect of early treatment on immune maturation.
Fig 3.
Seroconversion panels from treated and untreated subjects who were monitored for >300 days after infection. Seroconversion panels from 9 untreated and 9 treated individuals were monitored for evolution of LS and avidity-modified Vitros results over the first 500 days of infection. (A) All 9 untreated individuals reach the selected cutoff values for LS (S/C ratio of 20) and avidity (AI of 0.6) Vitros assays. (B) However, the threshold values for Vitros assays are not reached for 7 of the 9 individuals.
Challenging the accuracy of detecting recent infection.
A panel of long-term infected individuals on HAART or with AIDS (CD4 < 50) were studied to investigate whether the LS- and avidity-modified Vitros HIV assays at the various cutoffs would misclassify as “false-recent” samples that were prone to have lower antibody titers or lower antibody avidity. The results were compared to other currently used assays for the detection of recent infection. LS-Vitros, avidity-modified Vitros, and BED assays were performed on all individuals in these groups (Tables 2 to 4). Using a cutoff for LS-Vitros of 20 S/C, 14% (95% CI, 9, 21) of AIDS patients and 29% (95% CI, 22, 38) of individuals who were on HAART were misclassified as recently infected. Using a cutoff of 0.6 for BED, 22% (95% CI, 16, 30) and 12% (95% CI, 7, 9), respectively, of these AIDS patients and HAART-suppressed patients were identified as recently infected. Using a Vitros avidity index cutoff of 0.6 to identify individuals who were recently infected, we found 3% (95% CI, 0.8, 7.2) and 42% (95% CI, 33, 51), respectively, of these AIDS patients and HAART-suppressed patients were misclassified as recently infected. Combining the assays in an algorithm improved the FRR. Calculating the concordant results between both LS- and avidity-modified assays can create a RITA algorithm that yields an overall FRR of 15% (95% CI, 11, 20) (Table 5). Using additional assays, such as the BED assay, further improves the overall FRR to 5% (95% CI, 2.8, 8.4).
TABLE 5.
False-recent rates in challenge cohorts
| Cohorta | False-recent rate (%) [95% CI] in cohort by the following assay(s): |
||||
|---|---|---|---|---|---|
| BED (OD-n cutoff of 0.6) | LS-Vitros (S/C cutoff of 20) | Avidity-modified Vitros (AI cutoff of 0.6) | LS + avidity-modified Vitros | LS + avidity-modified Vitros + BED | |
| AIDS patients (n = 140) | 22 [16, 30] | 14 [9, 21] | 3.0 [0.8, 7.2] | 2.1 [0.4, 6.1] | 1.4 [0.2, 5.1] |
| HAART-treated patients (n = 134) | 12 [7, 19] | 29 [22, 38] | 42 [33, 51] | 28 [21, 37] | 9.0 [5, 15] |
| All patients (n = 274) | 17 [13, 22] | 21 [16, 26] | 21 [16, 26] | 15 [11, 20] | 5.1 [2.8, 8.4] |
The AIDS patients had a CD4 count of <50. The HAART-treated patients had a CD4 count of >400 and a viral load of <50.
DISCUSSION
To understand the current state of HIV infection dynamics in a population, one needs to calculate the incidence of infection in that population. This will be essential in directing where prevention programs are needed and to power clinical studies and vaccine trials designed to reduce transmission rates. Those individuals who recently acquired HIV infection have high viral load within the first weeks of infection, and this may put them in a high-risk category for transmission to others. Since the acute phase of HIV infection is short-lived and the clinical manifestations are similar to other viral infections, an individual with a recently acquired HIV infection may not visit a physician. By the time most individuals test positive for infection, it is difficult to know when the infection occurred. Assays for the detection of recent HIV infection will be essential in understanding the state of disease not only for the calculation of incidence in a population to drive health interventions but also potentially for use in a clinical setting to help stage infection acquisition times and direct treatment to guide public health clinical interventions.
The purpose of this study was to explore the possibility of using modified versions of the FDA-approved Vitros HIV 1+2 assay for the purpose of detection of recent HIV infection. Modification of a currently FDA-approved assay for clinical HIV detection provides some advantages for development of a test for recent infection. The Vitros system is available in clinical laboratories around the world not only for the detection of HIV infection but also for measuring a large variety of clinical analytes. The Vitros assay has a rapid turnaround time that enables swift clinical intervention if necessary. This would be especially useful in the clinical laboratory setting where it would be possible to run the sensitive HIV diagnostic assay and subsequently run the detuned or avidity-modified version of the assay. The LS- and/or avidity-modified assay results can be generated almost in real-time (within several hours of initial diagnostic testing) to enable identification of individuals who are not only infected but also recently infected.
In this study, we were able to modify the Vitros assay so that it could be used to discriminate between recently acquired and long-standing infections using two methods, a LS protocol that employs a dilution step and elevated cutoff and an avidity protocol that uses a chaotropic agent; these methods investigate both antibody titer and quality of antibody binding and hence could complement one another for application in RITAs. Using well-characterized samples from first-time and repeat HIV-1-positive blood donors with known interdonation intervals, we calibrated the assays against two previously used assays for detection of recent infection, the Vironostika LS-EIA and BED assays. The LS-Vitros cutoff was initially selected because it was within the same range as the cutoffs for the Vironostika assay. This comparison method was used during transition from the 3A11-LS EIA to the Vironostika LS-EIA assay (25). We subsequently proceeded with a more in-depth analysis using seroconversion panels to calculate the duration of recency and investigate the performance of the assays using various measurement cutoffs. Although the choice of cutoffs can be influenced by intended use of the assays, we chose cutoffs that gave mean durations of recent infection similar to those of the Vironostika assay. For purposes of recency estimation, we highlight a cutoff S/C ratio of 20 for the LS-Vitros, which gave an MDR of 215 days (±65 days), and a cutoff of 0.6 for the avidity index, which gave an MDR of 170 days (±44 days). Using the combined algorithm in which either of these cutoffs is reached and using the results from the seroconversion panels, we calculated a mean recency period of 146 days (±39 days) and a false-recent rate of 8%. The selection of cutoff values and their corresponding duration periods of recent infection (Table 2) will require further evaluation and may depend on the application of the assays. The result of lowering the cutoff of one or both of the assays is expected to lower the false-recent rate and shorten the MDR.
Although 80 seroconversion panels were used in calculating the duration of recent infection, only a small subset of samples was used in calculating the FRR of the study. A larger set of long-term infected, AIDS-free, and HAART-naïve individuals will be important in assessing the true FRR. One of the major problems with incidence assays is the misclassification of long-term infected individuals as recently infected. Individuals on HAART therapy may have low levels of circulating antigen; this affects the magnitude and quality of the antiviral antibody response. These individuals have a lower antibody titer which, when tested using detuned or avidity-modified assays, may result in the appearance of recent infection. In addition, individuals who are late in their infection with low CD4 counts have impaired mechanisms of producing antibodies and also may appear to be recently infected based on RITAs. By using samples from individuals with long-term infection that are on HAART with consequent low viral loads or samples from patients with low CD4 counts, we were able to challenge the new modified Vitros assays to investigate their ability to accurately distinguish long-term infection from recent infection. Using these challenge specimens, we found that both LS- and avidity-modified Vitros assays had difficulty correctly classifying samples from individuals with low viral load due to HAART treatment; however, the assays were better able to distinguish long-term infection in individuals with untreated, uncontrolled viral load and low CD4 count. This illustrates potential problems using serological assays to detect recent HIV infection.
The LS-Vitros assay performed relatively better on samples from individuals with AIDS, while the BED assay performed relatively better on samples from HAART-treated individuals. Reasons for differences in assay performance are difficult to identify; they may be due to the antigens used in the assays. The BED assay uses a branched gp41 peptide from clade B, E, and D envelope (Env); considering the high mutation rate of HIV Env, immune responses to HIV Env may evolve around the peptide epitope used in the assay. The Vitros assays use recombinant Env antigens, which may preserve the tertiary conformation of the proteins in the assay, enabling better antibody binding to native proteins; larger recombinant proteins are also likely to have multiple antibody epitopes and thus would be less likely to have a loss of antibody reactivity due to mutation. The Vitros assays also use the p24 antigen, which is a relatively conserved Gag protein and may preserve a stable antibody response. Recent work has shown that p24 continues to circulate on erythrocytes in individuals with undetectable viral load, as in elite controllers, enabling a boost of the anti-p24 antibody response (11). In chronic HIV infection, the loss or dysfunction of CD4+ T cells may impede effective antibody production; neutralizing antibodies have been shown to be restored with increased CD4 cell counts after successful HAART therapy (19, 26). Considering the more effective discrimination of false-recent samples in AIDS patients by the avidity-modified Vitros assays suggests that there may be maintenance of a high-avidity antibody response despite CD4+ T cell loss. With the changes in assays used for the detection of recent infection, substantial effort is being directed to validate existing RITA assays and to assess alternative approaches and serial test algorithms, with particular interest in developing methods for incidence estimation at the population level and to support intervention trials. Well-characterized panels of samples are needed in order to validate new methods in the future, maintain consistency of the definition of recent infection, and to calculate the MDR for new assays. A current initiative called the Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA), funded by the Bill and Melinda Gates Foundation, is in the process of developing large panels of well-characterized samples for this purpose (http://www.incidence-estimation.com/page/cephia-overview). The LS- and avidity-modified Vitros assays will be among the tests rigorously studied using these panels. Newer assays, including the limiting antigen assay (LAg), will also be incorporated into the assay evaluation program; this assay uses a lower concentration of coating antigen and a dissociation step to ensure that only highly avid antibodies remain bound, enabling better discrimination of long-standing HIV infections from recent HIV infections (10). The full evaluation of these and other assays with the CEPHIA panels will establish the best ways to test for recent HIV infection and to determine whether algorithms comprised of multiple incidence assays will be required.
ACKNOWLEDGMENTS
We thank Paul Contestable, Steven Edwards, and Ortho Clinical Diagnostics for assay reagents and Steven Ethridge (CDC), Bernie Branson (CDC), Pat Garrett, and Chris Pilcher for help acquiring panel specimens and advice. We thank the staff of the Wadsworth Center Bloodborne Viruses Laboratory for technical assistance.
This work was supported by the HIV Prevention Trials Network (HPTN) sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the NIH, DHHS (U01-AI46745, U01-AI48054, U01-AI068613, UM1-AI068613, NIH R01 DA11602, and R01 AA16893). Additional support was provided by the Division of Intramural Research, NIAID, NIH and CDC intramural funding.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Beyrer C, Nelson KE. 1997. Loss to follow-up effect in investigations of HIV-1 incidence. Lancet 349:649–650 [DOI] [PubMed] [Google Scholar]
- 2. Brookmeyer R. 2010. Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol. Rev. 32:26–37 [DOI] [PubMed] [Google Scholar]
- 3. Brookmeyer R, Quinn TC. 1995. Estimation of current human immunodeficiency virus incidence rates from a cross-sectional survey using early diagnostic tests. Am. J. Epidemiol. 141:166–172 [DOI] [PubMed] [Google Scholar]
- 4. Busch MP, et al. 2010. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS (London, Engl.) 24:2763–2771 [DOI] [PubMed] [Google Scholar]
- 5. Celum CL, et al. 2001. Early human immunodeficiency virus (HIV) infection in the HIV Network for Prevention Trials Vaccine Preparedness Cohort: risk behaviors, symptoms, and early plasma and genital tract virus load. J. Infect. Dis. 183:23–35 [DOI] [PubMed] [Google Scholar]
- 6. Chawla A, et al. 2007. Human immunodeficiency virus (HIV) antibody avidity testing to identify recent infection in newly diagnosed HIV type 1 (HIV-1)-seropositive persons infected with diverse HIV-1 subtypes. J. Clin. Microbiol. 45:415–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chetty C, et al. 2005. Vironostika HIV-1 Plus O Microelisa System for the detection of antibodies to HIV-1, including Group O, in serum, plasma, or dried blood spots. HIV Diagnostics: New Developments and Challenges, 28 February to 1 March 2005, Orlando, FL http://www.hivtestingconferencearchive.org/hivtesting2005/CA1.htm [Google Scholar]
- 8. Cimerman S, Sucupira MC, Lewi DS, Diaz RS. 2007. Less sensitive HIV-1 enzyme immunoassay as an adjuvant method for monitoring patients receiving antiretroviral therapy. AIDS Patient Care STDS 21:100–105 [DOI] [PubMed] [Google Scholar]
- 9. Diaz RS, Kallas EG, Castelo A, Rawal BD, Busch MP. 1999. Use of a new ‘less-sensitive enzyme immunoassay’ testing strategy to identify recently infected persons in a Brazilian prison: estimation of incidence and epidemiological tracing. AIDS (London, Engl.) 13:1417–1418 [DOI] [PubMed] [Google Scholar]
- 10. Duong YT, et al. 2012. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One 7:e33328 doi:10.1371/journal.pone.0033328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia MN, dos Ramos Farias MS, Avila MM, Rabinovich RD. 2011. Presence of p24-antigen associated to erythrocyte in HIV-positive individuals even in patients with undetectable plasma viral load. PLoS One 6:e14544 doi:10.1371/journal.pone.0014544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groopman JE, et al. 1985. Antibody seronegative human T-lymphotropic virus type III (HTLV-III)-infected patients with acquired immunodeficiency syndrome or related disorders. Blood 66:742–744 [PubMed] [Google Scholar]
- 13. Guy R, et al. 2009. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect. Dis. 9:747–759 [DOI] [PubMed] [Google Scholar]
- 14. Hallett TB, Ghys P, Barnighausen T, Yan P, Garnett GP. 2009. Errors in ‘BED’-derived estimates of HIV incidence will vary by place, time and age. PLoS One 4:e5720 doi:10.1371/journal.pone.0005720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hallett TB, et al. 2008. Estimating incidence from prevalence in generalised HIV epidemics: methods and validation. PLoS Med. 5:e80 doi:10.1371/journal.pmed.0050080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hargrove JW, et al. 2008. Improved HIV-1 incidence estimates using the BED capture enzyme immunoassay. AIDS (London, Engl.) 22:511–518 [DOI] [PubMed] [Google Scholar]
- 17. Highleyman L. 1999. Detuned assay used to track recent infections. Beta 12:6, 78 [PubMed] [Google Scholar]
- 18. Janssen RS, et al. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280:42–48 [DOI] [PubMed] [Google Scholar]
- 19. Kimura T, et al. 2002. Reconstitution of spontaneous neutralizing antibody response against autologous human immunodeficiency virus during highly active antiretroviral therapy. J. Infect. Dis. 185:53–60 [DOI] [PubMed] [Google Scholar]
- 20. Martro E, et al. 2005. Comparison of the avidity index method and the serologic testing algorithm for recent human immunodeficiency virus (HIV) seroconversion, two methods using a single serum sample for identification of recent HIV infections. J. Clin. Microbiol. 43:6197–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore RD. 1998. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 17(Suppl 1):S38–S41 [DOI] [PubMed] [Google Scholar]
- 22. Murphy G, Parry JV. 2008. Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill. 13:pii=18966. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=18966 [PubMed] [Google Scholar]
- 23. Parekh BS, et al. 2011. Determination of mean recency period for estimation of HIV type 1 incidence with the BED-capture EIA in persons infected with diverse subtypes. AIDS Res. Hum. Retroviruses 27:265–273 [DOI] [PubMed] [Google Scholar]
- 24. Parekh BS, et al. 2002. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res. Hum. Retroviruses 18:295–307 [DOI] [PubMed] [Google Scholar]
- 25. Rawal BD, et al. 2003. Development of a new less-sensitive enzyme immunoassay for detection of early HIV-1 infection. J. Acquir. Immune Defic. Syndr. 33:349–355 [DOI] [PubMed] [Google Scholar]
- 26. Samuelsson A, Brostrom C, van Dijk N, Sonnerborg A, Chiodi F. 1997. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection–correlation with clinical progression, viral load, and loss of humoral immunity. Virology 238:180–188 [DOI] [PubMed] [Google Scholar]