Abstract
Neisseria lactamica is a true commensal bacterium occupying the same ecological niche as the pathogenic Neisseria meningitidis, which is responsible for outbreaks and large epidemics, especially in sub-Saharan Africa. To better understand the epidemiology of N. lactamica in Africa and its relationship to N. meningitidis, we studied N. lactamica carriage in 1- to 29-year-old people living in three districts of Burkina Faso from 2009 to 2011. N. lactamica was detected in 18.2% of 45,847 oropharyngeal samples. Carriage prevalence was highest among the 2-year-olds (40.1%) and decreased with age. Overall prevalence was higher for males (19.1%) than females (17.5%) (odds ratio [OR], 1.11; 95% confidence interval [CI], 1.04 to 1.18), while among the 18- to 29-year-olds, carriage prevalence was significantly higher in women (9.1%) than in men (3.9%) (OR, 2.49; 95% CI, 1.94 to 3.19). Carriage prevalence of N. lactamica was remarkably homogeneous in the three districts of Burkina Faso and stable over time, in comparison with carriage of N. meningitidis (P. A. Kristiansen et al., Clin. Vaccine Immunol. 18:435–443, 2011). There was no significant seasonal variation of N. lactamica carriage and no significant change in carriage prevalence after introduction of the serogroup A meningococcal conjugate vaccine, MenAfriVac. Multilocus sequence typing was performed on a selection of 142 isolates. The genetic diversity was high, as we identified 62 different genotypes, of which 56 were new. The epidemiology of N. lactamica carriage and the molecular characteristics of carried isolates were similar to those reported from industrialized countries, in contrast to the particularities of N. meningitidis carriage and disease epidemiology in Burkina Faso.
INTRODUCTION
Neisseria lactamica is a lactose-fermenting Gram-negative diplococcus living in a commensal relationship with humans. The bacteria, frequently found in the upper respiratory tract, most commonly in young children, are transmitted between healthy individuals through close contact and are normally not a threat to humans, as only a few cases of clinical significance have been reported (9, 12, 36). Neisseria meningitidis, a closely related species, occupies the same ecological niche but may cause severe disease, such as meningitis and/or septicemia. Large epidemics can occur, with devastating consequences, especially in countries of the meningitis belt, a sub-Saharan area with modest yearly rainfall (17, 26). N. meningitidis possesses a protective polysaccharide capsule, from which the serogroup is determined. N. lactamica does not express a capsule, but subcapsular components, such as outer membrane proteins, are similar in these two species (3, 5, 18, 29). Early studies have shown that N. lactamica carriage is frequent in age groups with low prevalence of N. meningitidis asymptomatic carriage and vice versa (15, 32). It is believed that carriage of N. lactamica can protect against meningococcal infection by cross-reactive immunity (11), and meningococcal vaccines based on N. lactamica have been considered (14, 16).
Although N. lactamica is closely related to N. meningitidis, little is known about its epidemiology. A limited number of studies have reported carriage prevalence and distribution (1, 2, 4, 7, 8, 11, 19, 20, 27, 30, 32–34), some of them conducted within the African meningitis belt (7, 27, 30). To date, two studies have used molecular techniques to characterize N. lactamica isolates (1, 4), one using multilocus sequence typing (MLST) (4), the same scheme as currently recommended for N. meningitidis genotyping. Research on the relationship between the two commensals and on characteristics of the circulating strains may contribute to a better understanding of meningococcal colonization and virulence factors.
In the meningitis belt of Africa, N. meningitidis serogroup A belonging to the sequence type 5 (ST-5) clonal complex has been responsible for the majority of epidemics in the past decades, although serogroups X and W135 have also caused outbreaks (31). To eliminate meningococcal epidemics in the meningitis belt, an affordable serogroup A conjugate vaccine, MenAfriVac, was first introduced in a country-wide mass vaccination campaign in Burkina Faso in December 2010 (10, 24, 25) after a large-scale safety study had been conducted in the whole district of Kaya in September 2010. The impact of MenAfriVac vaccination on meningococcal carriage was evaluated in a large carriage study performed before and up to 13 months after mass vaccination (21, 22). In the course of that study, all lactose-fermenting Gram-negative diplococci were registered and some were kept for further investigation.
We present here the epidemiology of N. lactamica carriage in Burkina Faso before and after MenAfriVac vaccination and the genetic profile of N. lactamica isolates circulating in the country.
MATERIALS AND METHODS
Ethics.
The study obtained ethical clearance from the Norwegian Regional Committee for Medical Research Ethics, southern Norway (file no. S-08375a), the Ethical Committee for Health Research in Burkina Faso, and the Internal Review Board at Centers for Disease Control and Prevention (CDC), Atlanta, Georgia (file no. 5524).
Study population and data collection.
In a repeated cross-sectional study performed between 2009 and 2011, oropharyngeal swabs were collected from 1- to 29-year-old residents of Burkina Faso, as described previously (22). A multistage cluster sampling design ensured the enrollment of a representative proportion of the 1- to 29-year-old population from three health districts: the urban district of Bogodogo and the rural districts of Dandé and Kaya. Nine sampling campaigns, S1 (January and February 2009), S2 (April and May 2009), S3 (July and August 2009), S4 (October and November 2009), S5 (October and November 2010), S6 (February and March 2011), S7 (May 2011), S8 (August 2011), and S9 (October and November 2011), were performed simultaneously in the three districts within a 4-week period. The sampling campaigns covered meningitis epidemic seasons occurring in the dry period of the year (S1, S2, S6, and S7) and nonepidemic (rainy) seasons (S4, S5, S6, S8, and S9). For each sampling campaign, a random selection of households in the rural districts and city blocks in the urban district was made, and all healthy 1- to 29-year-olds in the selected households or city block were invited to participate in the study and to be swabbed. Epidemiological data on each participant such as the person's age and gender were collected on handheld computers in the field.
Sample collection and bacterial identification.
Each volunteer was swabbed at the posterior pharyngeal wall behind the uvula and at one tonsil, using a sterile cotton swab (Copan, Brescia, Italy). The swab was immediately plated onto selective agar (modified Thayer Martin V-C-N-T, containing 3 mg/liter vancomycin, 7.5 mg/liter colistin, 12.5 U/liter nystatin, 5 mg/liter trimethoprim lactate, and Vitox supplement; produced by WHO/Multi Disease Surveillance Centre, Burkina Faso) that supported the growth of both N. meningitidis and N. lactamica. Inoculated plates were placed in sealed jars in the field (Remel, GA) with CO2 generators (CO2 GEN; Oxoid, United Kingdom), and the temperature was monitored until the jars were set for incubation at 37°C within a maximum of 6 h after swabbing. In each district, a microbiological laboratory performed the bacterial identification: the Centre Hospitalier Universitaire Pédiatrique Charles de Gaulle, Ouagadougou, for Bogodogo; the Centre Hospitalier Universitaire Souro Sanou, Bobo-Dioulasso, for Dandé; and the Centre Hospitalier Universitaire Yalgado, Ouagadougou, for Kaya. After 24 and 48 h of incubation, one or two colonies growing on the selective agar plate with a typical Neisseria morphology were subcultured on blood agar plates (Reactivos Para Diagnostico, Spain) containing 5% defibrinated sheep blood (Fig. 1). Oxidase positive, Gram-negative diplococci were isolated and further characterized (22). Isolates with β-galactosidase (o-nitrophenyl-β-d-galactopyranoside [ONPG]) activity (Rosco Diagnostics, Denmark) were categorized as N. lactamica. The technicians working in the national laboratories received extensive training before the start of the study, and the quality of the laboratory analysis was monitored throughout the study period using a quality control system, as described previously (23).
Fig 1.
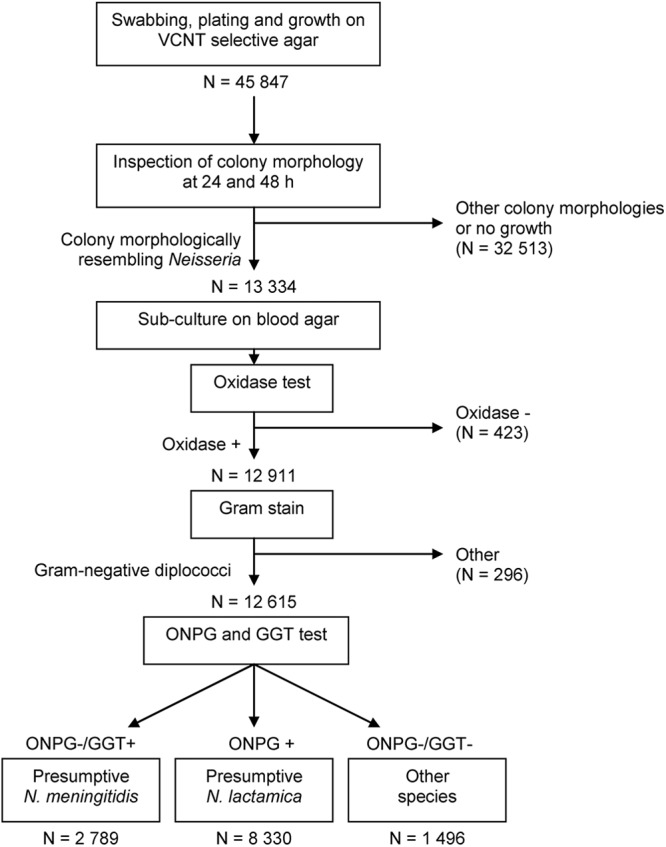
Flow diagram of laboratory analysis performed in Burkina Faso for the identification of N. lactamica in oropharyngeal swabs. ONPG, o-nitrophenyl-β-galactopyranoside (for β-galactosidase activity); GGT, γ-glutamyltransferase.
Selection of isolates for molecular characterization.
As part of the laboratory quality control system (23), 10% of all the isolates with an enzymatic profile different from that of N. meningitidis were controlled at the Norwegian Institute of Public Health (NIPH). Of these, isolates were confirmed as N. lactamica when the enzymatic profile was ONPG positive and γ-glutamyltransferase (GGT) negative (Rosco Diagnostics, Denmark). A subset of 142 (27.7%) confirmed N. lactamica isolates originating from sampling campaigns S1 to S4 performed in 2009 were randomly selected for molecular characterization to obtain a pool of isolates representative of this period. Isolates were selected from each of the visited villages at all four sampling campaigns. Within each village or city block, isolates were randomly selected from different compounds, both genders, and different age groups when possible.
Molecular characterization.
DNA from 142 N. lactamica isolates was isolated by suspending 1 loopful of bacteria in 200 μl Tris-EDTA (TE) buffer, pH 8.0, heating the mixture at 95°C for 10 min, and subjecting it to centrifugation at 16,000 × g for 5 min. The supernatants were stored at −20°C until use. MLST using seven housekeeping gene fragments was performed with the primers recommended by Alber et al. (1). Additional primer sets (fumC-P1, fumC-P2, fumC-P1B, and fumC-P2B) (6, 13) were needed for the amplification and sequencing of the fumC locus in some isolates. Each strain was assigned to a specific ST and ST complex (13, 28).
Data collection and analyses.
The epidemiological information collected in the field was combined with laboratory results from Burkina Faso and Norway. Of 46,196 oropharyngeal samples collected, 349 were excluded from statistical analysis due to data entry errors. Statistical analysis was done in Stata version 11.1. Odds ratios (OR) were calculated by bivariate logistic regression accounting for the cluster sampling design. Statistical significance was defined as P values of <0.05 and as 95% confidence intervals not including null. Phylogenetic analyses based on concatenated gene sequences of different STs were done by the unweighted-pair group method using arithmetic averages (UPGMA) generated in MEGA version 5 (35) with a bootstrap test (1,000 replicates). Bootstrapped UPGMA analysis was also used to compare STs identified in Burkina Faso with those reported to be found in the United Kingdom (4) and with all the N. lactamica STs registered in the MLST database (http://pubmlst.org/neisseria) by May 2012.
RESULTS
N. lactamica carriage prevalence.
From 9 sampling campaigns performed between 2009 and 2011, a total of 45,847 participants were included in the analysis. Of these, 43.5% were male and 50.9% <10 years old. A total of 8,330 (18.2%) participants were identified as N. lactamica carriers on the basis of colony morphology and biochemical properties of the isolates recovered from the pharyngeal samples collected by the national microbiology laboratories in Burkina Faso.
Accuracy of results.
From the sampling campaigns in 2009, 538 isolates classified as N. lactamica (ONPG positive) in Burkina Faso were sent to NIPH as part of the external quality control. Of these, 512 (95.2%) were confirmed as such at NIPH, while 11 (2.0%) were identified as N. meningitidis (23). Thus, carriage rates of N. lactamica presented here are likely to be slightly overestimated. Using the 95% accuracy rate, the overall carriage rate of N. lactamica was 17.3%.
Geographic and seasonal variation.
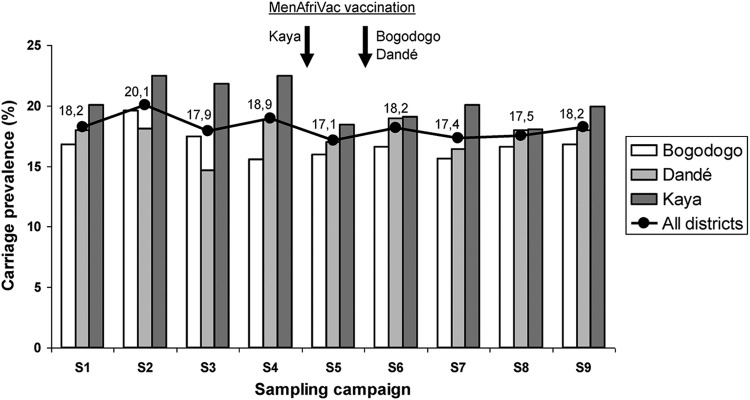
A total of 2,587 isolates were categorized as N. lactamica in Bogodogo (16.8%), 2,700 in Dandé (17.5%), and 3,043 in Kaya (20.3%). The carriage rate in Kaya was higher than in Bogodogo (OR, 1.26; 95% confidence interval [CI], 1.05 to 1.52), but the difference with Dandé was not significant (OR, 1.20; 95% CI, 0.95 to 1.50). Some fluctuations in carriage prevalence were seen between sampling campaigns (Fig. 2), but the differences between sampling time points were not statistically significant. Overall carriage prevalence in the dry seasons of 2009 and 2011 (S1, S2, S6, and S7) was 18.5%, while in the rainy seasons of 2009 and 2011 it was 18.1% (OR for dry versus rainy season, 1.02; 95% CI, 0.94 to 1.10).
Fig 2.
Geographic and temporal variation of N. lactamica carriage in three health districts in Burkina Faso, 2009 to 2011.
Age and gender distribution.
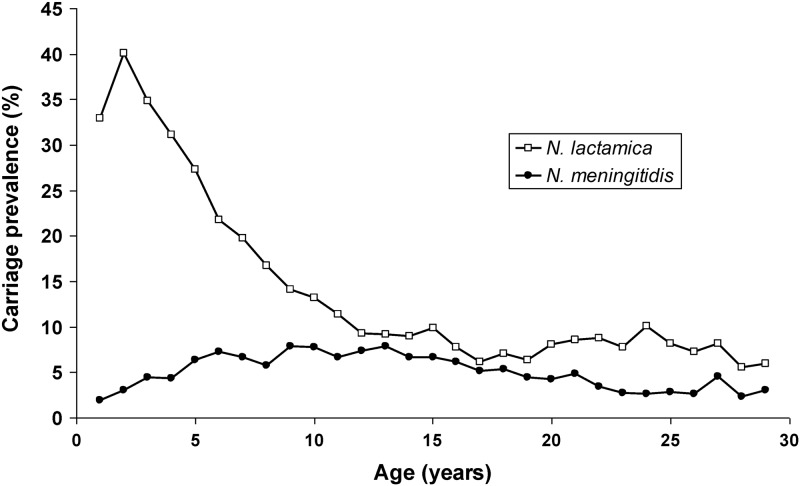
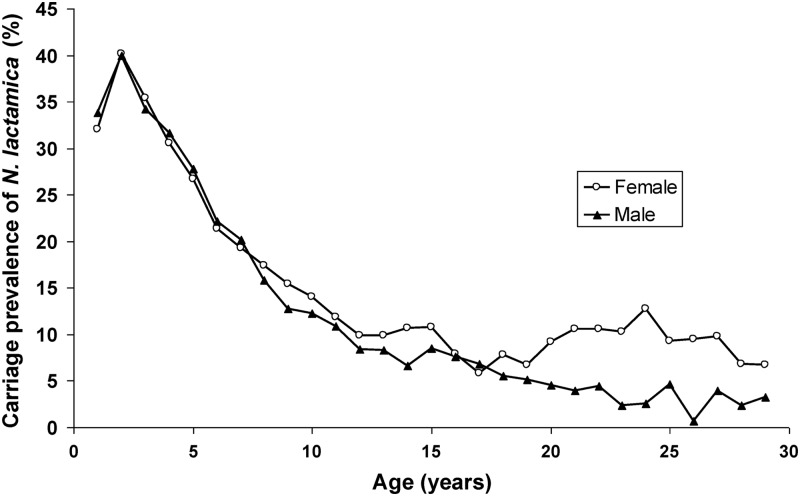
N. lactamica was carried by 33.0% of the 1-year-olds. N. lactamica carriage rate peaked at 2 years (40.1%) and then rapidly decreased with age but remained at around 5 to 10% in the older age groups. This is in contrast to a flatter age distribution curve for N. meningitidis carriage (22) in the same population (Fig. 3). This age distribution was similar in all three districts (data not shown). Overall carriage was significantly higher among male (19.1%) than among female (17.5%) participants (OR, 1.11; 95% CI, 1.04 to 1.18). The age distribution was remarkably different between genders, as carriage among women increased again from the age of 18, while it remained low among men (Fig. 4). In the age group of 18 to 29 years, carriage prevalence in women (9.1%) was significantly higher than in men (3.9%) (OR, 2.49; 95% CI, 1.94 to 3.19).
Fig 3.
Age distribution for carriers of N. lactamica and N. meningitidis in Burkina Faso, 2009 to 2011.
Fig 4.
Age distribution of female and male carriers of N. lactamica in Burkina Faso, 2009 to 2011.
Stability of N. lactamica carriage after MenAfriVac vaccination.
Overall carriage prevalence was 18.8% in 2009, before mass vaccination with MenAfriVac, and 17.8% in 2011, after vaccination, but the reduction was not statistically significant (OR, 0.94; 95% CI, 0.86 to 1.02). Compared to 2009, N. lactamica carriage in 2011 was lower in Bogodogo (OR, 0.93; 95% CI, 0.83 to 1.06), higher in Dandé (OR, 1.03; 95% CI, 0.93 to 1.15), and lower in Kaya (OR, 0.86; 95% CI, 0.68 to 1.08), but the differences were not significant in any of the districts. Carriage prevalence for male participants was 19.8% before vaccination and 18.7% after vaccination, while female participants had a carriage prevalence of 18.0% before vaccination and 17.1% after. The age distribution profile of N. lactamica carriers after vaccination was similar to the age profile before vaccination, although the prevalence in the 2-year-olds was lower (37.2%, down from 42.8%).
Molecular characterization.
A total of 142 confirmed N. lactamica isolates from sampling campaigns S1 to S4 in 2009 were randomly selected for molecular characterization and included 46 samples from Bogodogo, 51 from Dandé, and 45 from Kaya. None of the persons from which isolates were selected had been swabbed more than once.
In total, 62 STs were identified among the 142 isolates, of which 56 were new. Forty new alleles were registered in the MLST database. The majority of the isolates (103 isolates, 73%) were composed of 36 different genotypes belonging to four clonal complexes: ST-595 complex (n = 23), ST-613 complex (n = 40), ST-640 complex (n = 30), and ST-1494 complex (n = 10) (Table 1). The two dominant STs were ST-613 of the ST-613 complex and ST-9060 of the ST-640 complex, each representing 14.1% of the isolates. ST-9099 of the ST-595 complex was represented by 9 isolates (6.3%), and the remaining STs were represented by less than 5% of the isolates.
TABLE 1.
Distribution of sequence types among 142 N. lactamica strains isolated from 1- to 29-year-old carriers in the districts of Bogodogo, Dandé, and Kaya in Burkina Faso in 2009
| Clonal complex | % of isolates | STa | No. (%) of isolates |
|---|---|---|---|
| ST-595 | 16.2 | 595 | 4 (2.82) |
| 9099 | 9 (6.34) | ||
| 9146 | 4 (2.82) | ||
| 9271 | 2 (1.41) | ||
| Other | 4 (2.8) | ||
| ST-613 | 28.2 | 613 | 20 (14.1) |
| 9101 | 6 (4.23) | ||
| 9102 | 2 (1.41) | ||
| 9270 | 4 (2.82) | ||
| 9276 | 2 (1.41) | ||
| Other | 6 (4.2) | ||
| ST-640 | 21.1 | 640 | 2 (1.41) |
| 9060 | 20 (14.1) | ||
| Other | 8 (5.6) | ||
| ST-1494 | 7.0 | 9057 | 2 (1.41) |
| 9097 | 2 (1.41) | ||
| 9144 | 2 (1.41) | ||
| Other | 4 (2.8) | ||
| UAb | 27.5 | 1288 | 4 (2.82) |
| 8476 | 2 (1.41) | ||
| 9059 | 6 (4.23) | ||
| 9139 | 5 (3.52) | ||
| Other | 22 (15.4) |
ST, sequence type; Other, sequence types each representing less than 1% of the isolates.
UA, unassigned to any clonal complex.
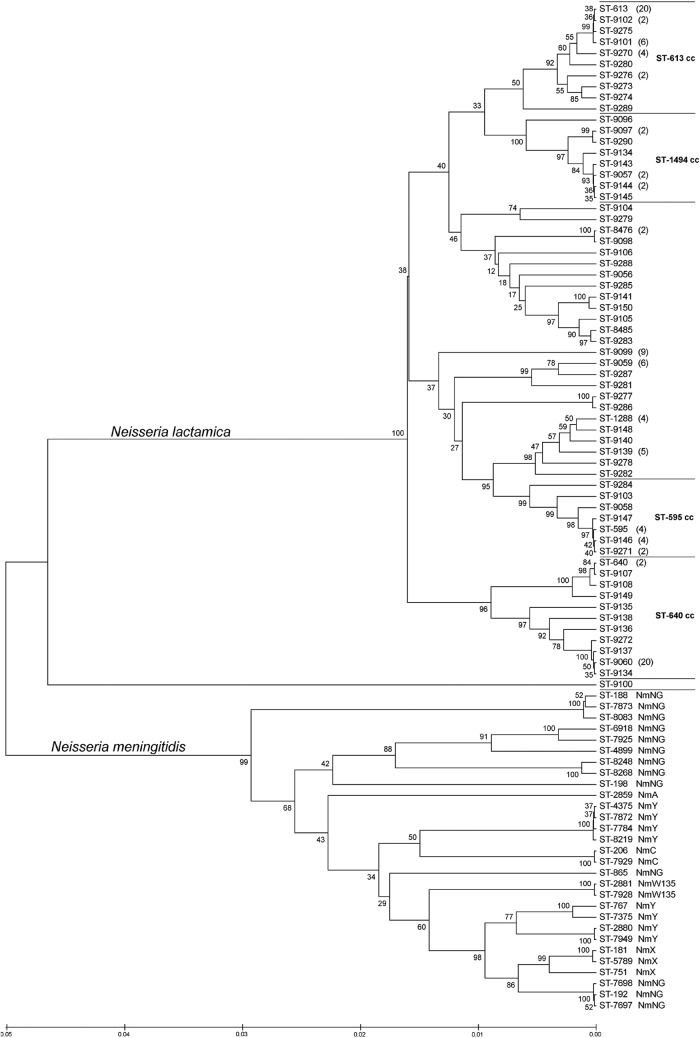
UPGMA analysis provided a visual representation of the genetic diversity of N. lactamica STs and the relative differences between ST complexes (Fig. 5). There were three main groups of STs not yet assigned to a clonal complex, one close to the ST-595 complex, one close to both the ST-613 and the ST-1494 complexes, and a single ST (ST-9100) represented by one isolate, distant from all the other N. lactamica STs. All the N. lactamica isolates were genetically distant from N. meningitidis carriage isolates retrieved from the same population in the same period (22) (Fig. 5).
Fig 5.
Linkage distance of 62 N. lactamica and 29 N. meningitidis sequence types (STs) identified among 1- to 29-year-old carriers from Burkina Faso in 2009, as determined by bootstrapped UPGMA analysis. For STs represented by more than one isolate, the number of isolates is given in parentheses. NmA, NmC, NmX, NmY, NmW135, and NmNG are abbreviations for serogroups A, C, X, Y, and W135 and nonserogroupable N. meningitidis, respectively. cc, clonal complex.
UPGMA analysis of N. lactamica STs identified among infants in the United Kingdom (4) combined with N. lactamica STs from Burkina Faso showed a high degree of genetic variation in both countries and no evident clustering of STs from Burkina Faso (Fig. 6).
Fig 6.
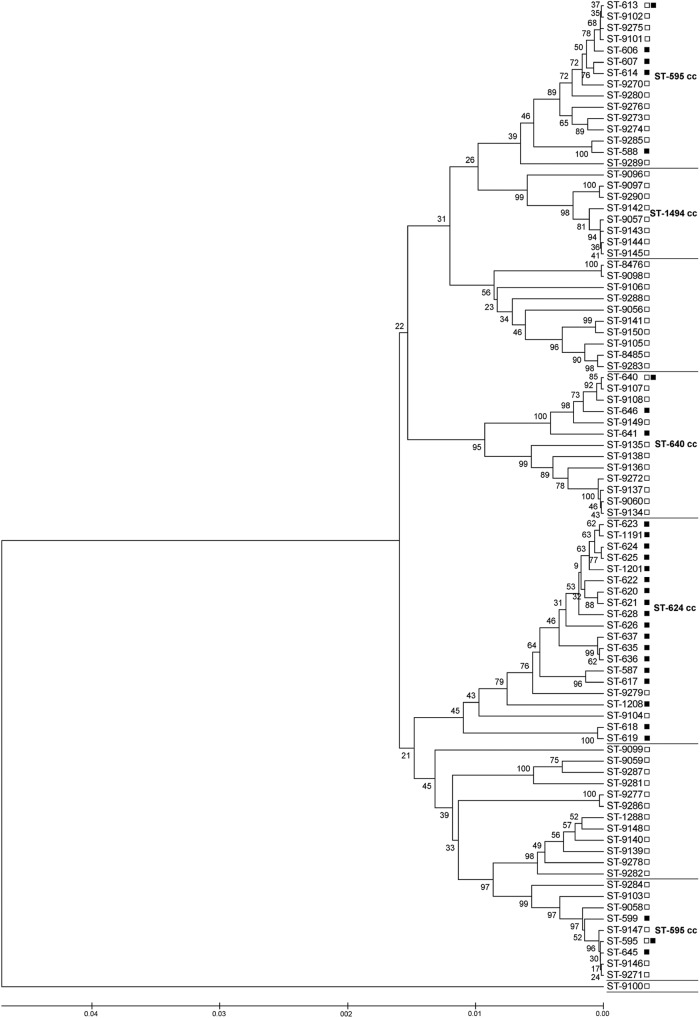
Comparison of sequence types of N. lactamica carriage isolates from Burkina Faso and from the United Kingdom, as determined by bootstrapped UPGMA analysis. Sequence types (STs) are followed by an empty square if found in Burkina Faso and a filled square if found in the United Kingdom. cc, clonal complex.
DISCUSSION
This study presents the epidemiology of N. lactamica carriage and the genetic diversity of the isolates recovered from 1- to 29-year-olds in Burkina Faso. The study shows little geographic variation of N. lactamica carriage and a stable prevalence over a 3-year period, also observed after mass vaccination with a serogroup A meningococcal conjugate vaccine.
The study was part of a large meningococcal carriage study, and the laboratory methodology was primarily aimed at finding N. meningitidis. We used an established sampling method, and extensive training and monitoring were an integral part of the study. To identify N. lactamica, we used results from the analytical steps common for both species. However, as only one or two colonies from the primary agar plate were further examined, cocolonization with N. meningitidis was not possible to estimate and the carriage prevalence of N. lactamica might have been underestimated. As meningococcal carriage prevalence (3.98% in 2009 and 6.95% in 2010-2011) (21, 22) was substantially lower than N. lactamica carriage prevalence and because of the difference in age distributions of the individuals colonized by the two species, the underestimation due to cocolonization is probably very small. On the other hand, the N. lactamica carriage rate might have been slightly overestimated, as 5% of the isolates classified as N. lactamica in Burkina Faso were not confirmed as such at NIPH. Among these isolates not confirmed as N. lactamica, one was identified as Moraxella catarrhalis while the remaining ones belonged to other Neisseria species as determined by their enzymatic profile and by 16S rRNA gene sequencing (data not shown).
Our estimate of 18.2% overall carriage, or 17.3% when considering the 95% confirmation rate at NIPH, is within the range of carriage rates reported elsewhere (1, 2, 4, 7, 8, 15, 19, 20, 32–34), and the characteristic age distribution with a maximum prevalence at 2 years of age is consistent with previous findings (4, 32, 34). Although the difference of overall carriage prevalence was calculated as significantly higher among men than women, this estimate might be biased due to the higher participation rate of young children, especially the 3- to 7-year-olds, where higher prevalence among male participants was found. The observation of a higher carriage prevalence among older women is consistent with results from other countries (8, 19), suggesting a closer contact between mother and child than between father and child. Interestingly, one study (4) has shown that children did not share the same isolates as their parents, but our results show that they do; in Burkina Faso the two dominant STs were carried by nearly all age groups (1 to 25 years for ST613 and 1 to 28 years for ST-9060). In households from which isolates from two participants swabbed the same day were selected for MLST, only a single ST was identified (3 of 3 households; data not shown).
The carriage prevalence of N. lactamica was remarkably homogeneous in Burkina Faso and was stable over time. A study conducted in Northern Ghana (27) also showed relatively stable N. lactamica carriage prevalence over a 6-year period, although the estimated prevalence was lower (4.7 to 9.3%) than that of our study. In Burkina Faso, meningococcal disease epidemiology evolved between 2009 and 2011: in 2009, the majority of invasive cases in Burkina Faso were due to N. meningitidis serogroup A, while in 2011, after MenAfriVac mass vaccination, the total number of cases was historically low; serogroup A disease was almost nonexistent, and serogroup X disease dominated. Carriage of N. meningitidis in the same population varied between 2009 and 2011, as serogroup Y dominated in 2009 (22) and serogroup X dominated in 2010 and 2011 (21). The seasonal and geographic stability of N. lactamica carriage in comparison to the significant variations observed for N. meningitidis carriage in the same population (22) and the consistency of N. lactamica carriage before and after MenAfriVac vaccination suggest that it was not affected by changing meningococcal carriage or by the epidemic context.
The genetic diversity of N. lactamica carriage isolates was consistent with previous findings (1, 4), and STs could be compared with those in a similar study performed in the United Kingdom (4). Three ST complexes were common in both studies, although within each of these common ST complexes, only one ST was represented in both populations (ST-595 for the ST-595 complex, ST-613 for the ST-613 complex, and ST-640 for the ST-640 complex). In both studies, ST-613 was one of the dominant STs. Although some STs from Burkina Faso were clustered, UPGMA analysis of STs for N. lactamica isolates from both countries reveals that there is as much genetic diversity within each of the two countries as there is between countries (Fig. 6).
The genetic diversity of N. lactamica isolates in Burkina Faso was higher than that of meningococcal carriage isolates retrieved from the same population, as only 29 different STs were identified from 809 meningococcal isolates (22) in comparison with 62 STs from 143 N. lactamica isolates. However, the diversity appears lower than in the United Kingdom, where 72 STs of N. lactamica were found among only 96 carriers. To date, a total of 340 N. lactamica STs have been registered in the MLST database (http://pubmlst.org/neisseria). UPGMA analysis of all 340 STs shows that the STs of isolates from Burkina Faso were not clustered (data not shown).
ST-9100, represented by a single isolate, was genetically distant from all the other N. lactamica isolates. Retesting of Gram staining and biochemical properties confirmed the initial classification as N. lactamica (oxidase positive, Gram-negative diplococcus with ONPG-positive and GGT-negative activity). The isolate was confirmed as N. lactamica by the API NM test (bioMérieux, France), while 16S rRNA sequencing revealed only 95% similarity to an uncultured Neisseria species clone (data not shown). Comparison of gene profiles for all the N. lactamica STs in the MLST database showed that ST-9100 was genetically close to a group of five STs registered as N. lactamica, the closest being ST-1528 (data not shown). These might represent an as-yet-unidentified species of the genus Neisseria.
Four isolates belonged to ST-1288, although in the MLST database, this ST was registered as N. meningitidis with an isolate from a carriage study in Ghana in 1999. The assignment of this ST to N. lactamica is supported by a genetic resemblance with other N. lactamica and not with N. meningitidis STs (Fig. 5).
The analysis of the genetic variation of isolates using the genome sequence of seven alleles provides the opportunity to study the variation of each ST within a certain ST complex, between ST complexes, and between species (Fig. 5). Although we found no resemblance between N. lactamica and N. meningitidis isolates by studying the MLST gene sequences, there might be similarities in other parts of the genome, such as the genes coding for outer membrane proteins. Common FetA variants have been found among Neisseria species in the United Kingdom (5), and further studies must be undertaken to verify if this is also true in Burkina Faso. Some STs that were not assigned to a clonal complex should probably be part of one: ST-9142 and ST-9290 should be assigned to the ST-1494 complex, and ST-9138 should be assigned to the ST-640 complex. Furthermore, ST-9279 and ST-9104 seem to be associated with the ST-624 complex (Fig. 6).
In summary, N. lactamica carriage in Burkina Faso showed little geographic and seasonal variation in comparison with that seen for carriage of N. meningitidis. Carriage of N. lactamica remained stable after the MenAfriVac vaccine introduction and was not affected by changing meningococcal carriage or by the epidemic context. The genetic diversity of N. lactamica isolates was high, and similarities with carriage isolates from the United Kingdom were documented. In contrast to major differences in the epidemiology of N. meningitidis carriage and disease, the epidemiology and the molecular characteristics of carried of N. lactamica isolates in Burkina Faso were similar to those reported from industrialized countries.
ACKNOWLEDGMENTS
We thank the residents in the districts of Bogodogo, Dandé, and Kaya for their participation in this study, the public health personnel from each district working in the field with mapping, recruitment, and survey, and all the laboratory technicians working in the study. We especially thank Elisabeth Fritzønn and Inger Marie Saga for excellent technical assistance, Cynthia Hatcher for supervision of laboratory activities in Burkina Faso, Flavien Aké, Stacey Martin, and Stanley C. Wei for assistance with data collection, and Lara Misegades for assistance with statistical analysis.
This publication made use of the Neisseria Multi Locus Sequence Typing website (http://pubmlst.org/neisseria) sited at the University of Oxford and funded by the Wellcome Trust and European Union.
The project was supported by the Research Council of Norway, grants no. 185784 and 196327 to D.A.C.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Alber D, et al. 2001. Genetic diversity of Neisseria lactamica strains from epidemiologically defined carriers. J. Clin. Microbiol. 39:1710–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bakir M, et al. 2001. Asymptomatic carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1400 children sampled. Eur. J. Epidemiol. 17:1015–1018 [DOI] [PubMed] [Google Scholar]
- 3. Bennett JS, Callaghan MJ, Derrick JP, Maiden MCJ. 2008. Variation in the Neisseria lactamica porin, and its relationship to meningococcal PorB. Microbiology 154:1525–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bennett JS, et al. 2005. Genetic diversity and carriage dynamics of Neisseria lactamica in infants. Infect. Immun. 73:2424–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett JS, Thompson EAL, Kriz P, Jolley KA, Maiden MCJ. 2009. A common gene pool for the Neisseria FetA antigen. Int. J. Med. Microbiol. 299:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Birtles A, et al. 2005. Multilocus sequence typing of Neisseria meningitidis directly from clinical samples and application of the method to the investigation of meningococcal disease case clusters. J. Clin. Microbiol. 43:6007–6014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blakebrough IS, Greenwood BM, Whittle HC. 1982. The epidemiology of infections due to Neisseria meningitidis and Neisseria lactamica in a northern Nigerian community. J. Infect. Dis. 146:626–637 [DOI] [PubMed] [Google Scholar]
- 8. Cartwright KAV, Stuart JM, Jones DM, Noah ND. 1987. The Stonehouse survey: nasopharyngeal carriage of meningococci and Neisseria lactamica. Epidemiol. Infect. 99:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denning DW, Gill SS. 1991. Neisseria lactamica meningitis following skull trauma. Rev. Infect. Dis. 13:216–218 [DOI] [PubMed] [Google Scholar]
- 10. Djingarey MH, et al. 2012. Effectively introducing a new meningococcal A conjugate vaccine in Africa: the Burkina Faso experience. Vaccine 30(Suppl 2):B40–B45 [DOI] [PubMed] [Google Scholar]
- 11. Evans CM, et al. 2011. Nasopharyngeal colonization by Neisseria lactamica and induction of protective immunity against Neisseria meningitidis. Clin. Infect. Dis. 52:70–77 [DOI] [PubMed] [Google Scholar]
- 12. Everts RJ, et al. 2010. Neisseria lactamica arthritis and septicemia complicating myeloma. J. Clin. Microbiol. 48:2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feil EJ, Maiden MCJ, Achtman M, Spratt BG. 1999. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol. Biol. Evol. 16:1196–1502 [DOI] [PubMed] [Google Scholar]
- 14. Finney M, et al. 2008. Characterization of the key antigenic components and pre-clinical immune responses to a meningococcal disease vaccine based on Neisseria lactamica outer membrane vesicles. Hum. Vaccines 4:23–30 [DOI] [PubMed] [Google Scholar]
- 15. Gold R, Goldschneider I, Lepow ML, Draper TF, Randolph M. 1978. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J. Infect. Dis. 137:112–121 [DOI] [PubMed] [Google Scholar]
- 16. Gorringe AR. 2005. Can Neisseria lactamica antigens provide an effective vaccine to prevent meningococcal disease? Expert Rev. Vaccines 4:373–379 [DOI] [PubMed] [Google Scholar]
- 17. Harrison LH, Trotter CL, Ramsay ME. 2009. Global epidemiology of meningococcal disease. Vaccine 27:B51–B63 [DOI] [PubMed] [Google Scholar]
- 18. Harrison OB, Maiden MCJ, Rokbi B. 2008. Distribution of transferrin binding protein B gene (tbpB) variants among Neisseria species. BMC Microbiol. 8:66 doi:10.1186/1471-2180-8-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kremastinou J, et al. 2003. Carriage of Neisseria meningitidis and Neisseria lactamica in northern Greece. FEMS Immunol. Med. Microbiol. 39:23–29 [DOI] [PubMed] [Google Scholar]
- 20. Kremastinou J, et al. 1999. Carriage of Neisseria meningitidis and Neisseria lactamica among ethnic Greek school children from Russian immigrant families in Athens. FEMS Immunol. Med. Microbiol. 23:13–20 [DOI] [PubMed] [Google Scholar]
- 21. Kristiansen PA, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin. Infect. Dis., in press [DOI] [PubMed] [Google Scholar]
- 22. Kristiansen PA, et al. 2011. Baseline meningococcal carriage in Burkina Faso before the introduction of a meningococcal serogroup A conjugate vaccine. Clin. Vaccine Immunol. 18:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kristiansen PA, et al. 2012. Laboratory quality control in a multicentre meningococcal carriage study in Burkina Faso. Trans. R. Soc. Trop. Med. Hyg. 106:289–297 [DOI] [PubMed] [Google Scholar]
- 24. LaForce FM, Konde K, Viviani S, Preziosi MP. 2007. The meningitis vaccine project. Vaccine 25:A97–A100 [DOI] [PubMed] [Google Scholar]
- 25. LaForce FM, Okwo-Bele J-M. 2011. Eliminating epidemic group A meningococcal meningitis in Africa through a new vaccine. Health Aff. (Millwood) 30:1049–1057 [DOI] [PubMed] [Google Scholar]
- 26. Lapeyssonnie L. 1963. La méningite cérébrospinale en Afrique. Bull. World Health Organ. 28(Suppl):3–114 [PMC free article] [PubMed] [Google Scholar]
- 27. Leimkugel J, et al. 2007. Clonal waves of Neisseria colonisation and disease in the African meningitis belt: eight-year longitudinal study in northern Ghana. PLoS Med. 4:e101 doi:10.1371/journal.pmed.0040101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maiden MC, et al. 1998. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc. Natl. Acad. Sci. U. S. A. 95:3140–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marri PR, et al. 2010. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 5:e11835 doi:10.1371/journal.pone.0011835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mueller JE, et al. 2007. Molecular characteristics and epidemiology of meningococcal carriage, Burkina Faso, 2003. Emerg. Infect. Dis. 13:847–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nicolas P, Norheim G, Garnotel E, Djibo S, Caugant DA. 2005. Molecular epidemiology of Neisseria meningitidis isolated in the African meningitis belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J. Clin. Microbiol. 43:5129–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Olsen SF, et al. 1991. Pharyngeal carriage of Neisseria meningitidis and Neisseria lactamica in households with infants within areas with high and low incidences of meningococcal disease. Epidemiol. Infect. 106:445–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saez-Nieto JA, Dominguez JR, Monton JL. 1985. Carriage of Neisseria meningitidis and Neisseria lactamica in a school population during an epidemic period in Spain. J. Hyg. (Lond.) 94:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simmons G, Martin D, Stewart J, Bremmer D. 2000. Carriage of N. lactamica in a population at high risk of meningococcal disease. Epidemiol. Infect. 125:99–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zavascki AP, et al. 2006. First case report of Neisseria lactamica causing cavitary lung disease in an adult organ transplant recipient. J. Clin. Microbiol. 44:2666–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]