Abstract
Elite controllers or suppressors (ES) are HIV-1-infected individuals who suppress viral replication to clinically undetectable levels without antiretroviral therapy. Understanding the mechanisms by which ES control viral replication may prove informative for the design of a therapeutic vaccine. Qualitative differences in the CD8+ T cell response have been implicated in control. Therefore, we isolated CD8+ T cells from ES and characterized the ability of sorted memory and activation subpopulations to control viral replication at various effector-to-target cell ratios using a novel modification of a CD8+ T cell suppression assay. The effector memory and terminal effector subpopulations of memory CD8+ T cells had the highest inhibitory potential over the course of a 3-day in vitro infection. Interestingly, after 5 days of infection, central memory CD8+ T cells were also very effective at suppressing viral replication. No significant correlation between the suppression of viral replication and the number of HIV-1-specific CD8+ T cells was observed. HLA-DR− CD38+ CD8+ T cells possessed the lowest inhibitory potential of the activation subpopulations. Taken together, our data suggest that there are key differences in the magnitude and kinetics of the suppression of HIV-1 replication by different CD8+ T cell subsets. These data should guide the development of an effective, cellular therapeutic vaccine that has the potential to elicit similar CD8+ T cell responses.
INTRODUCTION
The development of an effective vaccine against human immunodeficiency virus type 1 (HIV-1) is essential for the control of the HIV pandemic. In most HIV-1-infected individuals, known as chronic progressors (CP), high levels of viral replication lead to a progressive CD4+ T cell decline over a period of 5 to 10 years in the absence of antiretroviral therapy (ART). However, a unique subset of HIV-1-infected individuals termed elite suppressors (ES) are able to maintain clinically undetectable plasma HIV-1 RNA levels (<50 RNA copies/ml) in the absence of ART for the duration of infection (6). These remarkable individuals represent less than 1% of the HIV-1-infected population (41). Thus, ES provide a unique opportunity to better understand the mechanisms by which durable control is achieved. While these mechanisms are unclear, an improved understanding of the immune factors that enable this remarkable control can provide guidance for the development of an effective therapeutic vaccine for HIV-1 infection.
Many studies have linked an effective cytolytic T lymphocyte response with control of HIV-1 replication. Studies in the macaque model of elite suppression have shown that depletion of CD8+ T cells with monoclonal antibodies results in a loss of viral control (19, 44). HLA-B*57 and HLA-B*5801 are overrepresented in ES (14, 21, 29, 37, 38, 45, 50), and among HLA-B*57-positive patients, the preferential targeting of conserved HLA-B*57-restricted epitopes has been associated with control of HIV-1 replication (38). The targeting of conserved domains in Gag has been associated with escape mutations that may lead to viral attenuation, thus facilitating control of viral replication (30, 35). Additionally, genome-wide association studies have indicated that the HLA-B*57 and HLA-B*27 alleles are associated with viral control (9, 11, 16, 25, 32, 53).
While some ES do not have protective HLA alleles or strong HIV-1-specific CD8+ T cell responses (14, 40, 45, 49), the HIV-1-specific CD8+ T cell response in many ES has also been shown to be qualitatively more effective than the response in CP (23). CD8+ T cells from ES maintain a polyfunctional response after stimulation with HIV-1 peptides (2, 5, 17), and there is significantly higher expression of granzyme B and perforin by HIV-1-specific CD8+ T cells from ES than from CP (24, 36, 37). In addition, CD8+ T cells from ES are much more effective at suppressing HIV-1 replication in autologous CD4+ T cells in vitro than CD8+ T cells from CP (3, 7, 13, 37, 48, 49), and the inhibitory potential of CD8+ T cells has recently been shown to be predictive of the rate of CD4+ T cell decline early in viral infection (57).
Current analysis of the CD8+ T cell response in HIV-1 infection has focused primarily on unfractionated populations of CD8+ T cells. However, an in vitro study showed that stimulation of CD8+ T cells with HIV-1 peptides for 5 days greatly improved the inhibitory potential of these cells (37), and a recent report suggested that a vaccine that elicits effector memory (EM) CD8+ T cells was able to induce early and durable control of viral replication in simian immunodeficiency virus (SIV)-infected macaques (22). Herein, we report a novel suppression assay in which unstimulated CD8+ T cells are isolated directly ex vivo, sorted by flow cytometry into subsets based on expression of memory or activation markers, and tested for the ability to inhibit viral replication in autologous CD4+ T cells. The CD4+ T cells, which were not activated with antibodies or mitogens, were infected directly ex vivo and were maintained without exogenous cytokines to better recapitulate in vivo conditions. Thus, this variation of the CD8+ T cell suppression assay represents the most physiological analysis of the suppressive capacity of CD8+ T cells to date and the most detailed analysis of the relative inhibitory potentials of different memory and activation subsets from ES. The results provide guidance for the development of an effective cell-based vaccine against HIV-1 infection that can elicit immune responses and activation phenotypes like those observed in ES.
MATERIALS AND METHODS
Patients.
All individuals provided written informed consent prior to participating in this study, and all studies were approved by the Johns Hopkins Institutional Review Board. All 8 ES maintained undetectable plasma HIV-1 RNA levels for the duration of study (<50 copies/ml) and were positive for the HLA-B*57 allele. These patients had a mean CD4+ T cell count of 927/μl and had been infected for an average of 14 years. The 8 CP had CD4+ T cell counts ranging from 223 to 788/μl (median, 391 μl) and plasma HIV-1 RNA levels that ranged from 6,868 to 636,094 copies/ml (median 38,648 copies/ml). None of the CP enrolled were currently on antiretroviral therapy. Seronegative, healthy donors (HD) were 8 laboratory workers.
Isolation of CD4+ and CD8+ T cells.
Peripheral blood mononuculear cells (PBMCs) were isolated from whole blood by Ficoll gradient separation. CD8+ T cells were then purified by positive selection from PBMCs using human CD8+ microbeads (Miltenyi) according to the manufacturer's guidelines. CD8+ T cells were maintained in nonstimulating medium (RPMI 1640 with 10% fetal calf serum and without exogenous cytokines) on ice until cell sorting was performed. CD4+ T cells were then isolated from the CD8+ T cell-depleted PBMCs using human CD4+ Isolation Kit II (Miltenyi) according to the manufacturer's guidelines. CD4+ T cells were maintained in nonstimulating medium on ice until infection. Healthy donor CD4+ and CD8+ T cells were also isolated to test for nonspecific killing in the CD8+ suppression assay.
FACS.
CD8+ T cells from each patient were sorted into memory or activation CD8+ subsets. For the memory subsets, CD8+ T cells were stained with anti-CCR7-phycoerythrin (anti-CCR7-PE) (Biolegend) and anti-CD45RA-allophycocyanin (anti-CD45RA-APC) (Becton, Dickinson) antibodies by following the manufacturer's guidelines. The cells were then sorted using a FACS Aria (Becton, Dickinson) or a MoFlo (Beckman Coulter) cell sorter into the following 4 populations: naïve (N) (CCR7+ CD45RA+), central memory (CM) (CCR7+ CD45RA−), effector memory (CCR7− CD45RA−), and terminal effector (TE) (CCR7− CD45RA+) (10, 51). See Fig. 2 for a representative fluorescence-activated cell sorting (FACS) plot showing the memory cell sorting strategy. CD8+ T cells were also sorted separately using anti-HLA-DR-PE (Becton, Dickinson) and anti-CD38-APC (Becton, Dickinson) antibodies according to the manufacturer's guidelines. The cells were then sorted into the following 4 populations: HLA-DR+ CD38+, HLA-DR− CD38+, HLA-DR− CD38−, and HLA-DR+ CD38−. After being sorted, all cells were resuspended in nonstimulating medium at a concentration of 1 × 106 cells/ml. Cells were kept on ice until use in the CD8+ suppression assay. An aliquot of bulk CD8+ T cells was taken after staining and prior to cell sorting for comparison in the CD8+ suppression assay. Cell purity after sorting was routinely observed to be greater than 95% for each subset (data not shown). CD8+ T cells from healthy donors were also sorted for use as a negative control to test the specificity of suppression.
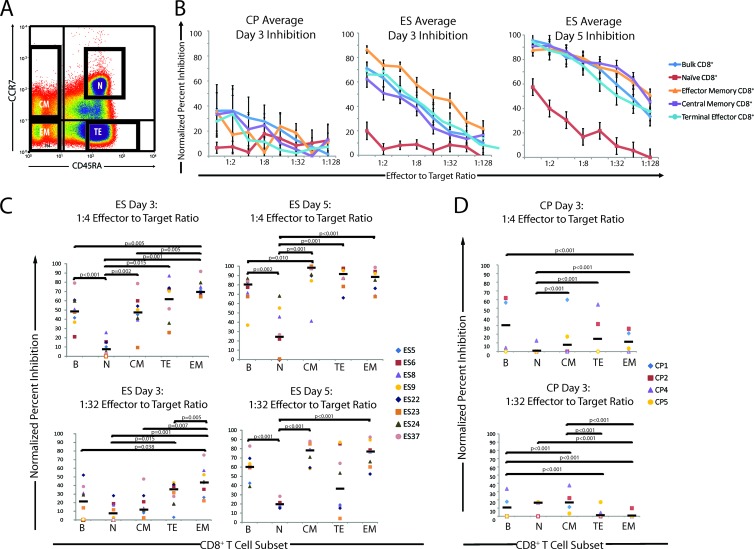
Fig 2.
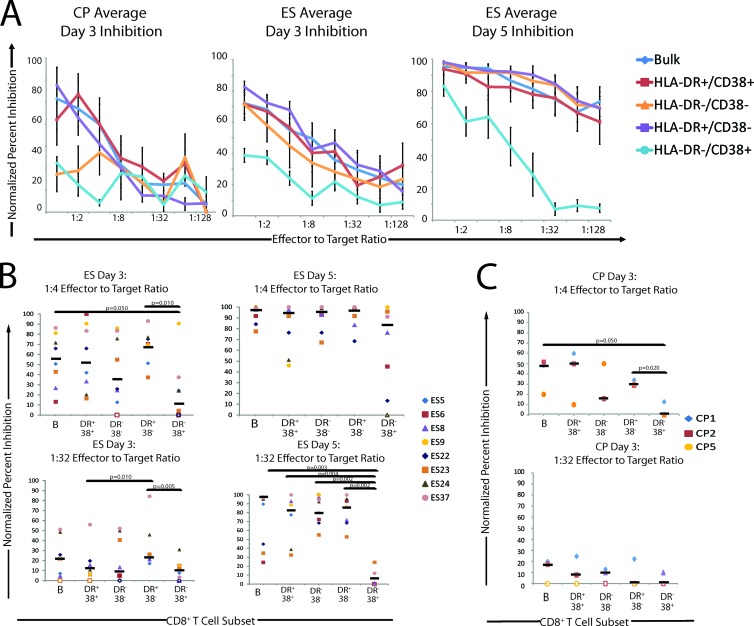
Analysis of normalized percent inhibition for CD8+ T cell memory subpopulations on days 3 and 5 after infection. (A) CD8+ T cells were isolated by magnetic bead separation from PBMCs. Cells were stained with anti-CCR7-PE and anti-CD45RA-APC antibodies and sorted. Representative data indicating the sorting strategy for one ES patient are shown. All four of the memory subpopulations were collected, and CD8+ T cells were then used at various E:T ratios in the CD8+ T cell suppression assay. (B) Average, normalized percent inhibition plots for ES (n = 8) and CP (n = 4) at different E:T ratios on day 3 for CP and ES and on day 5 for ES. The normalized values for inhibition of bulk CD8+ T cells (blue diamonds), naïve CD8+ T cells (red squares), EM CD8+ T cells (orange triangles), CM CD8+ T cells (purple squares), and TE CD8+ T cells (light blue circles) are shown. Open data points indicate a normalized percent inhibition of 0. Error bars represent the standard errors of the means. (C and D) Quantification and comparison of normalized percent inhibition for each subpopulation at 1:4 and 1:32 E:T ratios for the ES group (n = 8) (C) and the CP group (n = 4) (D). The normalized percent inhibition for each patient and each CD8+ T cell subpopulation is shown at a 1:4 E:T ratio (top) and a 1:32 E:T ratio (bottom) for day 3 (CP and ES) and day 5 (ES). Bulk (B), naïve (N), central memory (CM), terminal effector (TE), and effector memory (EM) CD8+ T cells were compared. Open circles indicate a normalized percent inhibition of 0. The median value of the normalized percent inhibition for each subpopulation is indicated. Only significant P values are indicated.
CD4+ T cell infection.
CD4+ T cells from each patient were infected ex vivo with a reporter virus by spinoculation at 1,200 × g for 2 h at room temperature (43). The virus used for infection, which has been routinely used by our lab group, was a replication-competent NL4-3 strain that was engineered to have green fluorescent protein (GFP) in the place of nef (NL43nGFP) (42). CD4+ T cells from all individuals were not stimulated prior to spinoculation and were subsequently cultured in nonstimulating medium. An aliquot of uninfected CD4+ T cells was kept on ice to be used as a negative control. Infection of CD4+ T cells was performed concurrently with the sorting of CD8+ T cells. CD4+ T cells from healthy donors were also infected to be used as a negative control to test the specificity of suppression.
CD8+ T cell suppression assay.
All cells were maintained in nonstimulating medium for the duration of the experiment. All CD4+ T cells and CD8+ T cells were isolated, sorted, infected, and cultured on the same day. Sorted CD8+ T cells (effector cells) and infected CD4+ T cells (target cells) were cocultured in a 96-well plate at various effector-to-target cell (E:T) ratios. The number of CD4+ T cells per well remained constant (100,000 cells per well), and the number of CD8+ T cells was varied. CD8+ T cells were serially diluted from a 1:1 effector-to-target cell ratio to a 1:128 effector-to-target cell ratio by 2-fold dilutions. If the number of CD8+ T cells available after cell sorting was not sufficient for a 1:1 effector-to-target cell ratio, lower initial dilutions were used and 2-fold dilutions were continued to a 1:128 effector-to-target cell ratio. CD8+ and CD4+ cocultures were maintained in a final volume of 200 μl of nonstimulating medium. Negative control wells with uninfected CD4+ T cells were present on each plate. Positive control wells with infected CD4+ target cells alone were also present on each plate. The percentage of infected cells in the positive controls ranged from 0.3 to 7.3 in CP versus 0.8 to 13.2 in ES and healthy donors, which is consist with our prior finding that CD4+ T cells from viremic patients were relatively resistant to infection in this assay (42, 46). Data from the inhibition assay were not used for the 4 CP in whom the infection rate of CD4+ T cells was less than 1.0% or for whom there was very poor cell viability. The 4 CP whose results in the inhibition assay were not used had higher plasma HIV-1 RNA levels than the 4 CP in whom superinfection of CD4+ T cells resulted in reasonable viability and infection rates (median of 297,882 versus 29,841 copies/ml). The median percentage of infected CD4+ T cells in the absence of CD8+ T cells for these 4 CP was 3.6%, compared to a median of 2.9% for HD and 8.6% for ES.
Each of the memory subsets (naïve, CM, EM, and TE) and the activation subsets (DR+ 38+, DR− 38+, DR− 38−, and DR+ 38−) were tested individually using this suppression assay. The percent infection in each well was calculated on day 3 after infection (when GFP expression can first be reliably detected) and 2 days later for comparison. Cells were stained with anti-CD3-Pacific blue (Becton, Dickinson) and anti-CD8-APCH7 (Becton, Dickinson) antibodies to distinguish target CD4+ T cells (CD3+ CD8−) and effector CD8+ T cells (CD3+ CD8+). For CM CD8+ T cells, cells were additionally stained with anti-CCR7-PE (Biolegend) and anti-CD45RA-APC (Becton, Dickinson) antibodies to analyze the changes in the CM population over the course of infection. For cytometric analysis, lymphocytes were gated by forward scatter and side scatter, and CD3+ CD8− target cells were gated and analyzed for the expression of GFP (see Fig. 1A), which is indicative of infection. The normalized percent inhibition was calculated as follows: (percent infection of wells with CD4+ T cells alone − percent infection of a well with CD4+ and CD8+ coculture)/(percent infection of CD4+ T cells alone) × 100. For example, in Fig. 1, the normalized percent inhibition for the representative data would be calculated as (8.4 − 2.2)/(8.4) × 100, resulting in a normalized percent inhibition of 73.8%. The normalized percent inhibition of each CD8+ T cell population at each effector-to-target ratio for each patient/healthy donor was calculated at day 3 and day 5. All cytometric analyses were performed using a FACS Canto II (Becton, Dickinson) and analyzed using the FACS Diva software. A minimum of 100,000 events per sample were recorded.
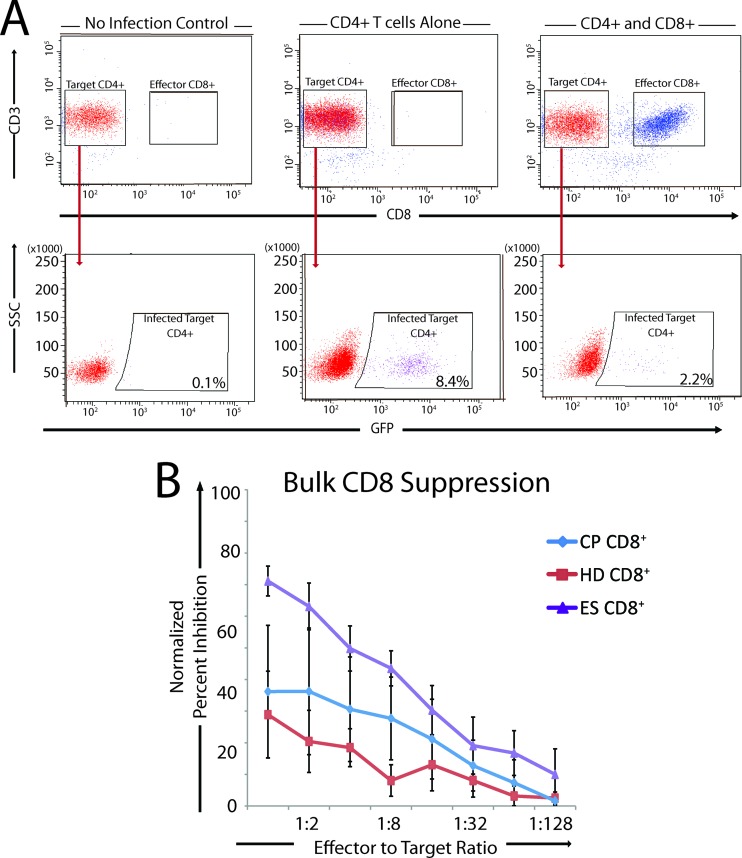
Fig 1.
Strategies for calculation of the normalized percent inhibition and bulk CD8 suppression of viral replication by HD, CP, and ES. (A) Representative data showing the strategy used to determine the normalized percent inhibition for each CD8+ T cell memory and activation subpopulation. Cells were stained with anti-CD3 and anti-CD8 antibodies to distinguish target CD4+ T cells (CD3+ CD8−) and effector CD8+ T cells (CD3+ CD8+). Target cells were then gated, and the percentage of cells that were GFP positive was calculated. Uninfected target CD4+ T cells were used as a negative control (left). Infected CD4+ T cells cultured without CD8+ T cells were used as a positive infection control (center). CD8+ T cell subpopulations were cultured with infected CD4+ T cells at various E:T ratios to allow the analysis of normalized percent inhibition (right). (B) Bulk CD8 T cells from HD (n = 8), CP (n = 4), and ES (n = 8) were used in a suppression assay with E:T ratios ranging from 1:1 to 1:128. Results for HD (red line), CP (blue line), and ES (purple line) are shown, and the error bars represent the standard errors of the means.
Intracellular cytokine staining.
Cytokine production was measured by intracellular cytokine staining as previously described (3). Briefly, bulk PBMCs from each patient were isolated directly ex vivo and stimulated overnight with overlapping Gag or Nef peptide mixtures that spanned the length of each protein at a concentration of 5 μg/ml. Prior to overnight incubation, cells were treated with a cocktail of anti-CD49d and anti-CD28, in addition to Golgi Plug and Golgi Stop (Becton, Dickinson) as recommended by the manufacturer. PBMCs treated in the same manner without HIV-1 peptides were analyzed to verify that the costimulatory factors alone did not result in stimulation. Cells were harvested after overnight incubation, and surface proteins were stained using anti-CD8-APCH7, anti-CCR7-PE, and anti-CD45RA-APC antibodies (Becton, Dickinson). The Cytofix/Cytoperm kit (Becton, Dickinson) was used to stain for intracellular cytokines according to the manufacturer's guidelines. Intracellular staining was performed using anti-gamma interferon-PeCy7 (anti-IFN-γ-PeCy7; Becton, Dickinson) and anti-tumor necrosis factor alpha-Pacific blue (anti-TNF-α-Pacific blue; Biolegend). Live lymphocytes were gated by forward and side scatter and then by CD8 expression, and they were then subdivided into the naïve, CM, EM, and TE CD8+ T cell subsets by CCR7 versus CD45RA expression. Expression of IFN-γ and TNF-α by each subset was then analyzed using an FACS Canto II (Becton, Dickinson). Data were analyzed using FACSDiva software.
Statistical analysis.
For the analysis of the significance of the difference between populations, the Mann-Whitney nonparametric t test was used. P values were calculated using SPSS software, and a P value of less than 0.05 was considered significant. Significant P values are indicated on the figures. For the correlation analysis, a Pearson's correlations analysis was used. Normalized percent inhibition is shown at an E:T ratio of 1:4 since enough effectors were available at this ratio for the different subsets for all experiments. An E:T ratio of 1:32 was randomly selected for comparison.
RESULTS
CD8+ T cells from ES effectively inhibit viral replication.
We used a novel modification of a CD8+ T cell suppression assay in which CD8+ T cells were isolated and assayed directly ex vivo. These unstimulated cells were cocultured at various E:T ratios with autologous, unstimulated, and freshly isolated target CD4+ T cells that had been infected with replication-competent HIV-1 (NL4-3 ΔNef/GFP) by spinoculation. The E:T ratio ranged from 1:1 to 1:128. The percent infection for targets alone was compared to the percent infection of targets cells cultured with CD8+ T cells to calculate a normalized percent inhibition for each subpopulation at each E:T ratio (Fig. 1A). CD8+ T cells from ES were markedly more effective than CD8+ T cells from CP and seronegative healthy donors at each E:T ratio (Fig. 1B). The effectiveness of CP CD8+ T cells shown here is likely biased by the fact that data from CP with higher plasma HIV-1 RNA levels could not be used because of very low levels of infection and/or very poor cell viability of target cells at day 3. We would expect that CD8+ T cells from these CP would be less effective at inhibiting viral replication.
The effector memory CD8+ T cells are the most effective subpopulation at suppressing viral replication.
To determine what memory population mediates the inhibition of viral replication in ES and CP, CD8+ T cells were isolated directly from 8 ES and 8 CP and stained using anti-CCR7 and anti-CD45RA antibodies. They were then sorted using a fluorescence-activated cell sorter into previously defined (10, 51) subpopulations defined by these markers: naïve (N), central memory (CM), effector memory (EM), and terminal effector (TE) (Fig. 2A). After 3 days of coculture, a dose-dependent relationship between the E:T ratio and the normalized percent inhibition was observed for each ES and CP population (Fig. 2B), whereas CD8+ T cells from an uninfected, healthy donor had no inhibitory effect (data not shown). The inhibition mediated by CD8+ T cells from ES on day 3 was markedly higher than that mediated by CD8+ T cells from CP, and an even greater level of inhibition mediated by ES CD8+ T cells was observed on day 5 (Fig. 2B).
The normalized inhibition values at E:T ratios of 1:4 and 1:32 were determined for each population. For ES, the bulk, EM, TE, and CM subsets had a significantly higher percent inhibition than did naïve CD8+ T cells at a 1:4 E:T ratio. EM cells also had a significantly higher percent inhibition than did bulk and CM CD8+ T cells at a 1:4 E:T ratio. Naïve CD8+ T cells had little inhibitory potential at either E:T ratio. At a 1:32 E:T ratio, EM CD8+ T cells caused significantly more inhibition of viral replication than did all other subpopulations and bulk CD8+ T cells, and TE CD8+ T cells inhibited viral replication significantly better than naïve CD8+ T cells.
Interestingly, differences in the patterns of inhibition by T cell subsets were observed after 5 days of coculture (Fig. 2C). Naïve CD8+ T cells showed some activity in the 5-day suppression assay, but it was relatively low compared to those of all other subtypes and bulk CD8+ T cells at a 1:4 E:T ratio. The CM, TE, and EM all showed similar levels of viral suppression, but only CM CD8+ T cells caused significantly higher inhibition than did bulk CD8+ T cells at a 1:4 E:T ratio. At a 1:32 E:T ratio, the EM and CM subsets and bulk CD8+ T cells suppressed viral replication significantly better than did naïve CD8+ T cells. The percent inhibition of TE CD8+ T cells was extremely variable at a 1:32 E:T ratio.
The EM CD8+ T cells consistently exhibited high levels of inhibition of viral replication, with a median percent inhibition of 69% at a 1:4 E:T ratio on day 3 after infection, which increased to 89% by day 5 after infection. While CM CD8+ T cells were observed to have a median percent inhibition of 48% at a 1:4 E:T ratio at day 3, the median percent inhibition increased to 94% by day 5 after infection. These data indicate potent suppression of viral replication mediated by ex vivo-isolated CD8+ T cells from ES. Overall, EM CD8+ T had a consistent and potent inhibitory effect at E:T ratios of both 1:4 and 1:32 that was maintained on both day 3 and day 5 after infection. CM CD8+ T cells, while initially producing lower inhibition than EM and TE cells, were observed to inhibit viral replication as effectively as EM CD8+ T cells by day 5 after infection. Naïve CD8+ T cells showed little to no inhibition of viral replication for the duration of the assay.
At a 1:4 ratio, bulk CD8+ T cells from CP (Fig. 2D) were more effective at viral inhibition than any sorted population of cells at day 3 of infection, and while all the memory subsets were more effective than naïve CD8+ T cells, there was no significant difference between EM, TE, and CM cell populations. Interestingly, at a 1:32 E:T ratio, CM cells were the most effective at inhibiting viral replication. We were not able to analyze the effects of different subpopulations on viral replication at day 5 of infection because of very poor viability of the target cells at this time point, consistent with our prior observation that CD4+ T cells from CP die more quickly than cells from ES and HD after superinfection (42).
A significant change in the phenotype of ES central memory CD8+ T cells occurs between days 3 and 5 of infection.
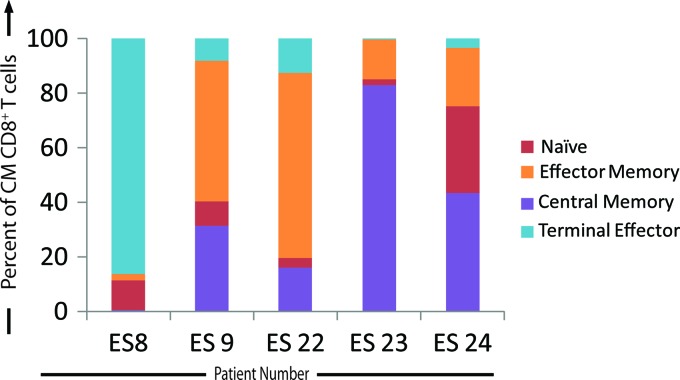
From day 3 to day 5 after infection, there was a dramatic increase in the percent inhibition by the ES CM CD8+ T cell subpopulation (Fig. 2C). Therefore, in a subset of the ES, the expression of CCR7 and CD45RA by the CM CD8+ T cells was also analyzed on day 5 after infection to determine if there were changes in the phenotype of the cells in culture after the initial culturing of the pure, sorted CD8+ T cell memory populations (Fig. 3). CM CD8+ T cells were the majority population in only one of the five ES that were analyzed. In a majority of patients, the majority cell population present was of the EM or TE phenotype. Thus, the increased suppressive ability after 5 days of infection for the sorted CM CD8+ T cell population may be a result of the differentiation of CM CD8+ T cells into effector CD8+ T cells.
Fig 3.
Changes in the CM CD8+ T cell population by day 5 after infection. Sorted CD8+ T cells were cocultured with infected CD4+ T cells on day 0. On day 5, CD8+ T cells were stained with anti-CCR7 and anti-CD45 RA antibodies, and the expression of these markers was analyzed and quantified on day 5 after infection (n = 5). The percentage of the CD8+ T cells expressing markers indicative of a TE (light blue bars), EM (orange bars), naïve (red bars), or CM (purple bars) phenotype is indicated for each ES analyzed.
Inhibitory potential is not significantly correlated with the quantity of HIV-specific CD8+ T cells.
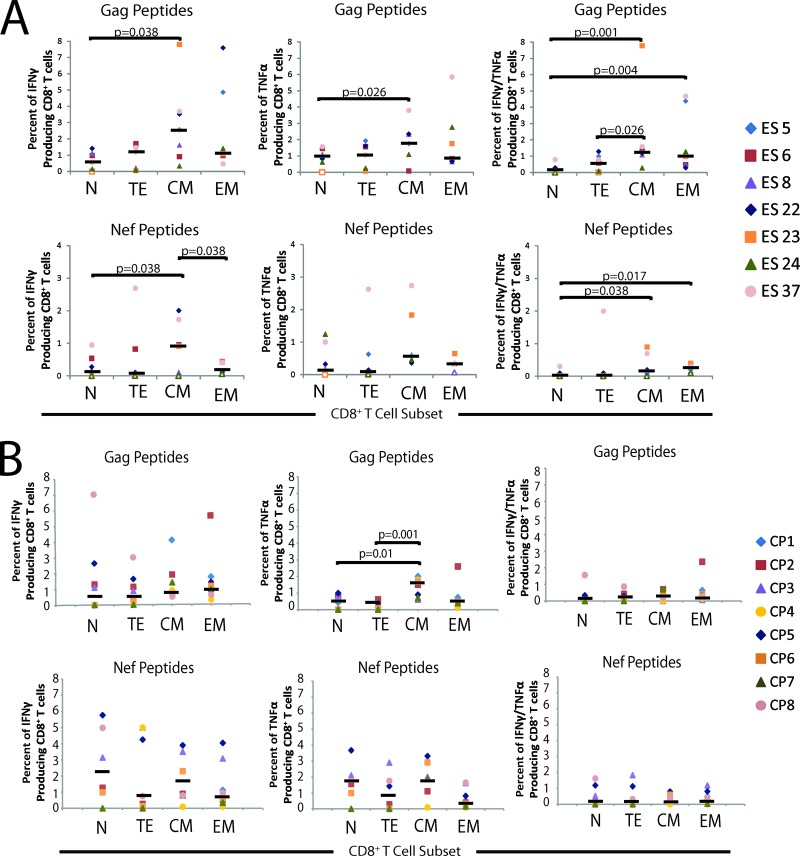
It is possible that in ES, EM and TE CD8+ T cells have an elevated inhibitory potential relative to the other memory subpopulations because there is a higher frequency of HIV-1-specific CD8+ T cells in this subset. Therefore, to further characterize the response of the memory subtypes in HIV-1 infection, we performed a 12-h intracellular cytokine staining assay to analyze the production of cytokines by different CD8+ T cells subsets after stimulation with Gag and Nef peptides. The percentages of IFN-γ, TNF-α, and TNF-α and IFN-γ dually positive cells were quantified for each memory subtype (Fig. 4A). CM cells contained significantly more IFN-γ, TNF-α, and dual-cytokine-secreting cells than naïve cells in response to Gag stimulation. The EM subset also contained a significantly higher fraction of cells that produced both TNF-α and IFN-γ compared to naïve CD8+ T cells, and the number of dual-cytokine-expressing HIV-1-specific T cells was significantly higher for CM cells than for TE CD8+ T cells. Similar trends were observed after stimulation with Nef peptides. The fraction of TNF-α and IFN-γ dually positive cells for the CM and EM populations was significantly greater than the fraction present in the naïve subpopulation. Generally, the fraction of HIV-1-specific CD8+ T cells in the EM and CM cell populations showed substantial patient-to-patient variability, and a number of patients had high levels of HIV-specific T cells in these populations. In contrast, in CP, the only significant difference in cytokine secretion seen was in TNF-α production: CM cells had significantly more cytokine-producing cells in response to Gag stimulation than TE and naïve cells (Fig. 4B).
Fig 4.
Analysis of antigen-specific production of cytokines by CD8+ T cell memory populations. PBMCs isolated directly ex vivo were stimulated overnight with Gag or Nef peptides. The percentage of IFN-γ (left), TNF-α (center), and IFN-γ and TNF-α dually positive CD8+ T cells (right) for each memory population was calculated. Results are shown as the percentage of CD8+ T cells for each memory subpopulation for each patient. (A) Gag stimulation of ES cells (n = 7) is shown at the top, and Nef stimulation is shown at the bottom. (B) Gag stimulation of CP cells (n = 8) is shown at the top, and Nef stimulation is shown at the bottom. The median value of the normalized percent inhibition for each subpopulation is indicated. Only significant P values are indicated.
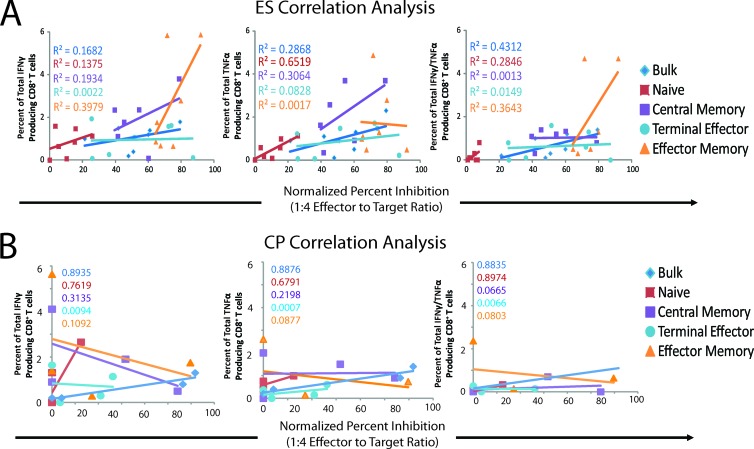
A correlation analysis was performed to determine whether there was a relationship between the frequency of HIV-1-specific CD8+ T cells and inhibitory potential in each memory subset. We compared the normalized percent inhibitions for each CD8+ T cell subpopulation at a 1:4 E:T ratio with the number of Gag-specific CD8+ T cells that produced IFN-γ, TNF-α, or both IFN-γ and TNF-α for ES (Fig. 5A) and CP (Fig. 5B). For each of the 4 memory subpopulations and bulk CD8+ T cells, no statistically significant correlations between viral inhibition at day 3 of infection and the percentage of CD8+ T cells that expressed cytokines in response to Gag stimulation was observed for either ES or CP. These data suggest that the inhibition of viral replication is not simply a function of the fraction of Gag-specific CD8+ T cells present.
Fig 5.
Correlation between the number of dually expressing HIV-1 Gag-specific CD8+ T cells and the normalized percent inhibition for ES (n = 7) (A) and CP (n = 4) (B). A Pearson's correlation analysis was performed to determine if a significant correlation existed. The correlations for bulk CD8+ T cells (blue diamonds), naïve CD8+ T cells (red squares), EM CD8+ T cells (orange triangles), CM CD8+ T cells (purple squares), and TE CD8+ T cells (light blue circles) are shown. The R2 values are color coded and indicated for each population.
CD8+ T cells with an HLA-DR− CD38+ phenotype are significantly less effective at suppressing viral replication.
Markers of CD8+ T cell activation have also been intensely studied in HIV-1 infection (8, 20, 33). Therefore, we determined which CD8+ T cell subsets defined by activation marker expression were most effective at mediating the suppression of viral replication in ES. A strategy similar to the memory subtype analysis was utilized. CD8+ T cells and CD4+ T cells were isolated directly ex vivo from PBMCs from ES and CP. All cells were maintained in nonstimulating medium for the duration of the experiment. The CD8+ T cells were stained using anti-HLA-DR and anti-CD38 antibodies and sorted using fluorescence-activated cell sorting into DR+ CD38+, DR− CD38−, DR+ CD38−, and DR− CD38+ activation subpopulations. The unstimulated, sorted CD8+ T cells were cocultured at various E:T ratios with freshly isolated, autologous, target CD4+ T cells that had been isolated directly ex vivo and infected with replication-competent NL4-3 ΔNef/GFP virus by spinoculation. The E:T ratio ranged from 1:1 to 1:128. The average percent infection for infected targets alone was compared to the percent infection seen in the presence of CD8+ T cells to calculate a normalized percent inhibition for each subpopulation at each E:T ratio (Fig. 1B).
A dose-dependent relationship between the E:T ratio and the normalized percent inhibition was observed for all ES and CP analyzed (Fig. 6A), while no response was seen for the CD8+ T cells from the uninfected, healthy donor (data not shown). The normalized inhibition values at E:T ratios of 1:4 and 1:32 are shown in Fig. 6B for the ES. Overall, the inhibitory potentials of the activation subpopulations were similar, with the DR− CD38+ population being the exception. This population had a significantly lower suppressive effect than the DR+ CD38− and bulk CD8+ T cells at a 1:4 E:T ratio at day 3 after infection. The DR+ CD38− population had the highest median inhibition, but this difference was not significant at the 1:4 E:T ratio. At a 1:32 E:T ratio, a significant difference remained between the DR+ CD38− cells and the DR− CD38+ CD8+ T cells.
Fig 6.
(A) Analysis of normalized percent inhibition for CD8+ T cell activation subsets on days 3 and 5 after infection. (B) Average, normalized percent inhibition plots for ES (n = 8) and CP (n = 3) at different E:T ratios on day 3 for CP and ES and day 5 for ES. The normalized values for inhibition of bulk CD8+ T (blue diamonds), HLA-DR+ CD38+ CD8+ T cells (red squares), HLA-DR− CD38− CD8+ T cells (orange triangles), HLA-DR+ CD38− CD8+ T cells (purple squares), and HLA-DR− CD38+ CD8+ T cells (light blue circles) are shown. Open data points indicate a normalized percent inhibition of 0. Error bars represent the standard errors of the means. (B and C) Quantification and comparison of normalized percent inhibition for each subpopulation at a 1:4 and a 1:32 E:T ratio for the ES group (n = 8) (B) and the CP group (n = 3) (C). The normalized percent inhibition for each patient and each CD8+ T cell subpopulation is shown at a 1:4 E:T ratio (top) and a 1:32 E:T ratio (bottom) for day 3 (CP and ES) and day 5 (ES). Bulk (B), HLA-DR+ CD38+ (DR+ 38+), HLA-DR− CD38− (DR− 38−), HLA-DR+ CD38− (DR+ 38−), and HLA-DR− CD38+ (DR− 38+) CD8+ T cells were compared. Open circles indicate a normalized percent inhibition of 0. The median value of the normalized percent inhibition for each subpopulation is indicated (n = 8). Only significant P values are indicated.
By day 5 after infection, the inhibitory effects of all of the activation populations were all approaching 100% (Fig. 6B). At a 1:4 E:T ratio, there were no significant differences between the subpopulations. However, at a 1:32 ratio, the DR− CD38+ CD8+ T cells produced much less inhibition than did all other populations. The inhibitory potential of the DR− CD38+ subset was significantly less than that of each of the other three activation subpopulations and bulk CD8+ T cells, with a median inhibition below 10%. CD8+ T cells expressing HLA-DR generally had a high inhibitory potential, but this was not statistically different from inhibition produced by DR− CD38− CD8+ T cells. It is interesting that at day 5 after infection, DR− CD38+ CD8 T cells had an inhibition similar to that of the other activation populations at a 1:4 ratio, but the suppression of viral replication was lost at lower E:T ratios.
The HLA-DR− CD38+ subpopulation of CD8+ T cells also had the lowest inhibitory potential of CP cells at an E:T ratio of 1:4 at day 3 of infection (Fig. 6C). No significant difference in inhibition of viral replication was seen in the different subpopulations at an E:T ratio of 1:32.
DISCUSSION
It is clear that cellular immunity is paramount in the control of viral replication by ES. However, the mechanisms by which ES mediate this remarkable control are still unclear. An improved understanding of these mechanisms can aid in the development of an effective HIV-1 vaccine, which is desperately needed for the control of the HIV-1 pandemic. Herein, we present an in-depth in vitro analysis of the control of viral replication by CD8+ T cells from 8 ES. After 3 days of infection and coculture, EM and TE CD8+ T cells were the most effective at inhibiting viral replication, suggesting that these cells represent the CD8+ T cell populations that respond most rapidly to HIV-1 infection. A recent study demonstrated early control of SIV replication in the macaque model of HIV-1 disease after monkeys were treated with a preventative vaccine that elicited an EM T cell response. In this study, the EM vaccine induced high-frequency anti-SIV EM CD8+ T cell responses, resulting in either complete control or persistent control for up to 1 year after infection (22). These data in combination with the data presented herein support a model in which an effective EM CD8+ T cell response is crucial to long-term control of HIV-1 infection.
In ES, CM CD8+ T cells showed less activity after 3 days of infection but demonstrated a remarkable increase in inhibition at all E:T ratios after 5 days of infection. This is consistent with studies in the lymphocytic choriomeningitis virus (LCMV) model that demonstrated that memory CD8+ T cells required reactivation before they effectively controlled viral replication (12). It is currently unclear if this increase in the normalized percent inhibition is mediated by cells with a CM phenotype or if CM CD8+ T cells underwent differentiation and expansion. It is possible that EM or TE CD8+ T cells are responsible for the increase in the suppressive capacity that is observed by 5 days after infection for the sorted CM cell population. This hypothesis is supported by data showing that by day 5, EM and TE CD8+ T cells were the majority population in 3 out of 5 ES that were analyzed, whereas CM CD8+ T cells were the majority population in only 1 of these ES.
It should be noted that we used a conservative sorting scheme that did not account for all CD8+ T cells, and recent studies have suggested that CD8+ T cells with HIV-1-inhibitory activity may have phenotypes different from those described here (18, 28). Functional studies of virus-specific CD8+ T cells have shown that the markers used in this study also do not perfectly distinguish between central and effector memory CD8+ T cells, and there is some plasticity in the expression of surface markers depending on the activation state (15, 26, 39, 52, 54–56). This may explain why bulk CD8+ T cells from CP were more effective than any of the sorted subsets of cells at inhibiting viral replication.
In a prior study, a correlation between HIV-1 inhibition and HIV-1-specific MIP-1 beta and CD107 CD8+ T cell responses was observed (18). In the current study, there was no significant correlation between IFN-γ and TNF-α HIV-specific CD8+ T cell responses and the normalized percent inhibition of viral replication for each memory subtype, although coproduction of these cytokines has been associated with cytotoxic potential of CD8+ T cells (31). While our findings are limited by the fact that we focused on Gag-specific T cell responses, studies have suggested that the cytotoxic T lymphocyte (CTL) response is predominantly concentrated on Gag in ES (27, 45, 47, 48). This finding agrees with previous studies demonstrating no correlation between the frequency of HIV-1-specific CD8+ T cells and viral load in cohort studies (1, 4). It has been previously been shown that qualitative differences in the HIV-1 CD8+ T cell response may be linked to control of HIV-1 replication (2, 5, 17, 24, 36, 37). Further support for this comes from the data presented here which show that EM and TE cells from ES were more effective at controlling viral replication than EM and TE subsets from CP, even though similar percentages of Gag-specific cells were present in these populations of cells.
Markers of cellular activation have also been intensely studied in HIV-1 infection, and cell activation has been associated with accelerated disease progression (8, 33, 34). However, it is unclear how immune activation specifically impacts HIV-1-specific immunity, and little is known about the suppressive ability of CD8+ T cells subpopulations expressing different activation markers. Interestingly, of the activation subpopulations defined by HLA-DR and CD38 staining, the HLA-DR− CD38+ population had a markedly decreased suppressive capacity on both day 3 and day 5 after infection. It is noteworthy that in a prior study, HIV-specific CD8+ T cells from viremic patients were shown to express high levels of CD38, whereas HIV-1-specific CTLs from ES expressed significantly lower levels of this marker (48).
It is clear that CD8+ T cells from ES show remarkable inhibition of viral replication. Many memory and activation cell subsets were able to mediate potent inhibition of viral replication after 3 and 5 days of viral infection, which is consistent with the results from a recent study of HIV-1 controllers with viral loads of <5,000 copies/ml in which CD8+ T cells with multiple different phenotypes were shown to be capable of inhibiting viral replication (18). Taken together, these data support a key role for the CD8+ T cell response in the control of viral replication and provide a detailed analysis of an effective immune response to HIV-1 infection. The development of an effective HIV-1 vaccine should stimulate EM and TE CD8+ T cell responses, which were shown to most consistently and most rapidly result in a decrease in viral replication. However, a potent CM response may also be needed to replenish these effector cells. Additionally, an HLA-DR+ CD8+ T cell activation phenotype should be elicited, as this phenotype was more effective than CD8+ T cells with the HLA-DR− CD38+ CD8+ T cell phenotype. In summary, while our study is limited by the relatively small number of patients we studied and the fact that all our ES were HLA-B*57 positive, the data presented here represent a novel application of the CD8+ T cell suppression assay and represent the most physiological study of CD8+ T cell activation and memory subsets to date. These data provide insight into an effective immune response against HIV-1 infection and could be used to guide the development of an effective vaccine.
ACKNOWLEDGMENTS
This work was supported by NIH grant R01 AI080328 (J.N.B.) and the Howard Hughes Medical Institute (R.F.S.).
We thank Hao Zhang and Lee Blosser at the Johns Hopkins Flow Cytometry Core Facilities for fluorescence-activated cell sorting.
We declare no competing financial conflicts of interest.
Footnotes
Published ahead of print 10 October 2012
REFERENCES
- 1. Addo MM, et al. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081–2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almeida JR, et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485 doi:10.1084/jem.20070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bailey JR, et al. 2008. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J. Virol. 82:7395–7410 doi:10.1128/JVI.00800-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Betts MR, et al. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983–11991 doi:10.1128/JVI.75.24.11983-11991.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Betts MR, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781–4789 doi:10.1182/blood-2005-12-4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blankson JN. 2011. The study of elite controllers: a pure academic exercise or a potential pathway to an HIV-1 vaccine? Curr. Opin. HIV AIDS 6:147–150 doi:10.1097/COH.0b013e3283457868 [DOI] [PubMed] [Google Scholar]
- 7. Buckheit RW, III, et al. 2012. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat. Commun. 3:716 doi:10.1038/ncomms1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carbone J, Gil J, Benito JM, Munoz-Fernandez A, Fernandez-Cruz E. 2001. Elevated levels of CD4+CD7− T cells in HIV infection add to the prognostic value of low CD4 T cell levels and HIV-1-RNA quantification. AIDS 15:2459–2460 [DOI] [PubMed] [Google Scholar]
- 9. Catano G, et al. 2008. HIV-1 disease-influencing effects associated with ZNRD1, HCP5 and HLA-C alleles are attributable mainly to either HLA-A10 or HLA-B*57 alleles. PLoS One 3:e3636 doi:10.1371/journal.pone.0003636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Champagne P, et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106–111 doi:10.1038/35065118 [DOI] [PubMed] [Google Scholar]
- 11. Dalmasso C, et al. 2008. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: the ANRS Genome Wide Association 01 Study. PLoS One 3:e3907 doi:10.1371/journal.pone.0003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ehl S, Klenerman P, Aichele P, Hengartner H, Zinkernagel RM. 1997. A functional and kinetic comparison of antiviral effector and memory cytotoxic T lymphocyte populations in vivo and in vitro. Eur. J. Immunol. 27:3404–3413 doi:10.1002/eji.1830271240 [DOI] [PubMed] [Google Scholar]
- 13. Elahi S, et al. 2011. Protective HIV-specific CD8+ T cells evade Treg cell suppression. Nat. Med. 17:989–995 doi:10.1038/nm.2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emu B, et al. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 82:5398–5407 doi:10.1128/JVI.02176-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faint JM, et al. 2001. Memory T cells constitute a subset of the human CD8+CD45RA+ pool with distinct phenotypic and migratory characteristics. J. Immunol. 167:212–220 [DOI] [PubMed] [Google Scholar]
- 16. Fellay J, et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947 doi:10.1126/science.1143767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferre AL, et al. 2009. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113:3978–3989 doi:10.1182/blood-2008-10-182709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freel SA, et al. 2010. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J. Virol. 84:4998–5006 doi:10.1128/JVI.00138-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedrich TC, et al. 2007. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J. Virol. 81:3465–3476 doi:10.1128/JVI.02392-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giorgi JV, et al. 1993. Elevated levels of CD38+ CD8+ T cells in HIV infection add to the prognostic value of low CD4+ T cell levels: results of 6 years of follow-up. The Los Angeles Center, Multicenter AIDS Cohort Study. J. Acquir. Immune Defic. Syndr. 6:904–912 [PubMed] [Google Scholar]
- 21. Han Y, et al. 2008. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS. 22:541–544 doi:10.1097/QAD.0b013e3282f470e4 [DOI] [PubMed] [Google Scholar]
- 22. Hansen SG, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473:523–527 doi:10.1038/nature10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hersperger AR, Migueles SA, Betts MR, Connors M. 2011. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr. Opin. HIV AIDS 6:169–173 doi:10.1097/COH.0b013e3283454c39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hersperger AR, et al. 2010. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 6:e1000917 doi:10.1371/journal.ppat.1000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International HIV Controllers Study 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 doi:10.1126/science.1195271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jagannathan P, et al. 2009. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J. Virol. 83:2728–2742 doi:10.1128/JVI.02128-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 doi:10.1038/nm1520 [DOI] [PubMed] [Google Scholar]
- 28. Killian MS, Johnson C, Teque F, Fujimura S, Levy JA. 2011. Natural suppression of human immunodeficiency virus type 1 replication is mediated by transitional memory CD8+ T cells. J. Virol. 85:1696–1705 doi:10.1128/JVI.01120-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambotte O, et al. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 41:1053–1056 doi:10.1086/433188 [DOI] [PubMed] [Google Scholar]
- 30. Leslie AJ, et al. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10:282–289 doi:10.1038/nm992 [DOI] [PubMed] [Google Scholar]
- 31. Lichterfeld M, et al. 2004. HIV-1-specific cytotoxicity is preferentially mediated by a subset of CD8(+) T cells producing both interferon-gamma and tumor necrosis factor-alpha. Blood 104:487–494 doi:10.1182/blood-2003-12-4341 [DOI] [PubMed] [Google Scholar]
- 32. Limou S, et al. 2009. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J. Infect. Dis. 199:419–426 doi:10.1086/596067 [DOI] [PubMed] [Google Scholar]
- 33. Liu Z, et al. 1998. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:332–340 [DOI] [PubMed] [Google Scholar]
- 34. Liu Z, et al. 1997. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:83–92 [DOI] [PubMed] [Google Scholar]
- 35. Martinez-Picado J, et al. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 80:3617–3623 doi:10.1128/JVI.80.7.3617-3623.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Migueles SA, et al. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061–1068 doi:10.1038/ni845 [DOI] [PubMed] [Google Scholar]
- 37. Migueles SA, et al. 2008. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29:1009–1021 doi:10.1016/j.immuni.2008.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Migueles SA, et al. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709–2714 doi:10.1073/pnas.050567397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller JD, et al. 2008. Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines. Immunity 28:710–722 doi:10.1016/j.immuni.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 40. Ndhlovu ZM, et al. 2012. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J. Virol. 86:6959–6969 doi:10.1128/JVI.00531-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ockulicz J, Lambotte O. 2011. 1. Epidemiology and clinical characteristics of elite controllers. Curr. Opin. HIV AIDS 6:163–168 [DOI] [PubMed] [Google Scholar]
- 42. O'Connell KA, Rabi SA, Siliciano RF, Blankson JN. 2011. CD4+ T cells from elite suppressors are more susceptible to HIV-1 but produce fewer virions than cells from chronic progressors. Proc. Natl. Acad. Sci. U. S. A. 108:E689–E698 doi:10.1073/pnas.1108866108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Doherty U, Swiggard WJ, Malim MH. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pandrea I, et al. 2011. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog. 7:e1002170 doi:10.1371/journal.ppat.1002170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pereyra F, et al. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571 doi:10.1086/526786 [DOI] [PubMed] [Google Scholar]
- 46. Rabi SA, et al. 2011. Unstimulated primary CD4+ T cells from HIV-1-positive elite suppressors are fully susceptible to HIV-1 entry and productive infection. J. Virol. 85:979–986 doi:10.1128/JVI.01721-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rolland M, et al. 2008. Broad and Gag-biased HIV-1 epitope repertoires are associated with lower viral loads. PLoS One 3:e1424 doi:10.1371/journal.pone.0001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sáez-Cirión A, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. U. S. A. 104:6776–6781 doi:10.1073/pnas.0611244104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sáez-Cirión A, et al. 2009. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J. Immunol. 182:7828–7837 doi:10.4049/jimmunol.0803928 [DOI] [PubMed] [Google Scholar]
- 50. Sajadi MM, et al. 2009. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J. Acquir. Immune Defic. Syndr. 50:403–408 doi:10.1097/QAI.0b013e3181945f1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708–712 doi:10.1038/44385 [DOI] [PubMed] [Google Scholar]
- 52. Sandberg JK, Fast NM, Nixon DF. 2001. Functional heterogeneity of cytokines and cytolytic effector molecules in human CD8+ T lymphocytes. J. Immunol. 167:181–187 [DOI] [PubMed] [Google Scholar]
- 53. van Manen D, et al. 2009. Association of HLA-C and HCP5 gene regions with the clinical course of HIV-1 infection. AIDS 23:19–28 doi:10.1097/QAD.0b013e32831db247 [DOI] [PubMed] [Google Scholar]
- 54. Wills MR, et al. 1999. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhigh in vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J. Immunol. 162:7080–7087 [PubMed] [Google Scholar]
- 55. Wills MR, et al. 2002. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J. Immunol. 168:5455–5464 [DOI] [PubMed] [Google Scholar]
- 56. Wilson JD, et al. 1998. Oligoclonal expansions of CD8(+) T cells in chronic HIV infection are antigen specific. J. Exp. Med. 188:785–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang H, et al. 2012. Antiviral inhibitory capacity of CD8+ T cells predicts the rate of CD4+ T-cell decline in HIV-1 infection. J. Infect. Dis. 206:552–561 doi:10.1093/infdis/jis379 [DOI] [PMC free article] [PubMed] [Google Scholar]