Abstract
Virus infection activates host cellular signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which regulates diverse cellular activities related to cell growth, survival, and apoptosis. The present study demonstrated for the first time that porcine circovirus type 2 (PCV2), a major causative agent of postweaning multisystemic wasting syndrome, which is an emerging and important swine disease, can transiently induce the PI3K/Akt pathway in cultured cells at an early step during PCV2 infection. Activation of the PI3K/Akt signal was also induced by UV-irradiated PCV2, indicating that virus replication was not required for this induction. Inhibition of PI3K activation leads to reduced virus yield, which is associated with decreased viral DNA replication and lower virus protein expression. However, inhibition of PI3K activation greatly enhanced apoptotic responses as evidenced by the cleavage of poly-ADP ribose polymerase and caspase-3 as well as DNA fragmentation using terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling staining during the early stage of PCV2 infection. Furthermore, the pancaspase inhibitor zVAD.fmk alleviated the reduction in Akt phosphorylation levels by inhibiting PI3K activation, indicating that the signaling promotes cell survival and thereby favors viral replication. These results reveal that an antiapoptotic role for the PI3K/Akt pathway induced by PCV2 infection to suppress premature apoptosis for improved virus growth after infection, extending our understanding of the molecular mechanism of PCV2 infection.
INTRODUCTION
Porcine circovirus (PCV), which belongs to the genus Circovirus in the family Circoviridae (59), was first recognized as a persistent contaminant of a continuous porcine kidney cell line (PK15) in 1974 in Germany (57). Two genotypes of PCV have been identified. PCV type 1 (PCV1) does not induce disease in pigs (1). In contrast, PCV type 2 (PCV2) is virulent for pigs (1). PCV2 infection is closely associated with postweaning multisystemic wasting syndrome (PMWS), now known as PCV2-associated diseases (PCVAD) (2, 39). PCVAD is clinically characterized by severe progressive weight loss, respiratory distress, dyspnea, tachypnea, anemia, diarrhea, and lymphadenopathy in pigs aged 5 to 18 weeks (10, 49). Severely PCV2-infected pigs may develop immunosuppression, inducing an increased susceptibility to other infectious diseases as well as poor immune response to vaccines. PCVAD is now one of the most important diseases in all swine-producing areas of the world and is increasingly recognized as a serious threat to global pig production (49).
PCV genome is a circular single-stranded DNA molecule of about 1.7 kb. Two major open reading frames (ORFs) have been recognized for PCV: ORF1, called rep, which encodes a protein of 35.7 kDa that is responsible for virus replication (34), and ORF2, called cap, which encodes the major immunogenic capsid protein of 27.8 kDa (8, 38). In addition to the replicase ORF1 and the capsid protein ORF2, a gene encoding a novel protein, ORF3, was detected in productive PCV2 infection and is involved in viral pathogenesis via an apoptotic function (31, 32).
The phosphatidylinositol 3-kinase (PI3K) pathway is an important signaling pathway that modulates diverse cellular activities, including cell survival, growth, proliferation, metabolism, migration, and apoptosis (11, 65). PI3K activates its downstream effector, the serine/threonine kinase Akt (also known as PKB), by promoting its phosphorylation at the residues at Thr308 and Ser473. Activated Akt phosphorylates a number of downstream substrates, such as caspase-9, BAD, glycogen synthase kinase 3β (GSK-3β), and FKHR, thereby leading to cell survival and growth, as well as the prevention of apoptosis by activating antiapoptotic factors and inactivating proapoptotic factors. Another downstream target of activated Akt is mTOR kinase, which is associated with two functionally distinct complexes in mammalian cells, known as mTORC1 and mTORC2 (47). It has been shown that mTORC1 controls cell growth and cap-dependent translation and mTORC2 regulates the actin cytoskeleton. For virus infections, the PI3K/Akt pathway plays an important role in virus life cycle, from virus entry through viral transcription and protein synthesis, and in enhanced cell survival by blockage of apoptosis in infected cells, oncogenic transformation, and virion assembly (5, 12, 16, 18, 19, 23, 25, 37, 43, 45, 50, 66).
PCV2 infection triggers apoptosis both in vitro and in vivo (7, 20, 26, 31, 32, 44, 48, 53). It has been further demonstrated that PCV2 infection induces apoptosis via activating the caspase-8 followed by the caspase-3 pathway (32). In a recent report, we demonstrated that PCV2 infection induces NF-κB activation in cultured cells and further elucidated the role of NF-κB activation in PCV2 replication and PCV2-induced apoptotic caspase activity (62). In addition, we demonstrated that PCV2 infection induces the activation of JNK and p38 kinase and that the activation of JNK and p38 pathway is involved in PCV2-induced apoptosis (63). As a role for the PI3K/Akt pathway in virus-induced apoptotic responses of many other viruses has been reported, we also wanted to know whether the PI3K/Akt pathway is involved in PCV2 infection and contributes to PCV2-induced cell survival and prevention of apoptosis, thus favoring virus growth. The possibility that the PI3K/Akt pathway participates in the preservation of host cell survival and blockage of apoptotic responses during viral infection prompted us to investigate the interaction between PCV2 and this signal pathway.
In the present study, we showed that Akt can be phosphorylated early during PCV2 infection in a PI3K-dependent manner. Inhibition of PI3K activation induced a lower PCV2 virus yield as well as decreased viral DNA accumulation and protein synthesis. However, this inhibition enhanced apoptotic responses in the PCV2-induced cells, as evidenced by the cleavage of poly-ADP ribose polymerase and caspase-3 as well as DNA fragmentation using terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling staining at the early stage of infection. Furthermore, this enhanced early apoptosis after inhibition of PI3K activation could be largely overcome by the pancaspase inhibitor zVAD.fmk. These results suggest that PCV2 infection activates the PI3K/Akt pathway to suppress premature apoptosis for improved virus growth after infection.
MATERIALS AND METHODS
Virus and cells.
The permanent PK15 cell line, which was free of PCV, was maintained in minimal essential medium (MEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS), 5% l-glutamine, 100 U of penicillin G/ml, and 100 μl of streptomycin/ml at 37°C in a humidified 5% CO2 incubator. The PCV2 virus used in the study was originally isolated from a kidney tissue sample of a pig with naturally occurring PMWS (strain BJW) (32).
For PCV2 infection, PK15 cells seeded the day before were infected with PCV2 strain BJW at a multiplicity of infection (MOI) of 1 50% tissue culture infective dose (TCID50) per cell. Cells were additionally treated with 300 mM d-glucosamine at 24 h after infection as described previously (58).
Reagents and antibodies.
The PI3K inhibitor LY294002 and wortmannin were purchased from Calbiochem (La Jolla, CA). The mTORC1 inhibitor rapamycin and a pancaspase zVAD.fmk inhibitor were purchased from Sigma. Rabbit, goat, or mouse antibodies against Akt1/2/3, poly-ADP-ribose polymerase (PARP), glycogen synthase kinase 3β (GSK-3β), phosphorylated Akt (p-Akt) (Ser473/Thr308), p-GSK-3β, cleaved caspase-3, and β-actin were purchased from Santa Cruz Biotechnology. Horseradish peroxidase (HRP)-linked secondary antibodies were purchased from Sigma. Fluorescein isothiocyanate (FITC)-conjugated secondary antibodies were purchased from Dako.
Quantitative real-time PCR.
Quantitative real-time PCR was performed to determine the PCV2 virus loads in cell culture supernatants collected from PCV2-infected PK15 cells at 48 and 72 h after treatment with 20 μM LY294002. The sense primer (5′-TCTGACTGTGGTTCGCTTG-3′) and the antisense primer (5′-ACGTATCCAAGGAGGCGTTA-3′) were used to amplify a 187-bp fragment from the ORF2 gene of PCV2. Viral DNA was isolated from 100 μl of cell culture supernatant by using a Qiagen DNeasy blood and tissue kit according to the manufacturer's instructions. The DNA extracted from the cell culture supernatants was resuspended in 25 μl of DNase-, RNase-, and proteinase-free water. The real-time PCR protocol followed the instructions of a iQ SYBR green Supermix kit (Bio-Rad). The PCR parameters consisted of total denaturation at 95°C for 5 min and 40 cycles of denaturation at 95°C for 10 s and annealing at 55°C for 10 s. For a standard curve, serial dilutions of plasmid pBSK (PCV2 genome cloned into pBluescript SK) were used to quantify the virus genomic copy number. Each assay was run in duplicate.
Indirect immunofluorescence assay (IFA).
PK15 monolayer cells seeded in 24-well culture plates were infected with PCV2 strain BJW. At 72 h, the cells were washed with phosphate-buffered saline (PBS) and fixed in 4% paraformaldehyde (PFA). After three washes, the cells were incubated with mouse anti-ORF2 antibody diluted in 3% bovine serum albumin (BSA)-PBS at room temperature (RT) for 1 h. After three further washes, cells were incubated with FITC-conjugated anti-mouse immunoglobulin G (Sigma) at RT for 1 h and washed with PBS three times. The cells were examined under a fluorescence microscope, and cells positive for PCV2 viral antigens were counted in six fields of view.
Virus infectivity assay.
PK15 cells were infected with PCV2 strain BJW at an MOI of 1 for 18, 24, 36, 48, 72, and 96 h in the presence of LY294002 (20 μM). Cell cultures were harvested at various times postinfection by three cycles of freeze-thawing followed by clarification. Virus infectivity was assayed by IFA as described previously (62).
Cell viability assay (XTT assay).
Cell viability in the presence or absence of different inhibitors was determined by a colorimetric sodium 3′-[1-(phenyl-aminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate (XTT)-based assay (Cell Proliferation kit II; Roche). Briefly, confluent cells in 96-well plates were incubated with different inhibitors or dimethyl sulfoxide (DMSO) for various times. At the end of each incubation period, the cells were treated with the XTT labeling mixture at 37°C for about 6 h, and absorbance was then measured by an enzyme-linked immunosorbent assay (ELISA) reader. The absorbance measured was directly proportional to the number of living culture cells. The cell viability in untreated controls at the same time point was defined as 100% survival.
Whole-cell lysates.
Whole-cell lysate extracts from PK15 cells after infection at various time points were prepared with a nuclear extract kit (Active Motif) according to the manufacturer's protocol.
Western blotting.
The whole-cell lysate extracts prepared were diluted in 2× sample buffer and boiled for 5 min. Twenty micrograms of each extract was resolved by 10 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose (NC) membranes with a semidry transfer cell (Trans-Blot SD; Bio-Rad). The membranes were blocked for 2 h at RT in TBST blocking buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.1% Tween 20) containing 5% skim milk powder to prevent nonspecific binding and then incubated with specific primary antibodies raised against ORF2, Akt1/2/3, GSK-3β, PARP, p-Akt (Ser473/Thr308), p-GSK-3β, cleaved caspase-3, and β-actin at RT for 2 h. The membranes were washed three times with TBST buffer and incubated for 2 h at RT with HRP-conjugated secondary antibodies diluted in blocking buffer. Immunoreactive bands were visualized by enhanced chemiluminescence system (Kodak Image Station 4000R).
TUNEL assay.
PK15 monolayer cells were infected with PCV2 at an MOI of 1 with or without the inhibitor LY294002 (20 μM) treatment. At various times postinfection, the cells were fixed with 4% PFA and permeabilized with 0.5% Triton X-100. A DeadEnd colorimetric TUNEL system kit (Promega, Madison, WI) was used to detect apoptosis for a TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling) assay following the instructions of the manufacturer. The cells were visualized with a light microscope. At least 300 cells in 10 randomly fields of view were scored to calculate the percentage of TUNEL-positive cells. The data are expressed as the average percentage of apoptotic cells.
Statistical analysis.
Results are presented as averages ± standard deviations or standard errors of the means. Statistical comparisons were made by using Student's t test, and differences between groups were considered significant if the P value was <0.05.
RESULTS
PCV2 infection transiently activates PI3K-dependent Akt phosphorylation.
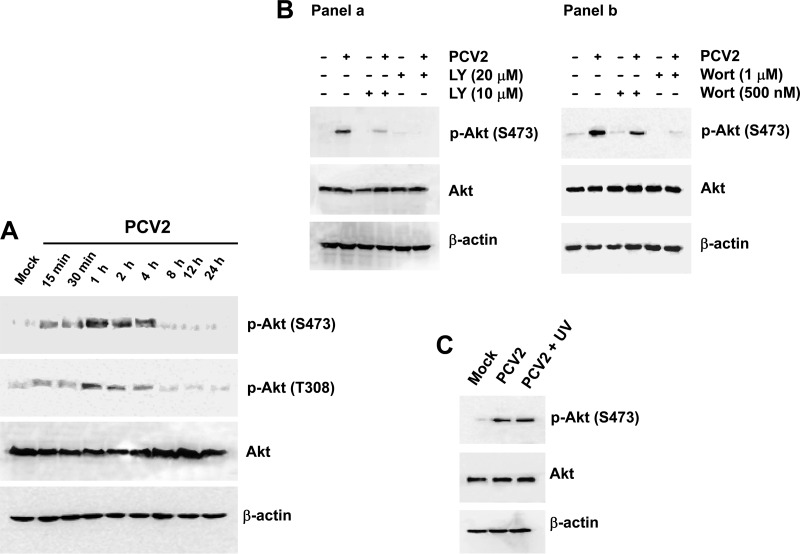
We initially determined whether Akt was phosphorylated upon PCV2 infection. Serum-starved PK15 cells were infected with PCV2 strain BJW at an MOI of 1 TCID50 per cell. Cells incubated with PBS served as mock-infected controls. Whole-cell lysates were analyzed at various times postinfection by Western blotting with specific anti-phospho(Ser473/Thr308)-Akt antibodies to evaluate the phosphorylation status of Akt. As shown in Fig. 1A, the amount of phosphorylated Akt at Ser473 was evident at 15 min. Activation was maximal at 1 h and then decreased; at 8 h, the amount of the phosphorylated Akt returned to background. The increased Thr308 phosphorylation was also concurrent with phosphorylation of Akt at Ser473 in the PCV2-infected cells (Fig. 1A). Thus, in subsequent experiments, we used the antibody against Ser473 phosphorylation to detect Akt activation. In contrast, the protein levels of total amounts of Akt remain unchanged in the PCV2-infected cells at various time points after infection compared to that in the mock-infected cells.
Fig 1.
PCV2 infection induces PI3K-dependent Akt phosphorylation. (A) Whole-cell lysates from serum-starved PK15 cells after infection with PCV2 strain BJW at an MOI of 1 TCID50. PCV2-infected cells were harvested at the indicated times postinfection, prepared, resolved by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted. The levels of Akt and its phosphorylated (Ser473/Thr308) form were analyzed. The amounts of β-actin were also assessed to monitor the equal loading of protein extracts. (B) Inhibition of PCV2-induced Akt phosphorylation by treatment with the PI3K inhibitor LY294002 (LY) (a) or wortmannin (Wort) (b). PK15 cells pretreated with LY294002 (10 and 20 μM) or Wort (500 nM and 1 μM) for 1 h were infected with the PCV2 strain BJW at an MOI of 1 for 60 min before the cell lysates were harvested at 1 h postinfection for Western blotting. (C) PI3K/Akt activation was not associated with PCV2 replication. PCV2-infected cell lysates as well as UV-irradiated PCV2-infected cell lysates 1 h postinfection were harvested at 1 h postinfection to examine Akt phosphorylation (Ser473).
We further investigated whether Akt activation in response to PCV2 infection occurred through the PI3K pathway by treating PK15 cells with a specific PI3K inhibitor, LY294002, at concentrations of 10 μM and 20 μM 1 h before they were mock or virus infected. The concentrations of the inhibitor were maintained during the adsorption period and PCV2 infection. Cell lysates were collected 1 h after infection and subjected to Western blot analysis for the detection of Akt phosphorylation (Fig. 1B, panel a). LY294002 inhibited Akt phosphorylation at both concentrations without altering total Akt levels. We also used another specific PI3K inhibitor, wortmannin, to treat the PCV2-infected cells and obtained results similar to those observed for LY294002 (Fig. 1B, panel b). Thus, the PCV2-induced phosphorylation of Akt involves a PI3K-dependent mechanism.
Based on the kinetics of Akt activation, we hypothesized that early events in viral infection were responsible for activation of the PI3K/Akt pathway. Therefore, we then used an UV light-irradiated PCV2 to determine whether phosphorylation of Akt was dependent upon PCV2 replication. Culture fluid from PCV2-infected PK15 cells was collected at 72 h postinfection and exposed to UV light (wavelength, 253 nm) for 20 min. The complete abolition of viral infectivity by UV light treatment was confirmed by immunofluorescence assay with undiluted viral suspension (data not shown). Cells were exposed to the inactivated viruses and cell lysates were then collected and subjected to Western blotting with anti-phospho-Akt antibody. In the cells exposed to the UV-irradiated PCV2 (corresponding to an MOI of 1 TCID50) at 1 h postinfection, the level of Akt phosphorylation was observed comparable to that seen in the cells infected with PCV2 alone (Fig. 1C). Thus, attachment of PCV2 to cell surface receptors alone is sufficient to trigger the phosphorylation of Akt in the absence of viral replication.
These results demonstrated that the activation of Akt signaling pathway induced by early PCV2 infection occurs through a PI3K-dependent mechanism, and this PI3K/Akt phosphorylation may be mediated by initial events of interaction of virus infection with host cells.
The PI3K/Akt signaling pathway is required for PCV2 growth.
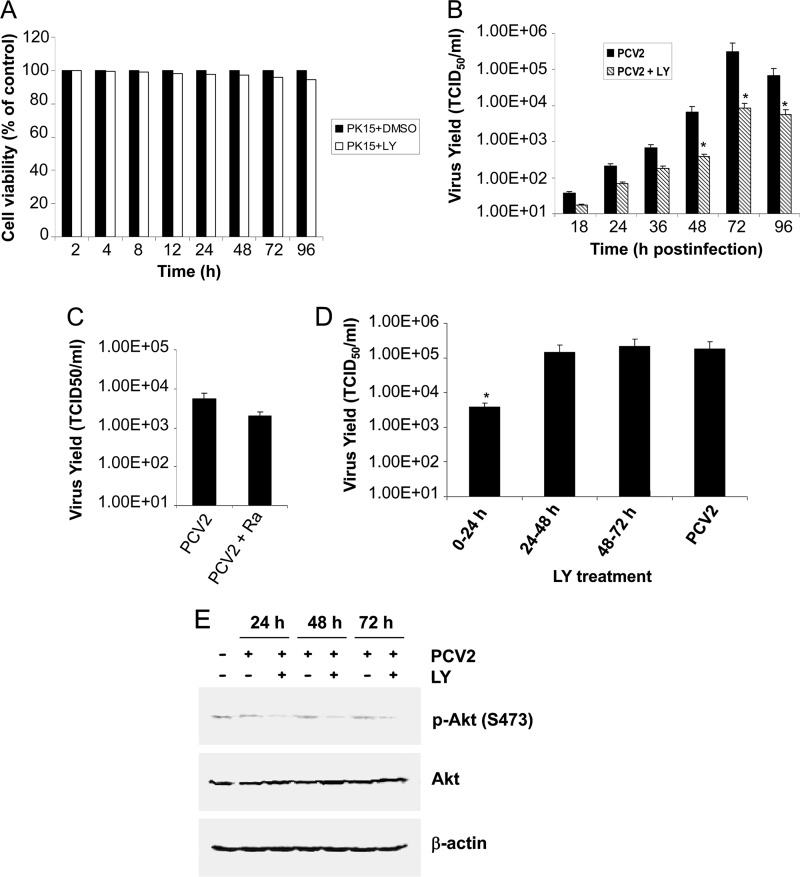
In order to investigate whether activated Akt plays any role in the replication of PCV2, we performed a one-step viral growth curve experiment in either the presence or the absence of the PI3K inhibitor LY294002. PK15 cells were infected with PCV2 at an MOI of 1 in the presence of LY294002 (20 μM), and viruses were then collected at 18, 24, 36, 48, 72, and 96 h postinfection. To rule out the possibility of reduced viral growth due to the cytotoxic effect of the inhibitor, the cell viability in the presence of either LY294002 or DMSO was first analyzed by the XTT assay. As shown in Fig. 2A, only 0.2 to 5.4% cellular toxicity was observed at the indicated time points after treatment with the inhibitor LY294002 at the indicated dose. Secondarily, viruses collected at the indicated time points postinfection after treatment with LY294002 were assayed for infectivity by using IFA. Figure 2B depicts the growth curve of virus (LY294002-treated versus non-LY294002-treated PCV2) at different times postinfection. A significant reduction in viral titers was observed when PCV2 infection was carried out in the continued presence of the PI3K inhibitor. Compared to the DMSO control, 1.25-, 1.58-, and 1.08-log reductions in PCV2 growth were observed in the presence of LY294002 for 48, 72, and 96 h, respectively. As expected, the UV-irradiated PCV2 failed to grow comparable to those seen in the mock-infected cells (data not shown).
Fig 2.
The PI3K/Akt signaling pathway is required for PCV2 growth. (A) The percentage of cell viability was measured by XTT assay in PK15 cells at the indicated time points after treatment with LY294002 (20 μM). The cell viability in untreated controls at the same time point was defined as 100% survival. Values are means ± standard deviations (SD) from three independent experiments. PK15 cells were treated for 60 min with 20 μM LY294002 (LY) (B) or 50 nM rapamycin (Ra) (C) prior to virus infection and infected with PCV2 strain BJW at an MOI of 1 in the continued presence of LY294002 for 18, 24, 36, 48, 72, and 96 h, or of rapamycin for 48 h. (D) LY294002 (20 μM) was added to PCV2-infected cells at 0 to 24, 24 to 48, and 48 to 72 h postinfection, and viral production was assayed at 72 h postinfection. Viruses were harvested at the indicated time points, and infectious titers (TCID50 per milliliter) were determined by IFA under a fluorescence microscope. Values are means ± SD of the results of three independent experiments. *, P < 0.05 for a comparison of PCV2-infected and LY294002 inhibitor-treated PCV2-infected cells. (E) PCV2-infected cell lysates for 24, 48, and 72 h were collected after treatment with LY294002 (20 μM) for 0 to 24, 24 to 48, and 48 to 72 h postinfection, respectively, and subjected to Western blotting to detect Akt phosphorylation (Ser473).
mTORC1, which serves as the downstream target of PI3K/Akt(T308), regulates key cellular events such as survival and translation. The maintenance of mTOR activity is beneficial to preserving viral cap-dependent translation. We further investigated whether mTORC1 was required for PCV2 replication. PK15 cells were incubated for 60 min prior to virus infection with rapamycin (50 nM), a specific inhibitor of mTORC1, and infected with PCV2 strain BJW at an MOI of 1 in the continued presence of rapamycin for 48 h for infectivity assay. As shown in Fig. 2C, the replication of PCV2 was only partially affected (0.45-log reduction) following mTORC1 inhibition. The result indicated that the PI3K/Akt(T308)/mTORC1 pathway may partially regulate PCV2 viral cap-dependent mRNA translation. However, this does not rule out the possibility that the other biological activities regulated by the PI3K/Akt signaling pathway, beyond mTORC1 activation, also participated to PCV2 infective cycle, thereby facilitating viral replication. The XTT assay was also used to determine cell viability of rapamycin treatment, and no obvious cellular toxicity was observed (data not shown).
To more specifically identify the stage of PCV2 infection that was targeted by the inhibition of PI3K/Akt activation, we quantified PCV2 viral growth after applying the inhibitor LY294002 (20 μM) for 0 to 24, 24 to 48, and 48 to 72 h postinfection. The PCV2-infected cells were incubated until 72 h postinfection, and viral production was assayed. As shown in Fig. 2D, addition of LY294002 for 0 to 24 h led to 1.67-log reduction in PCV2 growth compared to the DMSO-treated control. In contrast, no significant differences in viral growth were observed when the inhibitor was added for 24 to 48 or 48 to 72 h postinfection. Also, cell lysates collected at the indicated time points postinfection were subjected to Western blotting to detect Akt phosphorylation (Ser473). As shown in Fig. 2E, the amount of the phosphorylated Akt returned to background regardless of the presence or absence of the inhibitor LY294002 in the PCV2-infected cells compared to that in the mock-infected cells. This indicates that activation of PI3K/Akt induced by PCV2 infection indeed occurs at the early stage in PCV2 infection and that the initial transient activation of PI3K/Akt is required for PCV2 viral growth.
Thus, these results suggested that blockage of PI3K/Akt activation reduced virus growth, indicating that PCV2 has acquired the ability to activate cellular signaling to aid its replication.
Disruption of the PI3K/Akt pathway blocks viral DNA replication and protein synthesis.
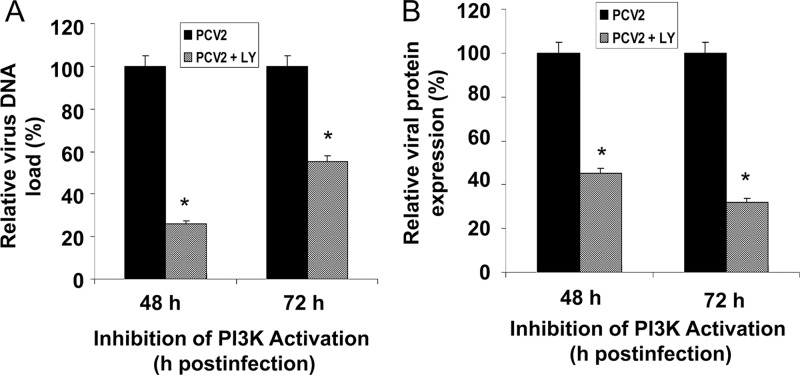
To gain insight into the mechanism underlying the decreased viral yield upon treatment with LY294002, we further examined the effect of PI3K inhibition on different stages of the virus life cycle in the cultured cells. PK15 cells were cultured either in the presence or in the absence of LY294002 (20 μM) and infected with PCV2 for 48 and 72 h. The supernatants were collected at various times for real-time PCR to calculate DNA replication. As shown in Fig. 3A, LY294002 significantly decreased viral DNA replication compared to control groups. Concurrently, the expression of PCV2 capsid protein encoded by ORF2 in PK15 cells infected with PCV2 in the presence or absence of inhibitor was analyzed by IFA. ORF2 protein expression was significantly reduced when cells were treated with the inhibitor, as demonstrated by the increased number of PCV2-positive cells observed in the infected cells (Fig. 3B). No significant differences were seen in the ORF2 protein expression between DMSO-treated infected cells and untreated infected cells (data not shown). No ORF2 protein expression was detected in the UV-irradiated PCV2-infected or mock-infected cells (data not shown). These results of reductions in PCV2 DNA replication and viral protein synthesis after inhibition of PI3K/Akt activation suggest that the PI3K/Akt pathway contributes to the virus life cycle and is beneficial for virus replication.
Fig 3.
Inhibition of the PI3K/Akt pathway decreases viral PCV2 DNA replication and protein synthesis. (A) Effect of PI3K/Akt inhibitor on PCV2 viral DNA replication. Real-time PCR analysis performed on DNA extracted from the supernatants of infected PK15 cells at 48 and 72 h postinfection after treatment with the inhibitor LY294002 (LY) (20 μM). Data for PCV2-infected cells at the indicated time points after treatment with the inhibitor are percentages of the value for PCV2-infected untreated cells (means ± SD of values from three independent experiments). *, P < 0.05 for the comparison of PCV2-infected and LY294002 inhibitor-treated PCV2-infected cells. (B) Effect of PI3K/Akt inhibitor on PCV2 protein expression. PCV2-infected PK15 cells were assayed by IFA after 48 and 72 h of treatment with LY294002 (20 μM) for the amount of PCV2 viral capsid expression. The amounts of PCV2 ORF2 protein expression are shown as percentages of the PCV2-positive signals in PCV2-infected untreated cells. Data are means ± SD from three independent experiments. *, P < 0.05 for the comparison of PCV2-infected and LY294002 inhibitor-treated PCV2-infected cells.
Inhibition of PI3K resulted in apoptotic responses early during PCV2 infection.
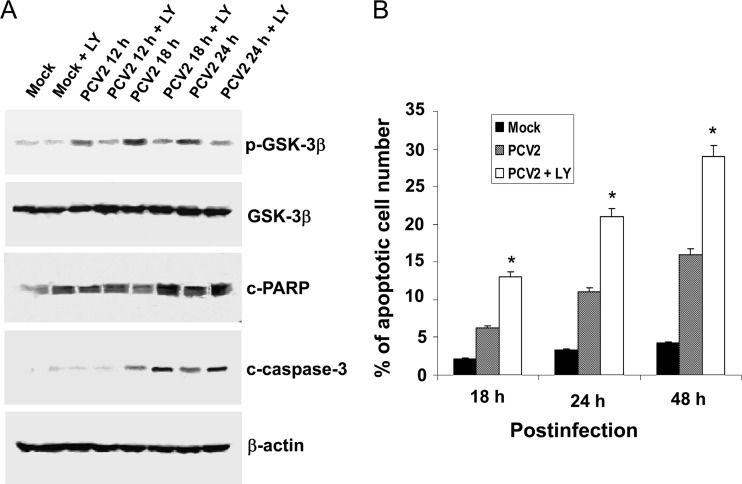
Phosphorylation and inactivation of apoptosis-promoting activity of GSK-3β has been shown to play an important role in suppressing apoptotic responses mediated by activation of PI3K/Akt signaling (41). To elucidate the role of the PI3K/Akt pathway in regulating PCV2-induced apoptosis in the cultured cells, the phosphorylation of GSK-3β was analyzed. PK15 cells were mock infected or infected with PCV2 at an MOI of 1 TCID50 for 12, 18, or 24 h in either the presence or the absence of LY294002 (20 μM). Cell lysates were then harvested and subjected to Western blotting. In the PCV2-infected cells, immunoblots of protein lysates probed with phospho-specific antibody against GSK-3β revealed increased levels of GSK-3β phosphorylation at 18 h postinfection which decreased thereafter (Fig. 4A). The increased levels of GSK-3β phosphorylation were significantly blocked by LY294002 treatment (Fig. 4A). We also measured the cleavage of host proteins associated with characteristic hallmark features of apoptosis, such as poly-ADP ribose polymerase (PARP) and caspase-3. As shown in Fig. 4A, PCV2 infection also caused the cleavage of PARP and caspase-3 at 24 h postinfection; however, when PI3K activation was blocked by LY294002, PCV2 induced the cleavage of PARP and caspase-3 at the earlier time of 18 h postinfection (Fig. 4A). These also coincide with a reduced phosphorylation of GSK-3β (Fig. 4A).
Fig 4.
PCV2 stimulation of the PI3K/Akt pathway suppresses viral apoptosis in host cells. (A) PK15 cells were treated with the PI3K inhibitor LY294002 (LY) (20 μM) or equal volumes of the solvent (DMSO) after infection with the PCV2 strain BJW at an MOI of 1 TCID50. At the indicated time points after infection, the cell lysates were harvested for Western blotting with specific antibodies to detect phosphorylation of GSK-3β, as well as the cleavage of PARP and of caspase-3. Equal protein loads were verified with β-actin blots. p, phosphorylated; c, cleaved. (B) Inhibition of the PI3K/Akt pathway activation enhances apoptosis caused by PCV2 infection by TUNEL staining. PK15 cells were infected with PCV2 in the absence or presence of PI3K inhibitor LY294002 (20 μM) for 18, 24, and 48 h and then processed for the TUNEL assay. In PCV2-infected LY294002-treated cells, increased TUNEL positivity was observed during earlier stages of infection compared to PCV2-infected untreated cells and mock-infected controls. Data are means ± SD from two independent experiments. *, P < 0.05 for the comparison of PCV2-infected and LY294002 inhibitor-treated PCV2-infected cells.
To firmly establish that the inhibition of the PI3K/Akt pathway by LY294002 results in PCV2-induced apoptosis in the cultured cells, a TUNEL assay was used to monitor apoptosis at the cellular level. PK15 cells were incubated with LY294002 (20 μM) for 1 h prior to viral infection with PCV2 at an MOI of 1 TCID50 for 18, 24, and 48 h, respectively. A brown signal was considered to be a TUNEL-positive cell. In spite of the reduced growth rate of the PCV2-infected cells after inhibition of PI3K activation, there was an increase in TUNEL-positive cells (apoptotic cells) as early as 18 h postinfection (13%) with a further increase (21 to 29%) by 24 to 48 h in the PCV2-infected LY294002-treated cells (Fig. 4B) compared to the 6.2 to 16% TUNEL positivity observed at 18 to 48 h in the PCV2-infected untreated cells. Only 2.1 to 4.2% positivity at 18 to 48 h postinfection was observed in the mock-infected cells.
These results indicate that inhibition of PI3K/Akt activation enhances the onset of premature virus-induced apoptotic responses at the early stage of PCV2 infection.
Inhibition of apoptosis enhances the PCV2-stimulated survival pathway.
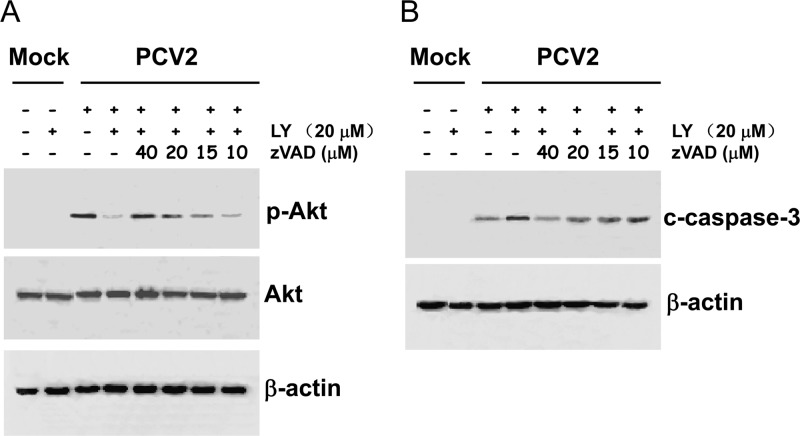
As described above, the blockage of the PI3K/Akt pathway by LY294002 enhanced PCV2-induced apoptotic responses. In order to further investigate the role of the PI3K/Akt pathway in the cell survival after PCV2 infection, PK15 cells were preincubated for 1 h with increasing concentrations (10, 15, 20, and 40 μM) of the pancaspase inhibitor zVAD.fmk in either the absence or the presence of LY294002 (20 μM). Cells were then infected with PCV2 at an MOI of 1 TCID50, and at 1 h postinfection, cell lysates were collected and subjected to Western blotting with anti-phospho-Akt antibody. As shown in Fig. 5A, incubation with the inhibitor zVAD.fmk prior to PCV2 infection increased the levels of Akt phosphorylation, which were even more pronounced than the level seen in the PCV2 infection alone. In contrast, the inhibition of the PI3K/Akt pathway resulted in decreased levels of Akt phosphorylation upon PCV2 infection. Remarkably, incubation with zVAD.fmk reversed the levels of PCV2-mediated Akt phosphorylation in a dose-dependent manner in the presence of LY294002.
Fig 5.
Inhibition of apoptosis enhances the PCV2-stimulated survival pathway. PK15 cells were preincubated for 1 h with increasing concentrations (10, 15, 20, and 40 μM) of the pancaspase inhibitor zVAD.fmk in either the absence or the presence of LY294002 (LY) (20 μM). Cells were then infected with PCV2 at an MOI of 1 TCID50, and cell lysates at the indicated times postinfection were collected and subjected to Western blotting with the indicated antibody. (A) The pancaspase inhibitor zVAD.fmk restores the levels of phosphorylated Akt in a dose-dependent manner. (B) Inhibition of apoptosis diminishes the levels of cleaved caspase-3 in a dose-dependent manner. p, phosphorylated; c, cleaved. An anti-β-actin antibody was used as an internal control for protein loading.
Inhibition of proapoptotic signals is accompanied by enhanced host survival signals upon PCV2 infection; therefore, we further investigated whether the cleavage of caspase-3 after exposure to zVAD.fmk would also be inhibited. PK15 cells were incubated with 10, 15, 20, and 40 μM zVAD.fmk for 1 h, in either the absence or the presence of LY294002 (20 μM), prior to viral infection at an MOI of 1 TCID50. At 18 h postinfection, cell lysates were collected and subjected to Western blotting with an antibody that specifically detects the cleaved form of caspase-3. As shown in Fig. 5B, the cleavage of caspase-3, as a consequence of the inhibition of the survival pathway by LY294002, was inhibited in a dose-dependent manner by the pancaspase inhibitor zVAD.fmk.
These data indicate that the inhibition of proapoptotic signals, accompanied by enhanced host survival signals upon PCV2 infection, plays an important role during the viral infective cycle.
Reduction of viral protein expression by inhibition of PI3K/Akt activation is caspase dependent.
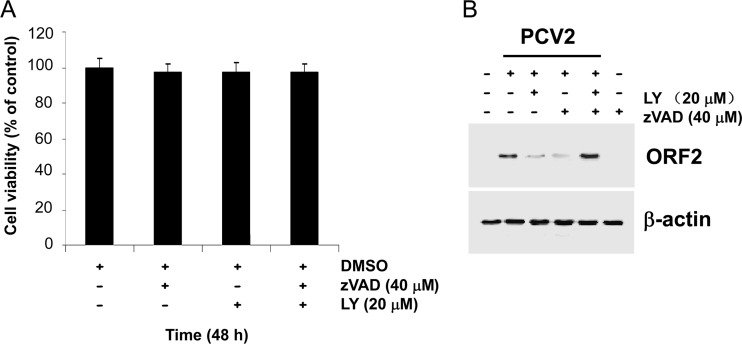
To investigate whether the LY294002-mediated inhibition of viral protein expression is due to an acceleration of apoptosis in the infected cells, we determined the levels of viral protein ORF2 expression in the presence of the pancaspase inhibitor zVAD.fmk. PK15 cells were incubated with 40 μM zVAD.fmk for 1 h, in either the absence or the presence of LY294002 (20 μM), prior to PCV2 infection at an MOI of 1 TCID50. The percentage of cell viability after treatment with 40 μM zVAD.fmk alone or together with 20 μM LY294002 for 48 h was measured by the XTT assay (Fig. 6A), showing that there was no observable cellular toxicity in the presence of zVAD.fmk and/or of LY294002 at the indicated doses. At 48 h postinfection, cell lysates were collected and subjected to Western blotting with an anti-ORF2 antibody. Remarkably, inhibition of apoptotic responses by the general pancaspase inhibitor zVAD.fmk reverses the inhibitor effect of LY294002 on viral ORF2 expression (Fig. 6B). The observation that zVAD.fmk is capable of blocking caspase-3 cleavage in the infected cells in association with the reversion of ORF2 expression, even in the presence of LY294002, indicates that the regulatory effect of the PI3K/Akt pathway exerted during PCV2 infection is a caspase-dependent event.
Fig 6.
Blockage of viral protein expression by LY294002 is caspase dependent. (A) The percentage of cell viability after treatment with 40 μM zVAD.fmk alone or together with 20 μM LY294002 was measured by the XTT assay. The cell viability in untreated controls at 48 h was defined as 100% survival. Values are means ± SD from three independent experiments. (B) PK15 cells were preincubated for 1 h with the pancaspase inhibitor zVAD.fmk (40 μM) in either the absence or the presence of LY294002 (LY) (20 μM). Cells were then infected with PCV2 at an MOI of 1 TCID50, and cell lysates were collected and subjected to Western blotting with using anti-ORF2 antibody. The pancaspase inhibitor zVAD.fmk reverses the blockage of LY294002 upon viral protein expression. Anti-β-actin antibody was used as an internal control for protein loading.
DISCUSSION
Virus manipulation of the cellular PI3K/Akt pathway serves as an important cellular survival signal for favoring a productive viral infection. Here, we demonstrated that PCV2 infection of the cultured cells induced the activation of the PI3K/Akt pathway, which is not dependent upon viral replication, and that inhibition of the PI3K/Akt activation leads to a reduction of viral activity, as determined by decreases in viral DNA replication, virus protein synthesis, and virus yield. We further demonstrated that PCV2 infection-induced apoptotic responses, as evidenced by cleavage of PARP and caspase-3 as well as DNA fragmentation by TUNEL staining, were enhanced during the early stage of viral infection, when activation of the PI3K/Akt pathway was inhibited. In addition, this enhanced early apoptosis after inhibition of PI3K activation could be largely overcome by a pancaspase inhibitor, zVAD.fmk, thereby indicating an involvement in cellular survival.
Research data have increasingly shown that a variety of viruses can induce activation of the PI3K/Akt signaling in host cells (9, 24). In the present study, we demonstrated that PCV2 stimulates Akt activation during the early stage of infection (Fig. 1A) by a mechanism that is dependent upon PI3K, as evidenced by a reduction in Akt activation levels in the PCV2-infected cells after inhibition of PI3K activation (Fig. 1B). Furthermore, the induction of PI3K/Akt activation does not require viral replication (Fig. 1C); virus receptor attachment and/or cell entry might be required for PI3K/Akt activation in the PCV2-infected cells, as described for some other viruses (28, 29, 40, 64). During their entry into cells, adenovirus, adenovirus-associated virus, respiratory syncytial virus, and Ebola virus all have the ability to promote PI3K/Akt signaling without expression of viral products (28, 29, 35, 40, 45, 64). Also, the PI3K/Akt pathway is simultaneously induced by intracellular viral proteins and particle adhesion during the internalization process of some viruses, including human papillomavirus (17), influenza A virus (14), respiratory syncytial virus (30), and bovine herpesvirus type 1 (66). The observation that phosphorylation of Akt occurs during the early stage of PCV2 infection (15 min to 8 h postinfection) (Fig. 1A and 2E), consistent with entry of PCV2 into host cells via clathrin-mediated endocytosis (36), further confirmed the hypothesis that the phosphorylation of Akt is an early event that occurs soon after viral attachment/entry.
Activation of the PI3K/Akt signaling pathway triggered by a number of viruses during the early stage of infection has been demonstrated to favor virus replication by prolonging survival of infected cells (9, 13, 24). In the present study, it was shown that inhibition of the PI3K/Akt pathway leads to a reduction of viral activity, as evidenced by decreases in viral DNA replication (Fig. 3A), viral protein synthesis (Fig. 3B), and viral yield (Fig. 2B and C), and further demonstrated that the initial transient activation of PI3K/Akt is required for PCV2 viral production (Fig. 2D). This indicates that activation of the PI3K/Akt pathway is beneficial to supporting PCV2 propagation as described in some other viruses, such as influenza A virus (14, 52), parainfluenza virus 5 (55), vaccinia and cowpox virus (54), infectious bursal disease virus (61), and so on. Many viruses exploit the PI3K/Akt pathway to facilitate various steps in their replication cycle, such as regulation of gene expression and genome replication. However, our study does not rule out the possibility that inhibition of PI3K/Akt activation blocks entry of PCV2 into host cells, as described for Ebola virus (45), thereby reducing viral DNA replication and viral protein synthesis and causing lower production of infectious virus particles in the PCV2-infected cells.
Many viruses adopt activation of the PI3K/Akt signaling as a strategy to counter apoptosis, especially during the early stages of infection for efficient virus propagation. Activated Akt phosphorylates a large number of substrates, such as GSK-3β. Our results showed that PCV2 infection, which activates the PI3K/Akt pathway, resulting in phosphorylation of GSK-3β, does not induce obvious cleavages of PARP and caspase-3 as well as DNA fragmentation at the early phase of virus infection (Fig. 4). However, significantly high levels of PARP and caspase-3 cleavages as well as enhanced DNA fragmentation were observed earlier in the PCV2-infected cells in the presence of PI3K inhibitor (Fig. 4). The role of the PI3K/Akt pathway in delaying apoptosis in the PCV2-alone infected cells was confirmed. This is consistent with the reports showing a role of the PI3K/Akt pathway in suppressing premature apoptosis during infection with respiratory syncytial virus (56), dengue virus and Japanese encephalitis virus (27), hepatitis C virus (35), influenza A virus (15, 51), hepatitis B virus (22), poliovirus (3), rotavirus (4), and infectious bursal disease virus (61). Therefore, we have expanded the data showing that the PI3K/Akt pathway plays an antiapoptotic role by suppressing the onset of premature virus-induced caspase activation and apoptosis in the early stage of PCV2 infection.
The PI3K/Akt signaling pathway has been demonstrated to play a pivotal role in cell survival and proliferation (11, 60), and its disruption results in a significant decrease in host cell viability (6, 42, 46, 60). Successful viral replication is critically dependent upon the maintenance of prosurvival and antiapoptotic signals in host cells. In the present study, the results that inhibition of Akt phosphorylation induced by PCV2 infection significantly enhances PARP and caspase-3 cleavage might indicate an antiapoptotic role for Akt during PCV2 infection. Furthermore, the pancaspase inhibitor zVAD.fmk has been shown to reverse the proapoptotic signals associated with the blockage of the PI3K/Akt pathway (Fig. 5A) and the cleavage of caspase-3 (Fig. 5B). Therefore, it is likely that the PI3K/Akt pathway triggered by PCV2 increases the viability of the infected host cells for efficient virus replication.
Cross talk between the PI3K/Akt and JNK as well as p38 MAPK pathways may help to retain the fine balance of cell survival and apoptosis. It has been shown that PI3K/Akt signaling inhibits p38-dependent apoptosis to promote endothelial cell survival (21). The PI3K/Akt-mediated survival pathway has been demonstrated to limit JNK activation during poliovirus infection (3). We previously showed that the phosphorylation and activation of JNK and p38 pathways triggered by PCV2 infection are required for viral replication and contribute to virus-induced apoptotic responses (63). In the present study, we found that the early signal transmitted by the PI3K/Akt pathway upon PCV2 infection does not require active replication of PCV2. Therefore, as described for other viruses, such as poliovirus (3) and influenza A virus (33), the early transient activation of the PI3K/Akt pathway triggered by PCV2 infection might contribute to JNK- and p38-mediated apoptotic responses in the PCV2-infected cells. However, the underlying mechanism regarding this matter needs further study.
In summary, the results presented here demonstrated that early PI3K/Akt signaling pathway activation triggered by porcine circovirus type 2 infection is required for efficient PCV2 replication as well as for suppression of premature apoptosis for improved virus growth after infection. Knowledge of the role of PI3K/Akt activation in regulating viral replication and mediating antiapoptotic responses in the cultured cells will contribute to important information about the molecular mechanism of PCV2 pathogenesis.
ACKNOWLEDGMENTS
This work was supported by grants from the Chinese National Science Fund for Distinguished Young Scholars (31025028) and the National Basic Research Program 973 (2010CB1347507).
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Allan GM, et al. 1995. Pathogenesis of porcine circovirus: experimental infections of colostrum deprived piglets and examination of pig fetal material. Vet. Microbiol. 44:49–64 [DOI] [PubMed] [Google Scholar]
- 2. Allan GM, et al. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the U. S. A. and Europe. J. Vet. Diagn. Invest. 10:3–10 [DOI] [PubMed] [Google Scholar]
- 3. Autret A, et al. 2008. Early phosphatidylinositol-3-kinase/Akt pathway activation limits poliovirus-induced JNK-mediated cell death. J. Virol. 82:3796–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bagchi P, et al. 2010. Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate virus growth by activating the cell survival pathways during early stages of infection. J. Virol. 84:6834–6845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benetti L, Roizman B. 2006. Protein kinase B/Akt is present in activated form throughout the entire replicative cycle of ΔUs3 mutant virus but only at early times after infection with wild-type herpes simplex virus 1. J. Virol. 80:3341–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. 2002. Inhibition of the phosphatidylinositol 3′-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol. Cancer Ther. 1:989–997 [PubMed] [Google Scholar]
- 7. Chang HW, et al. 2007. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet. Microbiol. 122:72–82 [DOI] [PubMed] [Google Scholar]
- 8. Cheung AK. 2003. Transcriptional analysis of porcine circovirus type 2. Virology 305:168–180 [DOI] [PubMed] [Google Scholar]
- 9. Cooray S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065–1076 [DOI] [PubMed] [Google Scholar]
- 10. Darwich L, Segales J, Mateu E. 2004. Pathogenesis of postweaning multisystemic wasting syndrome caused by porcine circovirus 2: an immune riddle. Arch. Virol. 149:857–874 [DOI] [PubMed] [Google Scholar]
- 11. Datta SR, Brunet A, Greenberg ME. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927 [DOI] [PubMed] [Google Scholar]
- 12. Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. 2003. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J. Biol. Chem. 278:3694–3704 [DOI] [PubMed] [Google Scholar]
- 13. Ehrhardt C, Ludwig S. 2009. A new player in a deadly game: influenza viruses and the PI3K/Akt signaling pathway. Cell. Microbiol. 11:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehrhardt C, et al. 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell. Microbiol. 8:1336–1348 [DOI] [PubMed] [Google Scholar]
- 15. Ehrhardt C, et al. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81:3058–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esfandiarei M, et al. 2004. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J. Virol. 78:4289–4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fothergill T, McMillan NAJ. 2006. Papillomavirus virus-like particles activate the PI3-kinase pathway via alpha-6 beta-4 integrin upon binding. Virology 352:319–328 [DOI] [PubMed] [Google Scholar]
- 18. Francois F, Klotman ME. 2003. Phosphatidylinositol 3-kinase regulates human immunodeficiency virus type 1 replication following viral entry in primary CD4+ T lymphocytes and macrophages. J. Virol. 77:2539–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukuda M, Longnecker R. 2004. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J. Virol. 78:1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galindo-Cardiel I, Grau-Roma L, Pérez-Maíllo M, Segalés J. 2011. Characterization of necrotizing lymphadenitis associated with porcine circovirus type 2 infection. J. Comp. Pathol. 144:63–69 [DOI] [PubMed] [Google Scholar]
- 21. Gratton JP, Morales-Ruiz M, Kureishi Y, Fulton D, Walsh K. 2001. Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells. J. Biol. Chem. 276:30359–30365 [DOI] [PubMed] [Google Scholar]
- 22. Guo HT, et al. 2007. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-Akt signal transduction pathway. J. Virol. 81:10072–10080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. He Y, et al. 2002. Subversion of cell signaling pathways by hepatitis C virus nonstructural 5A protein via interaction with Grb2 and P85 phosphatidylinositol 3-kinase. J. Virol. 76:9207–9217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji WT, Liu HJ. 2008. PI3K-Akt signaling and viral infection. Recent Pat. Biotechnol. 2:218–226 [DOI] [PubMed] [Google Scholar]
- 25. Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022–6032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiupel M, et al. 2005. Porcine circovirus type 2 (PCV2) causes apoptosis in experimentally inoculated BALB/c mice. BMC Vet. Res. 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee CJ, Liao CL, Lin YL. 2005. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J. Virol. 79:8388–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li E, Stupack D, Bokoch GM, Nemerow GR. 1998. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J. Virol. 72:8806–8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. 1998. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH kinase. J. Virol. 72:2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lindemans CA, et al. 2006. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-κB-dependent mechanism. J. Immunol. 176:5529–5537 [DOI] [PubMed] [Google Scholar]
- 31. Liu J, Chen I, Du Q, Chua H, Kwang J. 2006. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol. 80:5065–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu J, Chen I, Kwang J. 2005. Characterization of a previously unidentified viral protein of porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 79:8262–8274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu X, et al. 2010. The PI3K/Akt pathway inhibits influenza A virus-induced Bax-mediated apoptosis by negatively regulating the JNK pathway via ASK1. J. Gen. Virol. 91:1439–1449 [DOI] [PubMed] [Google Scholar]
- 34. Mankertz A, Mankertz J, Wolf K, Buhk HJ. 1998. Identification of a protein essential for replication of porcine circovirus. J. Gen. Virol. 79:381–383 [DOI] [PubMed] [Google Scholar]
- 35. Mannova P, Beretta L. 2005. Activation of the N-Ras-PI3K-Akt-mTOR pathway by hepatitis C virus: control of cell survival and viral replication. J. Virol. 79:8742–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Misinzo G, et al. 2005. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J. Gen. Virol. 86:2057–2068 [DOI] [PubMed] [Google Scholar]
- 37. Nair P, Somasundaram K, Krishna S. 2003. Activated Notch1 inhibits p53-induced apoptosis and sustains transformation by human papillomavirus type 16 E6 and E7 oncogenes through a PI3K-PKB/Akt-dependent pathway. J. Virol. 77:7106–7112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nawagitgul P, et al. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81:2281–2287 [DOI] [PubMed] [Google Scholar]
- 39. Opriessnig T, Meng XJ, Halbur PG. 2007. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 19:571–615 [DOI] [PubMed] [Google Scholar]
- 40. O'Shea CC, Choi S, McCormick F, Stokoe D. 2005. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle 4:883–888 [DOI] [PubMed] [Google Scholar]
- 41. Pap M, Cooper GM. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929–19932 [DOI] [PubMed] [Google Scholar]
- 42. Peruzzi F, et al. 1999. Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol. Cell. Biol. 19:7203–7215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rajala MS, Rajala RVS, Astley RA, Buutt AL, Chodosh J. 2005. Corneal cell survival in adenovirus type 19 infection requires phosphoinositide 3-kinase/Akt activation. J. Virol. 79:12332–12341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Resendes AR, et al. 2011. Apoptosis in postweaning multisystemic wasting syndrome (PMWS) hepatitis in pigs naturally infected with porcine circovirus type 2 (PCV2). Vet. J. 189:72–76 [DOI] [PubMed] [Google Scholar]
- 45. Saeed MF, Kolokoltsov AA, Freiberg AN, Holbrook MR, Davey RA. 2008. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 4:e1000141 doi:10.1371/journal.ppat.1000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sandra F, Matsuki NA, Takeuchi H, Ikebe T, Kanematsu T. 2002. TNF inhibited the apoptosis by activation of Akt serine/threonine kinase in the human head and neck squamous cell carcinoma. Cell Signal. 14:771–778 [DOI] [PubMed] [Google Scholar]
- 47. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–2101 [DOI] [PubMed] [Google Scholar]
- 48. Seeliger FA, et al. 2007. Porcine circovirus type 2-associated cerebellar vasculitis in postweaning multisystemic wasting syndrome (PMWS)-affected pigs. Vet. Pathol. 44:621–634 [DOI] [PubMed] [Google Scholar]
- 49. Segalés J, Allan GM, Domingo M. 2005. Porcine circovirus diseases. Anim. Health Res. Rev. 6:119–142 [DOI] [PubMed] [Google Scholar]
- 50. Shih WL, Kuo ML, Chuang SE, Cheng AL, Doong SL. 2000. Hepatitis B virus X protein inhibits transforming growth factor-beta-induced apoptosis through the activation of phosphatidylinositol 3-kinase pathway. J. Biol. Chem. 275:25858–25864 [DOI] [PubMed] [Google Scholar]
- 51. Shin YK, et al. 2007. SH3 binding motif 1 in influenza A virus NS1 protein is essential for PI3K/Akt signaling pathway activation. J. Virol. 81:12730–12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shin YK, Liu Q, Tikoo SK, Babiuk LA, Zhou Y. 2007. Effect of the phosphatidylinositol 3-kinase/Akt pathway on influenza A virus propagation. J. Gen. Virol. 88:942–950 [DOI] [PubMed] [Google Scholar]
- 53. Sinha A, Schalk S, Lager KM, Wang C, Opriessnig T. 2012. Singular PCV2a or PCV2b infection results in apoptosis of hepatocytes in clinically affected gnotobiotic pigs. Res. Vet. Sci. 92:151–156 [DOI] [PubMed] [Google Scholar]
- 54. Soares JAP, et al. 2009. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 83:6883–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun M, et al. 2008. Akt plays a critical role in replication of nonsegmented negative-stranded RNA viruses. J. Virol. 82:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Thomas KW, et al. 2002. Respiratory syncytial virus inhibits apoptosis and induces NF-κB activity through a phosphatidylinositol 3-kinase-dependent pathway. J. Biol. Chem. 277:492–501 [DOI] [PubMed] [Google Scholar]
- 57. Tischer I, Mields W, Wolff D, Vagt M, Griem W. 1986. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 91:271–276 [DOI] [PubMed] [Google Scholar]
- 58. Tischer I, Peters D, Rasch R, Pociuli S. 1987. Replication of porcine circovirus: induction by glucosamine and cell cycle dependence. Arch. Virol. 96:39–57 [DOI] [PubMed] [Google Scholar]
- 59. Todd D, et al. 2005. Circoviridae, p 327–334 In Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA. (ed), Virus taxonomy, VIIIth report of the International Committee for the Taxonomy of Viruses Elsevier/Academic Press, London, United Kingdom [Google Scholar]
- 60. Tsuruta F, Masuyama N, Gotoh Y. 2002. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 277:14040–14047 [DOI] [PubMed] [Google Scholar]
- 61. Wei L, et al. 2011. Infectious bursal disease virus activates the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by interaction of VP5 protein with the p85α subunit of PI3K. Virology 417:211–220 [DOI] [PubMed] [Google Scholar]
- 62. Wei L, et al. 2008. Porcine circovirus type 2 induces the activation of nuclear factor kappa B by IκBα degradation. Virology 378:177–184 [DOI] [PubMed] [Google Scholar]
- 63. Wei L, Zhu Z, Wang J, Liu J. 2009. JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J. Virol. 83:6039–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong WR, Chen YY, Yang SM, Chen YL, Horng JT. 2005. Phosphorylation of PI3K/Akt and MAPK/ERK in an early entry step of enterovirus 71. Life Sci. 78:82–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yao R, Cooper GM. 1995. Requirement for phosphatidylinostol-3 kinase in the prevention of apoptosis by nerve growth factor. Science 267:2003–2006 [DOI] [PubMed] [Google Scholar]
- 66. Zhu L, et al. 2011. Biphasic activation of PI3K/Akt and MAPK/Erk1/2 signaling pathways in bovine herpesvirus type 1 infection of MDBK cells. BMC Vet. Res. 42:57. [DOI] [PMC free article] [PubMed] [Google Scholar]