Abstract
Porcine circovirus type 2 (PCV2) capsid protein (CP) is the only protein necessary for the formation of the virion capsid, and recombinant CP spontaneously forms virus-like particles (VLPs). Located within a single CP subunit is an immunodominant epitope consisting of residues 169 to 180 [CP(169–180)], which is exposed on the surface of the subunit, but, in the structural context of the VLP, the epitope is buried and inaccessible to antibody. High levels of anti-CP(169–180) activity are associated with porcine circovirus-associated disease (PCVAD). The purpose of this study was to investigate the role of the immune response to monomer CP in the development of PCVAD. The approach was to immunize pigs with CP monomer, followed by challenge with PCV2 and porcine reproductive and respiratory syndrome virus (PRRSV). To maintain the CP immunogen as a stable monomer, CP(43–233) was fused to ubiquitin (Ub-CP). Size exclusion chromatography showed that Ub-CP was present as a single 33-kDa protein. Pigs immunized with Ub-CP developed a strong antibody response to PCV2, including antibodies against CP(169–180). However, only low levels of virus neutralizing activity were detected, and viremia levels were similar to those of nonimmunized pigs. As a positive control, immunization with baculovirus-expressed CP (Bac-CP) resulted in high levels of virus neutralizing activity, small amounts of anti-CP(169–180) activity, and the absence of viremia in pigs following virus challenge. The data support the role of CP(169–180) as an immunological decoy and illustrate the importance of the structural form of the CP immunogen in determining the outcome following infection.
INTRODUCTION
Porcine circovirus-associated disease (PCVAD) encompasses a variety of progressive disease syndromes that include a variety of clinical signs, such as respiratory distress, wasting, dermatitis, reduced growth performance, and reproductive failure (3, 4, 12). A new syndrome, acute pulmonary edema (APE), is characterized by the rapid onset of respiratory distress followed by death (5). Experimental studies demonstrate that the infection of pigs with porcine circovirus type 2 (PCV2) alone is necessary but not sufficient to induce PCVAD and requires additional cofactors, which can include infection with viruses or bacteria, immune stimulation following vaccination, and the genetics of the host (17, 20, 21, 27, 28, 33, 35). The contribution of cofactors in disease progression is likely related to immune modulation combined with increased numbers of proliferating lymphocytes, the primary targets of virus replication. One example is the experimental coinfection of pigs with PCV2 and porcine reproductive and respiratory syndrome virus (PRRSV). Coinfection results in increased PCV2 viremia and the appearance of clinical signs resembling PCVAD (1, 30, 36, 40, 43).
PCV2 isolates are placed in two major genotypes, termed PCV2a and PCV2b (37). A third genotype, PCV2c, was identified in archived tissues from Denmark (6). The virion is nonenveloped, with a 1.7-kb circular single-stranded DNA genome which is dominated by three open reading frames (ORFs) (10). The largest, ORF1, encodes the replicase proteins, Rep and Rep′ (23). ORF3, which is embedded within ORF1, is reported to be involved in apoptosis. However, a role for ORF3 in pathogenesis remains controversial (14, 18). ORF2 codes for the 233- or 234-amino-acid capsid protein (CP), which is responsible for forming the homopolymer icosahedral capsid (26). In addition, CP participates in the attachment, entry, and shuttling of the viral genome across the nuclear pore complex and into the nucleus, the site of virus replication (25, 39). CP expressed in baculovirus or Escherichia coli spontaneously forms a virus-like particle (VLP), demonstrating that CP alone is sufficient for capsid formation (15, 19, 45, 46). Recombinant vaccines incorporating baculovirus-expressed CP (Bac-CP) are effective in reducing viremia, improving growth performance, and protecting against PCVAD (7, 12, 16, 24). Another vaccine approach is the expression of CP using a PCV1 backbone (8). We showed that sera from pigs vaccinated with Bac-CP preferentially react with a single CP polypeptide fragment consisting of residues 43 to 233 [CP(43-233)] and possess strong virus neutralizing activity. PCVAD-affected pigs and a subset of pigs experimentally infected with PCV2 recognize CP(43-233) but also recognize a group of truncated polypeptides that contain a single epitope, 169-STIDYFQPNNKR-180, which is located within the epitope C region of CP (42, 44). Mahé et al. (22) identified a similar immunodominant oligopeptide. Results of alanine scanning mutagenesis showed that 173-Tyr, 174-Phe, 175-Glu, and, to a lesser extent, 179-Lys are important for antibody (Ab) recognition (42). Removal of a single key residue is sufficient to inhibit antibody recognition.
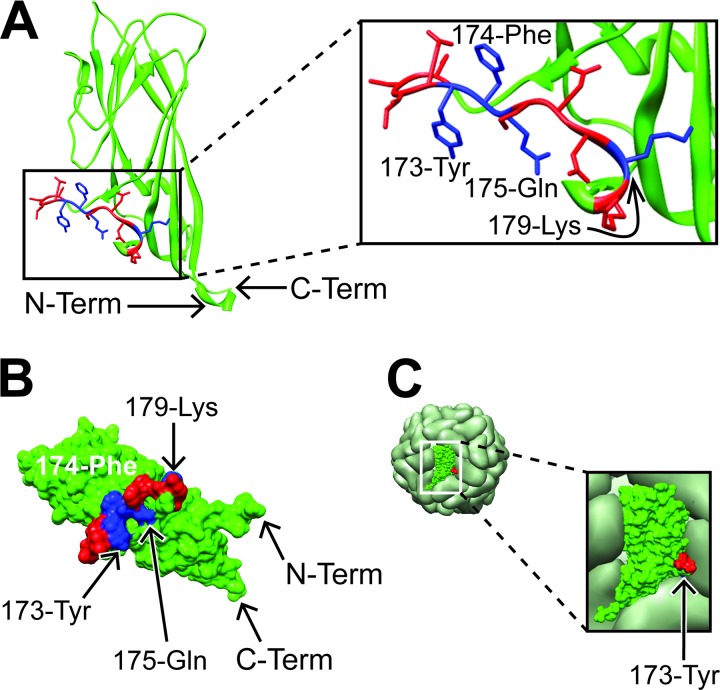
Recently, Khayat et al. (15) reported the crystal structure of CP(40-233). Maintenance of CP as a monomer required the presence of 20 mM 3-(cyclohexylamino)-1-propanesulfonic acid (CAPS) and 200 mM l-Arg. The monomer structures were assembled into a VLP model consisting of 60 CP subunits to form an icosahedron with T=1 symmetry, which was identical to a cryo-electron microscopy (EM) reconstruction of VLPs derived from Bac-CP. Representations of ribbon and space-filling structures for a single CP subunit are shown in Fig. 1A and B. The CP(169-180) domain forms an external loop structure, which protrudes from the outer surface of the CP subunit. The key antibody binding residues, 173-Tyr, 174-Phe, and 175-Glu, are located in the middle of a connecting loop domain and lie in a similar plane. The location and orientation of CP(169-180) within the VLP capsid are shown in Fig. 1C. The CP(169-180) region is located near the interface of the icosahedral 3-fold axis. Close examination reveals that, of the key residues, only 173-Tyr (blue residue in Fig. 1C) is visible on the surface of the VLP but is located at the bottom of a cleft formed by the junction of three CP monomers. The remaining antibody binding residues are not visible on the surface.
Fig 1.
Location of the CP(169–180) epitope within a single CP subunit and VLP. Depicted are the ribbon (A) and surface (B) maps of a single CP subunit. The blue and red residues form the epitope CP(169–180). The blue residues are important for antibody recognition (22). The VLP (C) shows the surface of the VLP with a single CP shown in green, red, and blue. The 173-Tyr residue is colored blue; however, due to it's location and orientation, it is not accessible on the surface of the VLP. The red and blue regions correspond to the same residues identified in panel A. Coordinates for the PCV2 CP(41–233) subunit and VLP were accessed through the RCSB Protein Data Bank (PDB code 3R0R) (2, 11) and loaded into the open source molecular visual program, Chimera (29). Term, terminus.
Combining previous experimental observations with the models for the CP monomer and VLP provides a structural basis for the function of CP(169-180) as a decoy epitope, i.e., an immunodominant epitope not involved in protection, and diverts the immune response away from protective epitopes. Therefore, we predicted that immunization of pigs with monomer CP should favor the production of antibodies against CP(169-180) and should not protect pigs following virus challenge. In this study, pigs were immunized with a stabilized form of CP monomer protein and then cochallenged with PCV2 and PRRSV. The results showed that immunization with the CP monomer induced high levels of PCV2-specific antibodies but low levels of PCV2 neutralizing activity and failed to protect pigs following virus challenge. Overall, the antibody response produced against the CP monomer was similar to that observed for pigs with PCVAD.
MATERIALS AND METHODS
Cloning and expression of proteins and polypeptides.
A PCV2b capsid gene fragment containing amino acid residues 43 to 233 was cloned and expressed in the E. coli vector pHUE as previously described (42). To obtain enhanced green fluorescent protein (EGFP), the coding region of EGFP was PCR amplified from the pEGFP-C3 vector using the forward primer 5′-CCGCGGTGGTATGGTGAGCAAGGGCGAGG and the reverse primer 5′-AAGCTTTTACTTGTACAGCTCGTCCATGC. SacII and HindIII restriction enzyme sites (underlined sequences) were added to 5′ primer ends. The PCR product was doubly digested with SacII and HindIII, cloned into pHUE (2), and then transformed into E. coli BL21 (Invitrogen) according to the manufacturer's instructions. For protein expression, bacteria were grown in Luria-Bertani (LB) broth plus ampicillin (0.01 mg/ml) and incubated at 37°C with shaking. When the optical density at 600 nm (OD600) reached 0.4 to 0.6, protein expression was induced with isopropyl β-d-1-thiogalactopyranoside (IPTG; 1 mM/ml final concentration), and bacteria were harvested 4 h later. Protein was purified using a USB PrepEase histidine-tagged protein purification kit (Affymetrix/USB) under nondenaturing conditions, according to the manufacturer's directions. Purity was assessed by SDS-PAGE, and total protein was measured using a Bio-Rad protein assay (Bio-Rad Laboratories, Inc.).
Size exclusion chromatography (SEC).
A 400-mm by 15-mm column (Fischer and Porter) was packed with Sephacryl G-200 high-resolution gel filtration medium (GE Healthcare) to a final bed volume of 60 ml. The column was equilibrated with elution buffer (EB) (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole, pH 8.0 [Affymetrix/USB]) and calibrated for a 1-ml/min flow rate, as recommended by the manufacturer. The void volume (V0) was determined using Blue Dextran (Sigma). Proteins of known size, including bovine serum albumin (BSA; New England BioLabs), lysozyme (Sigma), and GFP fused to ubiquitin (Ub-GFP) were included as standards. Proteins and Blue Dextran were diluted in EB to a final concentration of 1 mg/ml and loaded onto the column. Two-milliliter fractions were collected. Blue Dextran was measured by absorbance at 610 nm. Protein concentrations were measured in each fraction by protein assay (Bio-Rad). The peak fraction containing Ub-GFP was confirmed by GFP fluorescence. In addition to total protein, the relative concentration of Ub-CP(43–233) was estimated by immunoassay. Briefly, from an initial dilution of 1:2, serial 2-fold dilutions of each Ub-CP(43–233)-containing fraction were prepared in 0.05 M carbonate binding buffer (pH 9.6), 100 μl of each was added to a 96-well enzyme-linked immunosorbent assay (ELISA) plate (Costar), and the plate was incubated overnight at 4°C. After incubation, plates were washed with phosphate-buffered saline (PBS) containing 0.01% Tween 20 (PBST) and blocked for 2 h at room temperature with PBS containing 10% goat serum (PBS-GS). Plate-bound antigen was detected with serum from a pig with PCVAD. Serum, diluted 1:400 in PBS plus 10% goat serum, was added to each well (Colorado Serum Company). Following incubation and washing steps, 100 μl of peroxidase-labeled goat anti-swine antibody (Accurate Chemical and Scientific Corp.), diluted 1:2,000 in PBS-GS, was added to each well. After incubation at room temperature for 1 h, plates were washed with PBST, and 100 μl of the chromogenic substrate ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] (KPL) was added to each well. Peroxidase activity was detected by measuring the absorbance at 405 nm. A standard curve was constructed by plotting the elution volume (Ve) divided by V0 against the log10 of the formula weight of each standard.
Animal experiments.
Experiments involving animals were performed after approval by Kansas State University Institutional Animal Use and Biosafety committees. PCV2-negative pigs were derived as described by Opriessnig et al. (29). Briefly, offspring from pregnant dams possessing indirect fluorescence antibody (IFA) titers of less than 320 were selected. At 3 weeks of age, 26 pigs, confirmed PCR negative for PCV2 and with low IFA titers, were assigned to three treatment groups with eight pigs in each group, plus a reference control group of two pigs. The seven pigs that possessed maternally derived antibodies were divided equally between treatment groups. Table 1 and Fig. 2 show the experimental groups and the timeline for the experiment, respectively. Treatment groups included pigs immunized with Ub-CP, pigs immunized with Bac-CP (commercial vaccine), and pigs that were not immunized (nonimmunized, or non-IM). The reference control group contained two pigs that were neither immunized nor challenged. The Ub-CP immunogen consisted of Ub-CP(43-233) at a concentration of 50 μg/ml emulsified in Emulsigen-D adjuvant (20%, vol/vol; MVP Technologies), gentamicin (30 μg/ml), and thimerosal (0.01%) prepared in a 2-ml volume of minimal essential medium (MEM). Pigs were immunized intramuscularly with a 2-ml dose at 4 and 7 weeks of age. The Bac-CP immunogen consisted of a commercial vaccine (Intervet-Schering Plough) administered as a 2-ml dose at 4 and 7 weeks of age, according to the label instructions. The PCV2b-PRRSV preparation used for challenge was the same as described previously (42, 43). Pigs in each treatment group were intermingled and challenged with PCV2b-PRRSV at a dose of approximately 105 50% tissue culture infective doses (TCID50) for each virus. The 3-ml virus inoculum was administered intranasally, with 1.5 ml divided between nares. Following virus challenge, pigs were monitored daily for clinical signs, and blood samples were collected weekly. Weights were measured at the beginning of the study, at virus challenge, and at the termination of the study. At the end of the study, lung, kidney, and lymphoid tissues were collected for histopathology and PCV2 immunohistochemistry (IHC).
TABLE 1.
Treatment groups
| Group (n)a | Immunogen | PCV2/PRRSV |
|---|---|---|
| Non-IM (8) | None | Yes |
| Bac-CP (8) | Bac-CP vaccine | Yes |
| Ub-CP (8) | Ub-CP | Yes |
| Control (2) | None | No |
n, number of pigs.
Fig 2.
Study timeline. wks, weeks.
Immunohistochemistry for PCV2.
PCV2 antigen was detected by IHC staining on paraffin-embedded tissue sections. At necropsy, tissues were immediately placed in 10% buffered formalin. After processing, paraffin-embedded sections were mounted on slides, deparaffinized, and stained using an automated procedure (NexES IHC Staining Module; Ventana Medical). PCV2 antigen was detected using a rabbit anti-PCV2 polyclonal antibody (Veterinary Medical Research and Development, Inc.) Bound antibody was detected with biotinylated goat anti-rabbit IgG followed by avidin-horseradish peroxidase and diaminobenzidine (DAB) chromogen (Ventana Medical). Slides were counterstained with hematoxylin and eosin.
PCV2 PCR.
PCV2 in serum was measured using a semiquantitative TaqMan PCR assay, performed as routine diagnostic tests by personnel in the Kansas Veterinary Diagnostic Laboratory (KSVDL). Briefly, total DNA was isolated from serum using a MagMAX-96 Viral DNA Isolation Kit (Applied Biosystems) according to the manufacturer's instructions. The PCRs were carried out on a QST 7500 Real-Time PCR System (Applied Biosystems) in a 96-well format. For the construction of a standard curve, dilutions of template DNA were prepared and assayed concurrently with the samples. The assay results were reported as the log10 of PCV2 DNA copy number per 50-μl reaction volume.
Measurements of PCV2 and PRRSV antibodies.
Total PCV2 antibody in serum was measured by IFA as previously described (43). Briefly, rapidly dividing swine testicle (ST) cells, maintained in MEM with 7% fetal bovine serum (FBS) and gentamicin (30 μg/ml [MEM-FBS-Gent]) on 96-well plates, were infected with a laboratory isolate of PCV2b. Three days later, the plates were fixed in 80% acetone. Serum samples were added at an initial dilution of 1:40 followed by serial 1:2 dilutions in a total volume of 100 μl. Samples were diluted in PBS with 10% fetal bovine serum (PBS-FBS; Sigma) and incubated for 2 h at room temperature. After samples were washed with PBS, fluorescein isothiocyanate (FITC)-labeled anti-pig antibody (Jackson Laboratory) diluted 1:2,000 in PBS-FBS was added to each well. Plates were incubated for 2 h at room temperature, washed, and viewed on an inverted fluorescence microscope. The results were reported as the reciprocal of the last serum dilution showing fluorescence.
PCV2 neutralizing activity was measured as previously described (43). Briefly, serial 1:2 dilutions of serum were prepared in MEM-FBS-Gent. Four 100-μl replicates of each dilution were mixed with 100 TCID50 of PCV2b (100 μl each) and incubated for 1 h at 37°C. The well contents were transferred onto day-old ST cells in 96-well plates. Following incubation for 4 days at 37°C, wells were fixed in 80% acetone. Infection was detected with anti-PCV2 antibody (kindly provided by Ying Fang), diluted 1:2,000 in phosphate-buffered saline (PBS), followed by incubation with FITC-labeled anti-mouse antibody (Jackson Laboratory). The log2 50% neutralizing antibody (NA50) endpoint was calculated according to the method of Spearman and Karber (9).
Anti-CP(169-180) antibody was measured by ELISA on 96-well plates coated with 100 μl of CP(169-180) oligopeptide (21st Century Biochemicals) at a concentration of 4 μg/ml (42, 43). Plates were incubated overnight at 4°C. After being washed with PBS containing 0.05% Tween 20 (PBST), plates were blocked for 2 h at room temperature (RT) with PBS containing 10% goat serum (PBS-GS). Serum, diluted 1:400 in PBS-GS, was added to each well and incubated for 2 h at RT. Plates were washed with PBST, and 100 μl of peroxidase-labeled goat anti-swine antibody (Accurate Chemical and Scientific Corp.), diluted 1:2,000 in PBS-GS, was added to each well. After incubation at RT for 1 h, plates were washed with PBST, and 100 μl of ABTS was added to each well. Peroxidase activity was detected by measuring the absorbance at 405 nm on a FLUOstar Omega instrument (BMG Labtech). To compare results across experiments, each ELISA plate included an internal positive control as previously described (42, 43). Results were reported as the antibody binding ratio, calculated as the A405 value of the unknown sample minus background divided by the A405 value of the internal positive control minus background.
PRRSV-specific antibody was analyzed by a commercially available ELISA (PRRS X3; IDEXX). Samples were considered positive if the ratio of the sample to the positive control was greater than 0.39, as recommended by the manufacturer.
Statistical methods.
Statistical analysis was performed using GraphPad Prism, version 5.04, for Windows (GraphPad Software, San Diego, CA). Data incorporating repeated measures were analyzed by one-way analysis of variance followed by a Tukey posttest. Differences at specific time points were analyzed by a Kruskal-Wallis test. If significant differences were detected, specific groups at time points were assessed using Wilcoxon's test. Nonrepeated measures were analyzed using a Kruskal-Wallis test. If differences were identified, measures were further assessed by Wilcoxon's test.
RESULTS
SEC of Ub-CP(43–233).
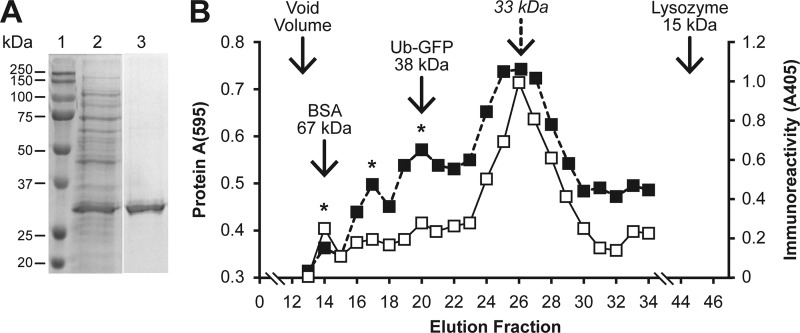
In this study, a 76-amino-acid ubiquitin molecule was fused to CP(43–233) as a means to maintain CP in a stable monomeric form. The removal of ubiquitin with the deubiquitylating enzyme, Usp-2cc (2), resulted in precipitation of CP(43-233) (data not shown). The expressed fusion protein was analyzed by SDS-PAGE. As shown in Fig. 3A, purified Ub-CP was present as a single 33-kDa protein, which is similar to the formula weight calculated for the 6×His-Ub-CP fusion protein. The structural form of Ub-CP was analyzed by size exclusion chromatography (SEC) on Sephacryl G-200. Monograms for total protein and immunoreactive Ub-CP are shown in Fig. 3B. Total and immunoreactive protein monograms were similar. Immunoreactive CP showed minor peaks in fractions 14, 17, and 20, which suggested the presence of a small quantity of Ub-CP in multimeric forms. Plotting the major peak against the standard curve showed that Ub-CP eluted as a 34.3-kDa molecule. Based on these results, the Ub-CP used for the immunization of pigs was present as a relatively pure monomer protein with only a small amount of Ub-CP present in a multimeric form.
Fig 3.
Size exclusion chromatography of CP(43–233). (A) SDS-PAGE gel showing affinity-purified Ub-CP. (B) Standard curve and size estimation for Ub-CP. A monogram of Ub-CP(43–233) eluted on a Sephacryl G-200 column was constructed by measuring total (solid line) and immunoreactive (dashed line) protein. Solid arrows show the location of the peaks for the BSA, Ub-GFP, and lysozyme standards. The dashed arrow shows the predicted peak for Ub-CP. The asterisks identify minor immunoreactive peaks, which may represent CP multimers. The elution volume of each fraction was 2 ml.
Clinical disease and outcome.
The overall design of the experiment is shown in Fig. 2. At approximately 7 days after virus challenge, pigs in all virus groups showed signs of mild respiratory distress, a clinical sign associated with acute porcine reproductive and respiratory syndrome (PRRS). The two pigs in the nonchallenged control group remained healthy. Serology confirmed that all virus-challenged pigs were positive for PRRSV (data not shown). At the end of the study, histopathology showed lesions in the lungs, including interstitial pneumonia with the infiltration of lymphocytes, macrophages, and neutrophils. IHC showed PCV2 antigen in lung tissue sections from all infected groups. PCV2 staining was localized in the bronchiolar epithelium. However, there was a difference in the number of pigs with antigen staining in lung. The greatest number of pigs positive for PCV2 antigen was in the Ub-CP group (six of eight pigs positive for PCV2 antigen), followed by the nonimmunized group (four of eight pigs), and the Bac-CP group (two of eight pigs).
Antibody response to PCV2.
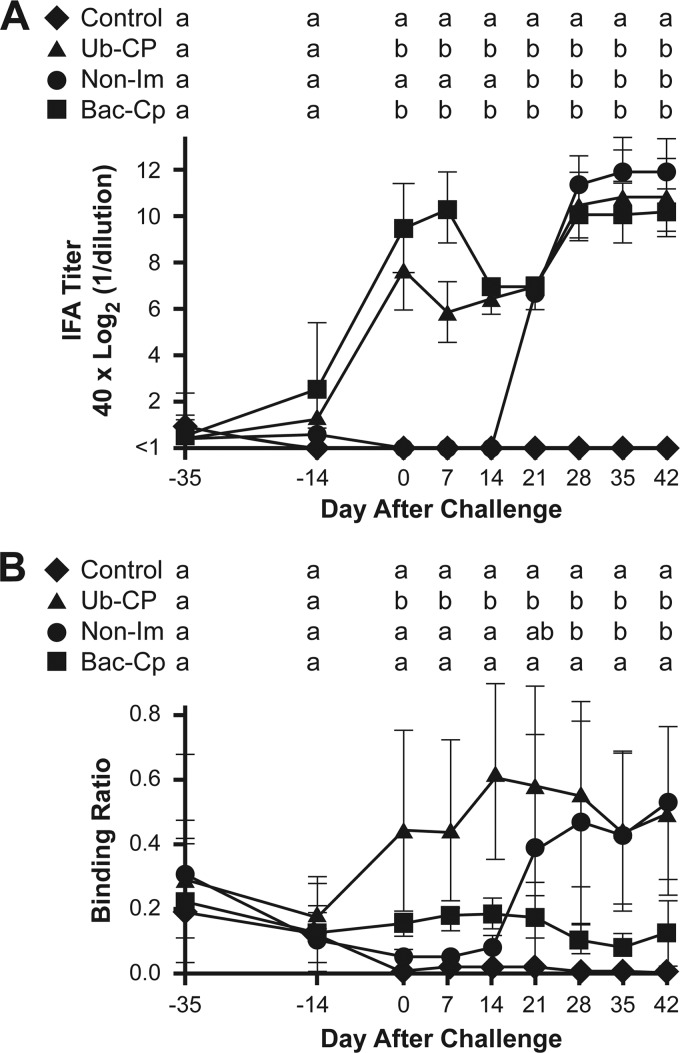
Serum samples, collected before and after immunization, were tested for total PCV2 antibody by IFA (Fig. 4). At the beginning of the study, low levels of PCV2-specific antibodies were detected in several pigs, likely the result of maternally derived antibody. For the Ub-CP group, a detectable increase in mean Ab titer was observed at the time of the second immunization (day −14), which peaked at the time of virus challenge and was followed by a second peak 28 days after challenge. Pigs immunized with the PCV2 vaccine, Bac-CP, showed a statistically similar antibody response. For the non-IM group, antibody was detected by 21 days after virus challenge and peaked by 28 days. For days 21 to 42 after infection, the IFA titers for the three challenge groups were similar and not statistically different. PCV2 antibody titers for the two nonchallenged control pigs remained below detectable levels throughout the study.
Fig 4.
Total PCV2 and CP(169–180) oligopeptide antibody response. Total antibody (A) was measured by indirect fluorescent antibody assay (IFA). CP(169–180) immunoreactivity (B) was determined by ELISA using plates coated with BSA-conjugated CP(169–180). Results show the mean value for each group. Means with the same letter are not significantly different (P > 0.05).
Serum samples were also reacted with the CP(169–180) oligopeptide. Significant amounts of anti-CP(169–180) binding activity were detected in the group Ub-CP pigs at the time of virus challenge and remained elevated throughout the remainder of the study period (Fig. 4B). In contrast, anti-CP(169–180) activity in the Bac-CP group remained near background levels and similar to the levels for the two noninfected control pigs. Following virus challenge, the non-IM group showed anti-CP(169–180) activity, which first appeared at 21 days after infection and peaked at the same level of binding activity as that of the Ub-CP group.
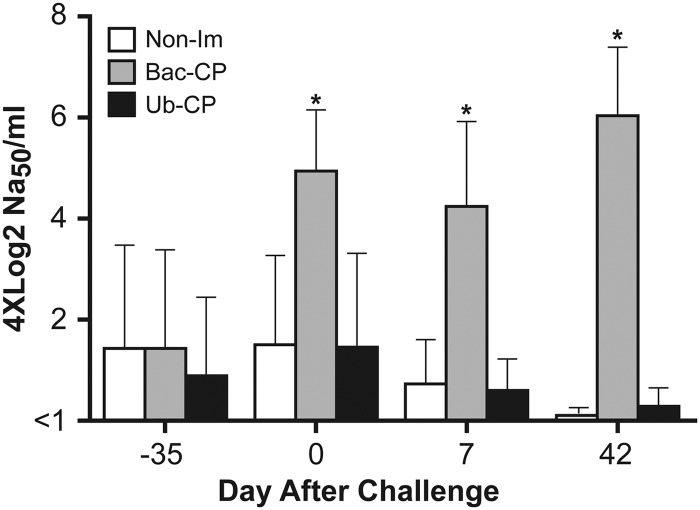
Results for PCV2 neutralizing activity for day −35 (time of first immunization), day 0 (time of virus challenge), 7 days after challenge, and at the termination of the study are presented in Fig. 5. At day −35, small amounts of neutralizing activity were detected, a likely result of maternal antibody present in the serum. After two immunizations with baculovirus-expressed CP, neutralizing activity was significantly higher than in the non-IM and Ub-CP groups. The level of neutralizing activity for the Bac-CP group remained significantly elevated throughout the remainder of the study. The decrease in the levels of neutralizing activity in the non-IM and Ub-CP groups likely reflects the gradual decay of maternal antibody.
Fig 5.
PCV2 neutralizing activity. The 50% neutralizing activity (NA50)/ml was determined by performing serum neutralization assays as described in Materials and Methods. The asterisk indicates means that are significantly different (P < 0.05).
PCV2 viremia.
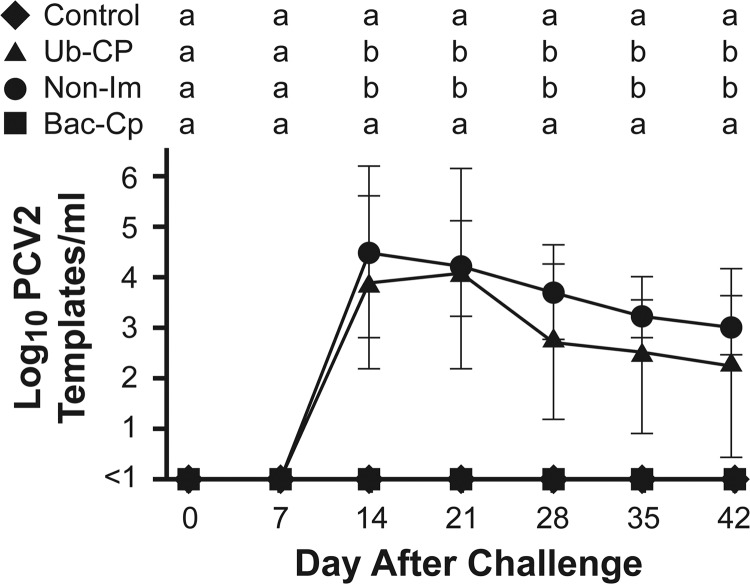
All pigs were negative for PRRSV and PCV2 by PCR at the beginning of the study. PCV2 DNA was first detected at 14 days after virus challenge in the Ub-CP and Non-Im groups (Fig. 6). Mean viremia levels for the two groups were similar, with no significant differences between groups on any day after infection. Viremia in the Bac-CP group remained below detectable levels throughout the study. After virus challenge, pigs from all three treatment groups were intermingled. Even though the Bac-CP group remained constantly exposed to viremic pigs, all pigs in the Bac-CP group remained negative for the presence of PCV2 DNA in serum.
Fig 6.
PCV2 viremia. Data are shown as the means ± 1 standard deviation. Means with the same letter are not significantly different (P > 0.05).
DISCUSSION
Previous work showed that the antibody in pigs with PCVAD is primarily directed toward an immunodominant oligopeptide epitope, CP(169–180), located within the epitope C region of CP (42). In this study, the antibody response of PCVAD pigs was mimicked by immunizing pigs with a monomer form of CP. The outcomes following immunization with monomer CP included high levels of anti-PCV2 antibody, antibody recognition of CP(169–180), undetectable levels of virus neutralizing activity, and the absence of protection following virus challenge. Since baculovirus- and E. coli-expressed CP spontaneously form VLPs, one of the first obstacles was to maintain the CP immunogen in a stable monomeric form. This was achieved by the fusion of CP to ubiquitin to form Ub-CP. In a similar manner, Yin et al. (46) incorporated SUMO for the expression of single CP subunits. Similar to the cleavage of Ub from Ub-CP, the removal of SUMO with the SUMO protease resulted in the spontaneous assembly of CP into VLPs. How fusion with Ub or SUMO functions to prevent the formation of a VLP is not clear. One possibility is that the fusion partner sterically blocks the interaction between CP subunits. Another possibility is that the addition of Ub or SUMO prevents subunit association through a conformational change in the CP monomer. Regardless of the mechanism for blocking VLP formation, the immunization of pigs with Ub-CP resulted in the production of antibodies against CP(169–180). As a positive control for these experiments, pigs were immunized with a commercial baculovirus-expressed CP vaccine. For the Bac-CP group, the total PCV2 antibody response followed a course similar to that of pigs immunized with Ub-CP (Fig. 4A). In contrast, the Bac-CP-vaccinated pigs possessed high levels of virus neutralizing activity and low levels of anti-CP(169–180) activity and showed no evidence of viremia after virus challenge.
In this study, the infection of pigs with a combination PCV2 and PRRSV did not result in mortality or the appearance of full-blown PCVAD, which was observed previously (1, 30, 36, 40, 43). It is difficult to faithfully reproduce PCVAD, even in the presence of known infectious disease cofactors. The absence of PCVAD in this study is likely the result of other, yet unknown factors that are involved in disease progression. The initial high health status of the pigs and high level of care may slow down disease progression and/or suppress clinical signs. Another possibility is that the experiment was terminated before PCVAD could develop. As shown in Fig. 6, virus was still present in sera of group Ub-CP and non-IM pigs. Continued virus replication may have eventually culminated in disease.
A potential role for anti-CP(169–180) antibodies in the development of PCVAD was evident by the number of pigs with antigen in the lung. For the non-IM and Bac-CP groups, four of eight and two of eight pigs, respectively, were positive for antigen staining in the lungs. In contrast, six of eight pigs in the Ub-CP group showed antigen staining in the lung. In all cases, PCV2 staining was localized in the bronchiolar epithelium. In PCVAD cases, the lung is one of the primary targets for PCV2 infection. For example, the APE syndrome is associated with massive quantities of PCV2 replication in the lung, which results in pulmonary edema (5). PCV2 antigen in lung is associated with the onset of porcine respiratory disease complex (3, 31). Further, Shen et al. (38) showed that PCV2 DNA was more prevalent in the lung than in the serum of pigs with PCVAD. One interesting observation in this study was the presence of PCV2 antigen in the lung epithelium of the pigs in the Bac-CP group, even in the absence of viremia. These results suggest that the lung represents a site for continuous shedding of virus, even in vaccinated populations.
The most intriguing feature of PCV2 infection is the dysregulation of the immune response, which culminates in a variety of disease outcomes. On one extreme is porcine multisystemic wasting syndrome (PMWS) which, in the end stage of the disease process, results in an almost complete elimination of B and T cells and the eventual decay of anti-PCV2 antibody (4). On the other extreme, porcine dermatitis and nephropathy syndrome (PDNS) is characterized by hyperimmunoreactivity, including immune complex formation and deposition of antigen-antibody complexes in organs, such as kidney and skin (3). We propose that antibody recognition of CP(169-180) functions similar to an immunological decoy, which directs the humoral response away from protective epitopes. The decoy antibody response is the result of host recognition of CP monomer or fragments produced during virus replication. Classic examples of immunological decoys are found in lentiviruses, such as feline immunodeficiency virus (FIV) and human immunodeficiency virus (HIV). As reviewed by Hosie et al. (13), the variable regions V3, V4, and V5 of the envelope (Env) protein of FIV are essential for virus infection, are immunodominant, and are targets for neutralization by antibody. However, continuous variation, as a result of shortening, lengthening, and/or peptide sequence hypervariability, produces large quantities of antibodies that are nonprotective. A similar mechanism occurs in the HIV glycoprotein 120 (gp120) (41). PRRSV possesses a hypervariable region in GP5 that may function in a similar manner by continually recruiting B cells that do not produce neutralizing antibody (32). An example of a nonessential protein functioning as a decoy is proposed for gp150 of gammaherpesvirus 68. The protein is immunodominant and results in a nonprotective antibody response (11). In sharp contrast to lenti- and herpesviruses, PCV2 possesses a rather small genome dominated by two relatively conserved proteins. Furthermore, CP is essential for virion integrity, infection, and replication. Therefore, when a decoy strategy is employed, the virus does not possess the luxury of peptide sequence hypervariability or the production of a nonessential protein. As illustrated in the model, presented in Fig. 7, we propose that PCV2 incorporates a unique strategy for employing a decoy epitope as a defense mechanism. Under normal circumstances, the immune response following PCV2 infection favors the production of antibodies directed against the whole virion. The resulting outcome is the control of virus replication and the generation of protective immunity. In contrast, the recognition of free CP and CP fragments, produced by infected cells, results in the production of antibodies against CP(169-180). Cofactors, such as coinfecting pathogens, may increase PCV2 replication and as a result may increase the level of non-VLP forms of CP. Recognition of CP(169-180) results in the production of nonprotective antibodies, thus allowing for increased PCV2 replication and the progression toward disease. Host genetics or coinfections may further modulate the host response, favoring the recognition CP(169-180). This model explains why the amount of circulating antibody fails to correlate with the outcome following PCV2 infection and provides important considerations in the design of PCV2 vaccines.
Fig 7.
Structural form of immunogen recognized by the host and relationship to outcome following PCV2 infection.
ACKNOWLEDGMENTS
This work was supported by National Pork Board grant 06-073 and USDA NRI grant 2009-35204-05290. Images of the PCV2 CP subunit and VLP were reproduced using the UCSF Chimera package from the Resource for Biocomputing, Visualization and Informatics at the University of California, San Francisco (supported by NIH P41 RR001081) (34).
We especially thank Ying Fang for the anti-PCV2 antibody.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Allan GM, et al. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 145:2421–2429 [DOI] [PubMed] [Google Scholar]
- 2. Catanzariti A-M, Soboleva TA, Jans DA, Board PG, Baker RT. 2004. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 13:1331–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chae C. 2005. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 169:326–336 [DOI] [PubMed] [Google Scholar]
- 4. Chae C. 2004. Postweaning multisystemic wasting syndrome: a review of aetiology, diagnosis and pathology. Vet. J. 168:41–49 [DOI] [PubMed] [Google Scholar]
- 5. Cino-Ozuna AG, et al. 2011. Notes: Characterization of a new disease syndrome associated with porcine circovirus type 2 in previously vaccinated herds. J. Clin. Microbiol. 49:2012–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dupont K, Nielsen EO, Baekbo P, Larsen LE. 2008. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case-control study supports a shift in genotypes with time. Vet. Microbiol. 128:56–64 [DOI] [PubMed] [Google Scholar]
- 7. Fachinger V, Bischoff R, Jedidia SB, Saalmüller A, Elbers K. 2008. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 26:1488–1499 [DOI] [PubMed] [Google Scholar]
- 8. Fenaux M, Opriessnig T, Halbur PG, Elvinger F, Meng XJ. 2004. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 78:6297–6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finney DJ. 1964. The Spearman-Karber method, p 524–530 In Finney DJ. (ed), Statistical method in biological assay, 2nd ed Charles Griffin, London, United Kingdom [Google Scholar]
- 10. Finsterbusch T, Mankertz A. 2009. Porcine circoviruses—small but powerful. Virus Res. 143:177–183 [DOI] [PubMed] [Google Scholar]
- 11. Gillet L, May JS, Colaco S, Stevenson PG. 2007. The murine gammaherpesvirus-68 gp150 acts as an immunogenic decoy to limit virion neutralization. PLoS One 2:e705 doi:10.1371/journal.pone.0000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horlen KP, et al. 2008. A field evaluation of mortality rate and growth performance in pigs vaccinated against porcine circovirus type 2. J. Am. Vet. Med. Assoc. 232:906–912 [DOI] [PubMed] [Google Scholar]
- 13. Hosie MJ, Pajek D, Samman A, Willett BJ. 2011. Feline immunodeficiency virus (FIV) neutralization: a review. Viruses 3:1870–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Juhan NM, LeRoith T, Opriessnig T, Meng XJ. 2010. The open reading frame 3 (ORF3) of porcine circovirus type 2 (PCV2) is dispensable for virus infection but evidence of reduced pathogenicity is limited in pigs infected by an ORF3-null PCV2 mutant. Virus Res. 147:60–66 [DOI] [PubMed] [Google Scholar]
- 15. Khayat R, et al. 2011. The 2.3-ångstrom structure of porcine circovirus 2. J. Virol. 85:7856–7862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kixmöller M, et al. 2008. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 26:3443–3451 [DOI] [PubMed] [Google Scholar]
- 17. Kyriakis SC, et al. 2002. The Effects of immuno-modulation on the clinical and pathological expression of postweaning multisystemic wasting syndrome. J. Comp. Pathol. 126:38–46 [DOI] [PubMed] [Google Scholar]
- 18. Liu J, Chen I, Du Q, Chua H, Kwang J. 2006. The ORF3 protein of porcine circovirus type 2 is involved in viral pathogenesis in vivo. J. Virol. 80:5065–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu L-J, et al. 2008. Efficient production of type 2 porcine circovirus-like particles by a recombinant baculovirus. Arch. Virol. 153:2291–2295 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. López-Soria S, et al. 2011. Post-weaning multisystemic wasting syndrome (PMWS) clinical expression under field conditions is modulated by the pig genetic background. Vet. Microbiol. 149:352–357 [DOI] [PubMed] [Google Scholar]
- 22. Mahé D, et al. 2000. Differential recognition of ORF2 protein from type 1 and type 2 porcine circoviruses and identification of immunorelevant epitopes. J. Gen. Virol. 81:1815–1824 [DOI] [PubMed] [Google Scholar]
- 23. Mankertz A, Hillenbrand B. 2001. Replication of porcine circovirus type 1 requires two proteins encoded by the viral rep gene. Virology 279:429–438 [DOI] [PubMed] [Google Scholar]
- 24. Martelli P, et al. 2011. One dose of a porcine circovirus 2 subunit vaccine induces humoral and cell-mediated immunity and protects against porcine circovirus-associated disease under field conditions. Vet. Microbiol. 149:339–351 [DOI] [PubMed] [Google Scholar]
- 25. Misinzo G, Delputte PL, Meerts P, Lefebvre DJ, Nauwynck HJ. 2006. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J. Virol. 80:3487–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nawagitgul P, et al. 2000. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81:2281–2287 [DOI] [PubMed] [Google Scholar]
- 27. Opriessnig T, et al. 2006. Evidence of breed-dependent differences in susceptibility to porcine circovirus type-2-associated disease and lesions. Vet. Pathol. 43:281–293 [DOI] [PubMed] [Google Scholar]
- 28. Opriessnig T, et al. 2003. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet. Pathol. 40:521–529 [DOI] [PubMed] [Google Scholar]
- 29. Opriessnig T, Yu S, Thacker EL. 2004. Derivation of porcine circovirus type 2-negative pigs from positive breeding herds. J. Swine Health Prod. 12:186–191 [Google Scholar]
- 30. Opriessnig T, Halbur PG. 2012. Concurrent infections are important for expression of porcine circovirus associated disease. Virus Res. 164:20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Opriessnig T, Meng X-J, Halbur PG. 2007. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J. Vet. Diagn. Invest. 19:591–615 [DOI] [PubMed] [Google Scholar]
- 32. Ostrowski M, et al. 2002. Identification of neutralizing and nonneutralizing epitopes in the porcine reproductive and respiratory syndrome virus GP5 ectodomain. J. Virol. 76:4241–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pallarés FJ, et al. 2002. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Invest. 14:515–519 [DOI] [PubMed] [Google Scholar]
- 34. Pettersen EF, et al. 2004. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- 35. Pogranichniy RM, Yoon K-J, Harms PA, Sorden SD, Daniels M. 2002. Case-control study on the association of porcine circovirus type 2 and other swine viral pathogens with postweaning multisystemic wasting syndrome. J. Vet. Diagn. Invest. 14:449–456 [DOI] [PubMed] [Google Scholar]
- 36. Rovira A, et al. 2002. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 76:3232–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segalés J, et al. 2008. PCV-2 genotype definition and nomenclature. Vet. Rec. 162:867–868 [DOI] [PubMed] [Google Scholar]
- 38. Shen H-G, Halbur PG, Opriessnig T. 2012. Prevalence and phylogenetic analysis of the current porcine circovirus 2 genotypes after implementation of widespread vaccination programmes in the USA. J. Gen. Virol. 93:1345–1355 [DOI] [PubMed] [Google Scholar]
- 39. Shuai J, et al. 2008. Mapping of the nuclear localization signals in open reading frame 2 protein from porcine circovirus type 1. Acta Biochim. Biophys. Sin. (Shanghai) 40:71–77 [DOI] [PubMed] [Google Scholar]
- 40. Sinha A, et al. 2010. Porcine reproductive and respiratory syndrome virus infection at the time of porcine circovirus type 2 vaccination has no impact on vaccine efficacy. Clin. Vaccine Immunol. 17:1940–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stamatatos L, Morris L, Burton DR, Mascola JR. 2009. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat. Med. 15:866–870 [DOI] [PubMed] [Google Scholar]
- 42. Trible BR, et al. 2011. Antibody recognition of porcine circovirus type 2 capsid protein epitopes after vaccination, infection, and disease. Clin. Vaccine Immunol. 18:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Trible BR, et al. 2012. Antibody responses following vaccination versus infection in a porcine circovirus-type 2 (PCV2) disease model show distinct differences in virus neutralization and epitope recognition. Vaccine 30:4079–4085 [DOI] [PubMed] [Google Scholar]
- 44. Trible BR, Rowland RRR. 2012. Genetic variation of porcine circovirus type 2 (PCV2) and its relevance to vaccination, pathogenesis and diagnosis. Virus Res. 164:68–77 [DOI] [PubMed] [Google Scholar]
- 45. Wu P-C, et al. 2012. Characterization of porcine circovirus type 2 (PCV2) capsid particle assembly and its application to virus-like particle vaccine development. Appl. Microbiol. Biotechnol. 95:1501–1507 [DOI] [PubMed] [Google Scholar]
- 46. Yin S, et al. 2010. Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol. J. 7:166 doi:10.1186/1743-422X-7-166 [DOI] [PMC free article] [PubMed] [Google Scholar]