Abstract
Two novel picornaviruses were serendipitously identified in apparently healthy young domestic animals—cattle (Bos taurus) and, subsequently, sheep (Ovis aries)—in Hungary during 2008 and 2009. Complete genome sequencing and comparative analysis showed that the two viruses are related to each other and have identical genome organizations, VPg + 5′ UTRIRES-II[L/1A-1B-1C-1D-2ANPG↓P/2B-2C/3A-3BVPg-3Cpro-3Dpol] 3′ UTR-poly(A). We suggest that they form two novel viral genotypes/serotypes, bovine hungarovirus 1 (BHuV-1; GenBank accession number JQ941880) and ovine hungarovirus 1 (OHuV-1; GenBank accession number HM153767), which may belong to a potential novel picornavirus genus in the family Picornaviridae. The genome lengths of BHuV-1 and OHuV-1 are 7,583 and 7,588 nucleotides, each comprising a single open reading frame encoding 2,243 and 2,252 amino acids, respectively. In the 5′ untranslated regions (5′ UTRs), both hungaroviruses are predicted to have a type II internal ribosome entry site (IRES). The nucleotide sequence and the secondary RNA structure of the hungarovirus IRES core domains H-I-J-K-L are highly similar to that of human parechovirus (HPeV) (genus Parechovirus), especially HPeV-3. However, in the polyprotein coding region, the amino acid sequences are more closely related to those of porcine teschoviruses (genus Teschovirus). Hungaroviruses were detected in 15% (4/26) and 25% (4/16) of the fecal samples from cattle and sheep, respectively. This report describes the discovery of two novel picornaviruses in farm animals, cattle and sheep. The mosaic genetic pattern raises the possibility that hungaroviruses, human parechoviruses, and porcine teschoviruses may be linked to each other by modular recombination of functional noncoding RNA elements.
INTRODUCTION
Picornaviruses (family Picornaviridae), which are small, nonenveloped viruses with single-stranded, positive-sense genomic RNA, are currently divided into 12 genera: Aphthovirus, Avihepatovirus, Cardiovirus, Enterovirus, Erbovirus, Hepatovirus, Kobuvirus, Parechovirus, Sapelovirus, Senecavirus, Teschovirus, and Tremovirus (16). Picornaviruses are found in humans and a wide variety of animals, in which they can cause respiratory, cardiac, hepatic, neurological, mucocutaneous, and systemic diseases of various severities; however, most infections are apparently asymptomatic.
In general, the 7.2- to 9.1-kb-long picornavirus genomes have a common organization. Between the 5′ and 3′ untranslated regions (UTRs), they encode a single polyprotein that can be divided into three parts: P1, P2, and P3. The P1 gene region encodes the viral capsid proteins (VP4-VP2-VP3-VP1), whereas the P2 and P3 gene regions encode nonstructural proteins involved in protein processing (2Apro, 3Cpro, and 3CDpro) and genome replication (2B, 2C, 3AB, 3BVPg, 3CDpro, and 3Dpol) (16, 24). The nonstructural 3Dpol region contains the highly conserved viral RNA-dependent RNA polymerase (RdRp) gene, which encodes an essential enzyme protein that catalyzes the replication of all RNA viruses with no DNA stage. In addition, aphthoviruses, cardioviruses, erboviruses, kobuviruses, sapeloviruses, senecaviruses, and teschoviruses encode a leader (L) protein before the P1 region.
In the last few years, there has been a surge in the number of novel picornaviruses discovered and genomes sequenced (4, 13, 14, 15, 17, 23, 33). In 2008, a novel picornavirus (porcine kobuvirus) was serendipitously identified by our laboratory from domestic pigs (25). Based upon the conserved nucleotide sequences of the 3D RdRp regions of the three prototype kobuviruses (Aichi virus, bovine kobuvirus, and porcine kobuvirus), we designed primers (UNIV-kobu-F/R) for kobuvirus screening (26). Shortly thereafter, it became clear that these primer pairs were more generic for picornaviruses and less specific for kobuviruses. Using the primers, a novel picornavirus (quail picornavirus) from domestic quail was discovered (22), which is not related to the members of the genus Kobuvirus and may belong to an unclassified picornavirus genus.
In this study, we report two further novel picornaviruses, which are related to each other but distinct from kobuviruses, in cattle and sheep that were identified using the UNIV-kobu-F/R primers.
MATERIALS AND METHODS
Sample collection.
In February 2008, a total of 26 fecal samples were collected from cattle (Bos taurus) under the age of 20 days in a closed herd with 870 animals located in Aba in central Hungary (28). On the sampling dates, no clinical history of diarrhea was reported on the farm. The cattle were kept in captive breeding and had no contact with sheep.
Fecal samples from approximately 3-week-old domestic sheep (Ovis aries) were collected from a farm located in Tárnok, central Hungary, in March 2009 (n = 8) and in April 2010 (n = 8) (27). At this farm, Merino ewes from Hungary were mated with blackhead meat rams from Germany. None of the sampled animals showed any signs of clinical symptoms at sample collection. The sheep were kept in captive breeding and had no contact with cattle.
RNA extraction, RT-PCR, and complete genome determination.
RNA was extracted from 150 μl of fecal suspension (35 to 40% [vol/vol] in 0.1 M phosphate-buffered saline) using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The initial aim of the study was to detect and follow up on kobuviruses at the farms by reverse transcription (RT)-PCR using the generic kobuvirus primer pair UNIV-kobu-F/UNIV-kobu-R (Table 1) designed for the conserved RdRp gene of kobuviruses and amplifying a 216-bp-long PCR fragment (26).
TABLE 1.
| Reaction no. | Reaction type | Primer name | Primer sequence (5′ to 3′) | Size of PCR product (bp) |
|---|---|---|---|---|
| BHuV | ||||
| 1 | RT-PCR | UNIV-kobu-R | ATG TTG TTR ATG ATG GTG TTG A | 216 |
| UNIV-kobu-F | TGG AYT ACA AGT GTT TTG ATG C | |||
| 2 | 3′ RACE | BHuV-6917-F | CAA CCC ACA TTT GGG ATG ATG A | 666 |
| 3 | 3′ RACE | BHuV-7164-F | AGC TTC AAA GAG TGG AAC CTT | 419 |
| 4 | RT-PCR | BHuV-6893-R | TTC AAG GCC ACA TCA TCA AAT CC | 1,339 |
| BHuV-5554-F | TTT GAS ACK GGW GCT CHT G | |||
| 4b | RT-PCR | BHuV-6277-R | GGT CAC CAA CGT GTT TAG CCA | 723 |
| BHuV-5554-F | TTT GAS ACK GGW GCT CHT G | |||
| 5 | RT-PCR | BHuV-5908-R | CTA CGG ACT CCC AGG TCT TG | 318 |
| BHuV-5590-F | GTT GTA ATG AAC TTC CAC TT | |||
| 6 | 5′ RACE | BHuV-5908-R | CTA CGG ACT CCC AGG TCT TG | 894 |
| BHuV-5866-R | TCT CTG CTT GAA ACA TAA AGC | |||
| BHuV-5694-R | GCC GTT TGC AGC GGG TCT CAC | |||
| 7 | 5′ RACE | BHuV-4867-R | TGG GTT CCA ATG CTT TTG CC | 1,441 |
| BHuV-4818-R | CGA TTG TCC TGC TTC AAC TTC | |||
| BHuV-4771-R | GTG CTC CAG GAT CTG CAA TTG | |||
| 8 | 5′ RACE | BHuV-3487-R | GGG CAC AAT CAG TCA ATT | 1,278 |
| BHuV-3463-R | AAC TTC TCT GCA TAA TTT CA | |||
| BHuV-3395-R | CTA AGA ATC GGA GGT CCT GG | |||
| 9 | 5′ RACE | BHuV-2264-R | TAC AAC ATC CTA ATC AGA ATA CTG | 781 |
| BHuV-2230-R | GTG CAA AAC CCC GAG ACA CTG | |||
| BHuV-2208-R | TTC CAA GCT AGT GTT CAA | |||
| 10 | 5′ RACE | BHuV-1517-R | CCC AAG GCA CCA AAA GGA GTG | 908 |
| BHuV-1491-R | AGT CAA GGG TAC CCA AGG CAG | |||
| BHuV-1465-R | AAG CCC ATC CAG AGT AGA C | |||
| 11 | 5′ RACE | BHuV-653-R | GTA AAG CAG CGT AGA GTG AGC | 443 |
| BHuV-630-R | CTG CTT AGA TCC ATA GTG TC | |||
| BHuV-592-R | ACC GTC AGG GCA TCC TTC AC | |||
| 12 | 5′ RACE | BHuV-232-R | GAA GTA CATG TTT CAC TCC AT | 181 |
| BHuV-209-R | GAA TGA ACTT TTC CTC CTA T | |||
| BHuV-181-R | AAG CGG CTT CGA CTGA AG | |||
| 13 | RT-PCR | BHuV-106-F | GTG ACC CCA TGC GAA GTA GTG | 1,411 |
| BHuV-1517-R | CCC AAG GCA CCA AAA GGA GTG | |||
| 14 | RT-PCR | BHuV-1403-F | GAT TTA TAA CAT TAC CAG TTG GTA | 861 |
| BHuV-2264-R | TAC AAC ATC CTA ATC AGA ATA CTG | |||
| 15 | RT-PCR | BHuV-2137-F | GTA CCA AAG CAA GCG CTG CTG ACT | 1,350 |
| BHuV-3487-R | GGG CAC AAT CAG TCA ATT | |||
| 16 | RT-PCR | BHuV-3305-F | CAC CAA TCC AAC AGT CTT CTG GTG | 1,562 |
| BHuV-4867-R | TGG GTT CCA ATG CTT TTG CC | |||
| 17 | RT-PCR | BHuV-4669-F | GCT AGT TTG AAT GAT AAA GGG ATC | 1,239 |
| BHuV-5908-R | CTA CGG ACT CCC AGG TCT TG | |||
| 3′/5′ RACE | VIAL 8 (Roche) | GAC CAC GCG TAT CGA TGT CGA C T(16)V | ||
| 3′/5′ RACE | VIAL 9 (Roche) | GAC CAC GCG TAT CGA TGT CGA C | ||
| OHuV | ||||
| 1 | RT-PCR | UNIV-Kobu-R | ATG TTG TTR ATG ATG GTG TTG A | 216 |
| UNIV-Kobu-F | TGG AYT ACA AGT GTT TTG ATG C | |||
| 2 | 3′ RACE | OHuV-6927-F | CAA CCC ATA TTT GGG ATG ATG A | 661 |
| 3 | 3′ RACE | OHuV-7174-F | AGC TTC AAA GAC AGG AAC TTT | 414 |
| 4 | RT-PCR | OHuV-6903-R | TTC AAG GCA ATT TCA TCA AAA CC | 327 |
| OHuV-6576-F | CCT TTM TYA ARG ATG ARC | |||
| 5 | RT-PCR | OHuV-6631-R | TCT TGT CTT ACC TTG TTT CAC | 1,511 |
| OHuV-5120-F | CCC AGV AGG AAG GAB GTT G | |||
| 6 | RT-PCR | OHuV-5164-R | TGC GAT TGC AAG GTC ACA CCA | 713 |
| OHuV-4451-F | CCT GGI CAR GGN AAR TCT GT | |||
| 7 | RT-PCR | OHuV-4500-R | GCA AAC ATT TGT GCC AAC AT | 1,172 |
| OHuV-3328-F | AAR CWA GCD GGA GAT GTT GA | |||
| 8 | RT-PCR | OHuV-3390-R | AAT TTC CTA GCA ATG CTG AG | 1,139 |
| OHuV-2251-F | TGG KAA YCA GAT GCA RAA | |||
| 9 | RT-PCR | OHuV-2298-R | GTG GCA GCA TAC ACA CCA CC | 771 |
| OHuV-1527-F | GTT GGV WTG TVC ARG TGC A | |||
| 10 | RT-PCR | OHuV-1584-R | ACA CCC AGA GCC CCA CCG TG | 562 |
| OHuV-1022-F | GTG ATM AAT TAY AAM TTT | |||
| 11 | RT-PCR | OHuV-1069-R | ATC AAC CGA ATT CTG CCA CTG | 645 |
| OHuV-424-F | TGC CTC WGG GGC CAA AAG | |||
| 12 | 5′ RACE | OHuV-592-R | ACT TGT TAC CTA TGG GTA CCG | 469 |
| OHuV-537-R | ATG CCA AGA TAC CAG TAC | |||
| OHuV-469-R | AAC TAG TAG GGT GCT GTT AAC | |||
| 3′/5′ RACE | VIAL 8 (Roche) | GAC CAC GCG TAT CGA TGT CGA C T(16)V | ||
| 3′/5′ RACE | VIAL 9 (Roche) | GAC CAC GCG TAT CGA TGT CGA C |
Locations are included in primer names.
Samples were selected for complete picornavirus genome amplification using the long-range RT-PCR method and the primer-walking technique. All reagents for the long-range RT-PCR were purchased from Promega (Madison, WI) unless otherwise specified. The cDNA synthesis was carried out in a 20-μl final volume containing 8 μl of RNA extract, 10 mM deoxynucleoside triphosphate (dNTP), 4 μl 5× Maxima RT buffer (Fermentas, St. Leon-Rot, Germany), 10 pmol of the antisense primer, 40 U RNasin, 200 U Maxima Reverse Transcriptase (Fermentas, St. Leon-Rot, Germany). The reverse transcription was performed at 50°C for 1 h. The RNA template was degraded at 37°C for 20 min with RNase H. The PCR was conducted in a 50-μl final volume using 5 μl of the RT reaction mixture as a template. The PCR mixture contained 5 μl 10× Long PCR buffer, 10 mM dNTP, 10 pmol/μl of each sense/antisense primer, and 2.5 U of Long PCR Enzyme mixture (Fermentas, St. Leon-Rot, Germany). The PCR was conducted under the following conditions: 1 cycle at 94°C for 1 min and 40 cycles of 94°C for 30 s, (primer melting temperature [Tm] − 5)°C for 30 s, and 68°C for 60 s/kb, followed by a final elongation step of 68°C for 10 min. To determine the 5′ and 3′ ends of the genome, a series of 5′ and 3′ rapid amplification of cDNA ends (RACE) reactions were conducted using two types of 3′/5′ RACE techniques: (i) terminal deoxynucleotidyl transferase enzyme based (Roche Diagnostics, Mannheim, Germany), as described previously (7), and (ii) adaptor ligation using a T4 RNA ligase-based system (19). Primer sequences and their locations are presented in Table 1. A generic primer pair (Hungaro-3D-R, 5′-CATYACYGGGCGAACAAG; Hungaro-3D-F, 5′-GAYTATTCKGGATTTGATGC) was designed based upon the nucleotide sequences of the 3D region of bovine hungarovirus 1 (BHuV-1) (corresponding to nucleotides [nt] 6799 to 6818 [F] and 7246 to 7263 [R]) and ovine hungarovirus 1 (OHuV-1) (corresponding to nt 6809 to 6828 [F] and 7256 to 7273 [R]) for screening. The PCR product was 465 nt long. Possible coinfection with porcine teschovirus (PTV) was tested by RT-PCR as described previously (21). The PCR products were sequenced directly with the BigDye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Warrington, United Kingdom) using sequence-specific primers and run on an automated sequencer (ABI Prism 310 Genetic Analyzer; Applied Biosystems, Stafford, TX).
Viral culture.
Original fecal samples positive for bovine hungarovirus and ovine hungarovirus by RT-PCR were propagated separately for virus isolation. Vero (African green monkey kidney; ATCC CRL-1586) cells were used as a broad-purpose cell line for catching most cultivable virus. Specimens (2.5 ml each) were stored in viral transport medium at −80°C. After thawing, they were clarified by centrifugation and filtered with 0.45-μm sterile filters (Millex-HV; Millipore, Bedford, MA). The clarified inoculum was added to 25-cm2 tissue culture flasks by adsorption inoculation, which involved decanting of the culture medium and direct application of the inoculum to the cell monolayer. After 1.5 h of adsorption at 37°C in a horizontal position, the excess inoculum was discarded and replaced with minimum essential medium (Sigma, Steinheim, Germany) with 1% fetal calf serum. Negative controls without fecal specimens were also used. The cultures were incubated at 37°C. They were inspected daily by inverted microscopy for cytopathic effect (CPE). After 12 days of incubation, subculturing to a fresh cell line was performed twice. At the end of the incubation, the cells were frozen and thawed once, and the culture lysates were collected for RNA extraction. Viral RNA was detected by RT-PCR as described above.
Sequence and phylogenetic analyses.
Reference picornavirus sequences were obtained from the GenBank database, the study sequences were aligned using Clustal X software ver. 2.0.3 software (31), and similarity calculations were performed with GeneDoc ver. 2.7 software (20). Phylogenetic trees of deduced amino acid sequence alignments were created using the maximum-likelihood method based on the Whelan and Goldman (WAG) substitution model (gamma distributed with invariant sites [G+I] and using all sites) (32) employing MEGA5 software (30). The selection of WAG+G+I as a substitution model was based on the results of “Find Best-Fit Substitution Model” of MEGA5 (30). Bootstrap values (based on 1,000 replicates) for each node are given if they are >50%. Possible polyprotein cleavage sites were predicted using the NetPicoRNA program (5). The secondary structure of the 5′ UTR was predicted (but not confirmed by biochemical probing) using the Mfold program (34), and a two-dimensional (2D) model was drawn using Corel Draw Graphics Suite ver. 12.
Nucleotide sequence accession numbers.
The nucleotide sequences of bovine hungarovirus 1 (BHuV-1/2008/HUN) and ovine hungarovirus 1 (OHuV-1/2009/HUN) have been submitted to GenBank under accession numbers JQ941880 and HM153767.
RESULTS
Detection and characterization of a novel picornavirus (bovine hungarovirus 1) in cattle.
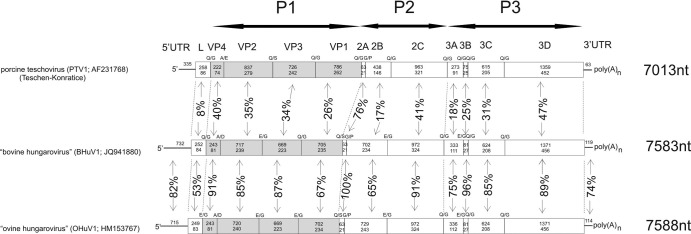
A PCR product of the correct amplicon size (216 nt) for kobuviruses was detected using the UNIV-kobu-F/UNIV-kobu-R primers in 1 (4%) of the 26 fecal samples collected from cattle. Interestingly, the 173-nt-long nucleotide sequence (without the primer sequences) had no similarity to kobuviruses in the GenBank database. However, a conserved amino acid motif, GLPSG, and some similarity to PTV RdRp sequences were found by using GenBank BLAST. The partial RdRp sequence of BHuV-1/2008/HUN, provisionally called BHuV-1, had 54% amino acid identity to PTV type 7 (PTV-7) (AF296092) and 52 to 54% amino acid identity to the 11 PTV serotypes. The complete RNA genome of BHuV-1/2008/HUN (JQ941880), which was found to have the same organization as some other picornaviruses, i.e., 5′ UTR-L-P1 (VP4-2-3-1)-P2 (2A-B-C)-P3 (3A-B-C-D)-3′ UTR, consisted of 7,583 nt, excluding the poly(A) tail (Fig. 1). A large open reading frame (ORF) of 6,732 nt was predicted to encode a potential polyprotein of 2,243 amino acids (aa) starting at nt 733. This was preceded by a 5′ UTR of 732 nt and followed by a 3′ UTR of 119 nt and a poly(A) tail. The L protein was 252 nt (84 aa) long. The complete P1 (2,334 nt; 778 aa), P2 (1,737 nt; 579 aa), and P3 (2,409 nt; 802 aa) regions were most closely related to PTV-1 Teschen-Konratice (AF231768), showing 31%, 32%, and 38% amino acid identity, respectively (Table 2). VP1 was 235 aa long and had only 24% amino acid identity to PTV-1. The following conserved picornavirus amino acid motifs were found: the N881PG↓P motif (↓ represents a “ribosomal skipping” site) in 2A, the GXXGXGKS (G1236KPGQGKS) motif for NTP binding in 2C, the DDLXQ (D1285DLGQ) motif for putative helicase activity in 2C, the active-site cysteine in GXCG (G1739FCG) in 3Cpro, the Y2113GDD motif of the 3Dpol active site, and the well-conserved K1948DELR, G2076LPSG, and F2161LKR in 3Dpol. A detailed genome structure and the organization and comparison of BHuV-1 gene regions to those of PTV-1 are shown in Fig. 1.
Fig 1.
Genome organization of BHuV-1 (JQ941880) and OHuV-1 (HM153767) and comparison of the structure to that of PTV-1 (AF231768). P1 (VP4-VP2-VP3-VP1; shaded) represents viral structural proteins, and P2 (2A-2B-2C) and P3 (3A-3BVPg-3Cpro-3Dpol) represent nonstructural proteins. Nucleotide (upper numbers) and amino acid (lower numbers) lengths are indicated in each gene box. Amino acid identity between the two hungaroviruses and PTV-1 are indicated (amino acid percentage) for each gene. Nucleotide sequence identities (percent) between the 5′ UTRs and between 3′ UTRs of hungaroviruses are indicated. Predicted N-terminal cleavage sites are indicated above the borders between genes.
TABLE 2.
Genomic features of BHuV-1 (JQ941880) and OHuV-1 (HM153767) and representatives of picornavirus genera
| Picornavirus genus | Virus (type) name | Genome features |
Pairwise amino acid identity (%)a |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Accession no. | Size (nt) | G+C (%) | BHuV-1 |
OHuV-1 |
||||||||||
| P1 | P2 | P3 | 3Cpro | 3Dpol | P1 | P2 | P3 | 3Cpro | 3Dpol | |||||
| Aphthovirus | Foot-and-mouth disease virus C | NC_002554 | 8,115 | 54 | 21 | 24 | 23 | 20 | 36 | 21 | 24 | 23 | 19 | 35 |
| Avihepatovirus | Duck hepatitis A virus 1 | NC_008250 | 7,687 | 43 | 12 | 11 | 17 | 13 | 20 | 11 | 10 | 17 | 15 | 20 |
| Cardiovirus | Encephalomyocarditis virus 1 | NC_001479 | 7,835 | 49 | 23 | 17 | 29 | 29 | 37 | 22 | 18 | 30 | 29 | 38 |
| Enterovirus | Human enterovirus C PV-1 | NC_002058 | 7,440 | 46 | 16 | 14 | 22 | 17 | 29 | 16 | 15 | 22 | 17 | 30 |
| Erbovirus | Equine rhinitis B virus 2 | NC_003077 | 8,821 | 50 | 24 | 22 | 25 | 14 | 39 | 24 | 21 | 25 | 13 | 39 |
| Hepatovirus | Hepatitis A virus 1 | NC_001489 | 7,478 | 38 | 12 | 11 | 16 | 14 | 21 | 11 | 11 | 16 | 13 | 20 |
| Kobuvirus | Aichi virus 1 | NC_001918 | 8,251 | 59 | 16 | 13 | 21 | 13 | 28 | 16 | 14 | 20 | 14 | 28 |
| Parechovirus | Human parechovirus 2 | NC_001897 | 7,348 | 39 | 13 | 13 | 15 | 9 | 20 | 13 | 12 | 15 | 9 | 20 |
| Sapelovirus | Porcine sapelovirus 1 | NC_003987 | 7,491 | 41 | 18 | 13 | 24 | 13 | 32 | 17 | 13 | 24 | 14 | 32 |
| Senecavirus | Seneca valley virus 1 | NC_011349 | 7,310 | 51 | 22 | 20 | 28 | 23 | 37 | 22 | 21 | 27 | 21 | 36 |
| Teschovirus | Porcine teschovirus 1 | NC_003985 | 7,117 | 45 | 31 | 32 | 38 | 31 | 47 | 31 | 33 | 37 | 30 | 46 |
| Tremovirus | Avian encephalomyelitis virus 1 | NC_003990 | 7,055 | 45 | 13 | 12 | 16 | 11 | 20 | 13 | 13 | 15 | 12 | 19 |
| Unclassified | Bovine hungarovirus 1 | JQ941880 | 7,583 | 46 | 81 | 81 | 86 | 85 | 89 | |||||
| Unclassified | Ovine hungarovirus 1 | HM153767 | 7,588 | 46 | 81 | 81 | 86 | 85 | 89 | |||||
Amino acid sequence identity (%) based upon the P1, P2, P3, 3Cpro, and 3Dpol regions. Boldface indicates the highest levels of amino acid identities.
Using specific hungarovirus screening primers (Hungaro-3D-R/F) and three additional bovine fecal samples, a total of 4 (15%) of the 26 specimens showed the presence of hungarovirus. PTV was not detected in any bovine samples.
Detection and characterization of a novel picornavirus (ovine hungarovirus 1) in sheep.
Six out of eight (75%) and two out of eight (25%) of the fecal samples collected in March 2009 and April 2010 from sheep gave a specific (216-nt-long) PCR product by RT-PCR and agarose gel electrophoresis using the universal kobuvirus primers (UNIV-kobu-F/UNIV-kobu-R). Seven of these sequences were characterized as kobuviruses (27). However, one had no similarity to kobuviruses, and the 57-residue-long picornavirus RdRp amino acid sequence of OHuV-1/2009/HUN had 85% identity to that of BHuV-1/2008/HUN as the closest match. The complete RNA genome of OHuV-1/2009/HUN (HM153767) was found to have the same organization as BHuV-1 and consisted of 7,588 nt, excluding the poly(A) tail (Fig. 1). A large single ORF of 6,759 nt was predicted to encode a polyprotein of 2,252 aa starting at nt 716. This was preceded by a 5′ UTR of 715 nt and followed by the 3′ UTR of 114 nt and a poly(A) tail. The L protein was 249 nt (83 aa) long. The complete P1 (2,334 nt; 778 aa), P2 (1,764 nt; 588 aa), and P3 (2,412 nt; 803 aa) regions showed 81%, 81%, and 86% amino acid identity to corresponding sequences of BHuV-1 (Table 2). VP1 was 234 aa long and had 67% amino acid identity to BHuV-1. The detailed genome structure and organization and comparisons with those of BHuV-1 are shown in Fig. 1.
Using specific hungarovirus screening primers (Hungaro-3D-R/F), a total of 4 (25%) of the 16 fecal samples from sheep were PCR positive. PTV was not detected in the ovine samples.
Characterization of 5′ and 3′ untranslated regions of hungaroviruses.
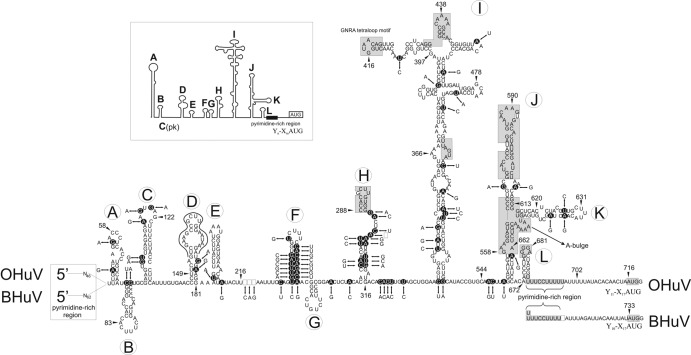
The 5′ UTRs of BHuV-1 and OHuV-1 were 732 and 715 nt long, respectively. Predicted translation from the 9th (BHuV-1) and 11th (OHuV-1) AUG codons resulted in a long polyprotein. These initiation codons (underlined) were set in nearly optimal Kozak contexts (18) (A/GNNAUGG): UAUAUGG in BHuV-1 at nt positions 733 to 735 and CUAAUGG in OHuV-1 at nt positions 716 to 718. The potential AUG codons in both viruses are preceded by a significant polypyrimidine tract (UUUUCCUUUU for BHuV-1 and UUUUCCUUUUU for OHuV-1), which is located in the RNA 17 nt upstream of the AUG initiation codon (Fig. 2) at nt positions 706 to 715 in BHuV-1 and 688 to 698 in OHuV-1. The Yn-Xm-AUG formula, which corresponds to lengths of pyrimidine tract (Y)/spacer sequence (X)/AUG initiation codon, is Y10-X17-AUG of BHuV-1 and Y11-X17-AUG of OHuV-1 (Fig. 2). In the GenBank database, nucleotide sequence similarity (up to 69%) was found between the 3′ end of the hungarovirus 5′ UTR region (from nt 415 to nt 732 of BHuV-1 and from nt 397 to nt 715 of OHuV-1) and core domains I-J-K-L of the internal ribosomal entry site (IRES) of members of the genus Parechovirus, especially the human parechoviruses (HPeV) (Fig. 2). The longest nucleotide sequence alignment and the closest match were found between OHuV-1 and human parechovirus type 3 (HPeV-3) (GQ183027), which was selected for further comparison. Higher nucleotide identities (up to 78%) were observed with domain J within the IRES region of human parechoviruses from nt 558 to nt 662 of OHuV-1 (from nt 576 to nt 680 of BHuV-1) (Fig. 2). At nt positions 558 to 620 (domain J), 559 to 613 (domain J), 559 to 613 (domain J), and 672 to 702 (domain L and the 3′-end pyrimidine-rich region) OHuV-1 had 84%, 84%, 86%, and 100% nucleotide identity to Ljungan virus 1 (EF202833), foot-and-mouth disease virus SAT 1 (HM067706), bovine rhinitis A virus 2 (JN936206), and human cosavirus B1 (FJ438907) (3, 12, 14). The apical 22 nucleotides in domain D of hungaroviruses are completely identical to Ljungan virus 4 (EU854568) nt 2 to 23 (Fig. 2). Based upon these data and the predicted secondary structure of the 5′ UTR, both hungaroviruses have a potential type II IRES (Fig. 2). This type of IRES comprises five major core domains from H to L and conserved nucleotide motifs that are also recognizable in bovine and ovine hungaroviruses (Fig. 2). Comparisons of the OHuV-1 and BHuV-1 5′ UTRs show a nucleotide identity of 82% (Fig. 1). In addition, the majority of the nucleotide mutations maintained the base pairing (and therefore the predicted secondary RNA structures) regardless of the Watson-Crick or wobble nature of base pairing (Fig. 2). The extreme 5′ end of the 5′ UTR (nucleotide positions 1 to 46 in OHuV-1 and nt positions 1 to 62 in BHuV-1) is pyrimidine rich in both hungaroviruses, and secondary RNA structure was not found (data not shown). This suggests that there may be some upstream sequence that we could not determine using 5′ RACE methods (7, 19), in spite of extensive efforts. This is a common problem with picornaviruses, and, for example, the 5′ ends of the genomes of erboviruses, teschoviruses, and some aphthoviruses remain to be determined (16). The 3′ UTRs of BHuV-1 and OHuV-1 are 119 nt and 114 nt long, respectively, and the nt identity is 74%. They are predicted to have extensive secondary RNA structure with a long double-stranded hairpin stem (data not shown). Nucleotide sequences similar to the hungarovirus 3′ UTRs were not found in GenBank.
Fig 2.
Predicted RNA secondary structure of the OHuV-1 (HM153767) 5′ UTR, including the IRES, as determined using the Mfold program. The RNA secondary structure of OHuV-1 is supplemented with the BHuV-1 (JQ941880) 5′ UTR nucleotide sequence for comparison. The positions of nucleotide differences are indicated by double arrows between OHuV-1 (dark circles) and BHuV-1; however, the pyrimidine-rich region at the extreme 5′ end and the Yn-Xm-AUG motif, which corresponds to the lengths of the pyrimidine tract/spacer sequence/AUG initiation codon at the 3′ end of the 5′ UTR, are indicated for both viruses. Nucleotide deletions/insertions are indicated by empty squares. The complete structure of the 5′ UTR, including the domains from A to L and the type II IRES, has been annotated as previously proposed for human parechoviruses (inset) (9). The central five IRES domains are labeled from H to L to maintain the continuity of the current nomenclature. The positions of conserved type II IRES motifs; the nucleotides with high identity to human parechoviruses in IRES domains H, I, J, and L; the pyrimidine-rich region at the 3′ end; and the predicted polyprotein AUG start codon are indicated by shaded boxes. In domain D, the continuous black line shows the positions of the 22 nucleotides that are identical in hungaroviruses and Ljungan virus 4 (EU854568).
Comparative, recombination, and phylogenetic analyses of bovine hungarovirus 1, ovine hungarovirus 1, and representative picornaviruses.
The genomes of BHuV-1 and OHuV-1 are approximately the same length, 7,588 nt and 7,583 nt, respectively (Fig. 1), and the G+C content is 46% in both hungaroviruses (Table 2). Except for the L/VP4 and the VP3/VP1 predicted cleavages, where Q/G is present in BHuV-1 and E/G is present in OHuV-1 in both positions, cleavage sites are similar in the two hungaroviruses (Fig. 1). Greater than 1% nucleotide sequence length differences were found in the 5′ UTR (2.3%), L (1.2%), 2B (3.7%), and 3′ UTR (4.2%) between the two hungaroviruses (Fig. 1). The longest sequence length difference (27 nt/9 aa) was found in the 2B region between OHuV-1 and BHuV-1 (Fig. 1). The amino acid identity range of the individual gene regions of the two hungaroviruses is between 53% (Leader) and 100% (2A) (Fig. 1). The amino acid lengths of the P1 regions (structural proteins) among the representative picornaviruses (Table 2) are between 731 aa (aphthoviruses and avihepatoviruses) and 880 aa (enteroviruses). Hungaroviruses have relatively short ORFs for P1 components (778 aa). Hungaroviruses had the highest identity to porcine teschoviruses in all coding regions except the short 2A region, where they had a higher (90%) amino acid identity to equine rhinitis B virus 1 (NC_003983) in the genus Erbovirus. The highest amino acid identity was found in the 2A (76%) and 3Dpol (47%) regions between BHuV-1 and PTV-1 (Table 2). The amino acid identities are less than 40% through the three P regions between hungaroviruses and the representative picornavirus genera (see Discussion below), with a maximum of 38% in the P3 region (Table 2). Similar nucleotide or amino acid sequences were not found in GenBank when searched with the leader, 3A, 3BVPg, and 3′ UTR sequences. In the L protein, neither the catalytic dyad (Cys and His), which is conserved in a papain-like thiol protease found in the foot-and-mouth disease virus L protein (10), nor the putative zinc-binding-like domain (Cys-His-Cys-Cys), found in the encephalomyocarditis virus L protein (8), could be identified.
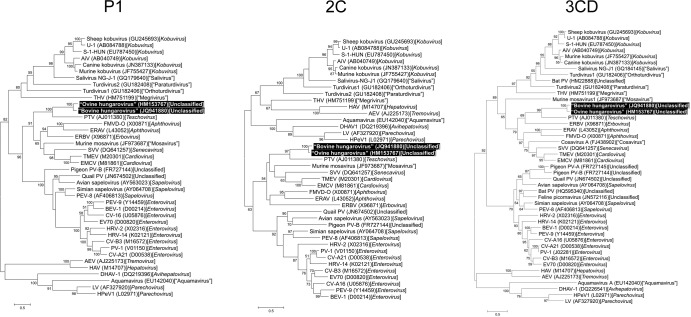
Figure 3 shows the phylogenetic analysis of hungaroviruses and representative picornaviruses based upon the amino acid sequences of the picornavirus P1, 2C, and 3CD proteins.
Fig 3.
Phylogenetic relationship between BHuV-1 (JQ941880) and OHuV-1 (HM153767) (black-shaded boxes) and other reference picornaviruses based upon the complete amino acid sequences of picornavirus P1, 2C, and 3CD coding regions. The phylogenetic tree was constructed by the maximum-likelihood clustering method using the WAG substitution model (MEGA5) (30). Bootstrap values (based on 1,000 replicates) are given for each node if they are >50%. Reference strains were obtained from GenBank. The scale bar indicates nucleotide substitutions per site.
Viral culture.
Neither CPE nor hungarovirus RNA replication could be detected in Vero cells even after serial passage of bovine and ovine hungaroviruses.
DISCUSSION
This study reports two novel picornaviruses apparently detected from domestic animals. Serendipitously, the generic kobuvirus primers designed to amplify, by RT-PCR, part of the kobuvirus RNA-dependent RNA-polymerase region (26) actually amplified novel picornavirus 3Dpol sequences, with 25% sensitivity, from fecal specimens collected from cattle (bovine) and sheep (ovine). This low sensitivity of the UNIV-kobu-F/R primers may reflect the incomplete nucleotide match between the primer and the target regions and/or a lower nucleic acid concentration of hungarovirus than of kobuvirus, which could result in a disadvantageous competition during RT-PCR in different samples. At the same time, these results repeatedly underscore the nonspecific (22) and more generic nature of the UNIV-kobu-F/R primers for kobuviruses (26) and for other picornaviruses.
According to the current International Committee on Taxonomy of Viruses(ICTV) Picornaviridae Study Group taxonomy guidelines (http://www.picornastudygroup.com/definitions/genus_definition.htm), novel picornavirus genera are defined if the amino acid identities in the P1, P2, and P3 regions are less than 40%, 40%, and 50%, respectively. Based upon the results of the complete genome-sequencing and comparative analyses, these novel picornaviruses, whose genome organizations are VPg + 5′ UTRIRES-II[L/1A-1B-1C-1D-2ANPGP/2B-2C/3A-3BVPg-3Cpro-3Dpol]3′ UTR-poly(A), are related to each other and may form two genotypes/serotypes within a candidate species in a novel picornavirus genus.
Interestingly, BHuV-1 and OHuV-1 appear to have a modular genome composition. In the 5′ UTR, the nucleotide sequence and secondary RNA structure of hungaroviruses are more similar to those of human parechoviruses (genus Parechovirus); however, in the coding region, the amino acid sequences are more closely related to those of porcine teschoviruses (genus Teschovirus). Both BHuV-1 and OHuV-1 appear to possess a type II IRES, based upon the sequence and secondary-structure similarities to human parechoviruses and Ljungan virus in the genus Parechovirus (9), with high nucleotide similarity in IRES core domains H-I-J-K-L. While teschoviruses, identified only in domestic pigs (1) and wild boar (6), have a type IV IRES (12), the high nucleotide identity of hungarovirus IRESs to the IRES of human parechoviruses, especially to that of HPeV-3, is particularly surprising and interesting. Apart from HPeV-1 and HPeV-6, which have been found in monkeys in China (29), human parechoviruses (or the 5′ UTR genome part of parechoviruses) have not yet been reported from nonhuman hosts. HPeV-3 is thought to be unique and the most virulent parechovirus in humans, with specific association with severe sepsis-like syndrome and encephalitis in young infants (11). In addition, despite the frequent recombination events in human parechoviruses, recombination was much more restricted among HPeV-3 sequences (2, 3). Since hungarovirus IRESs are most closely related to HPeV-3, this suggests the possibly that potential common evolutionary partners could be found more easily among picornaviruses of nonhuman origin. The independent development of a highly similar nucleotide sequence and secondary RNA structure of the IRES (and 5′ UTR) for the same function as found in HPeV and in the hungaroviruses is also possible, but the mosaic genetic pattern raises the possibility that hungaroviruses, human parechoviruses, and porcine teschoviruses (or a presently unknown ancestor) may have a close evolutionary connection to each other and that modular recombination of functional noncoding RNA elements between parental virus sequences may have occurred in the past. This may have had consequences for the host species switch and spectrum, too. In light of these results, more intense studies are needed to investigate the host species and genetic diversity of these three picornaviruses to confirm the possibility of a common evolutionary origin within the family Picornaviridae.
The host range of hungaroviruses and their pathogenicity in cattle and sheep remains to be determined. Apparently healthy animals (cattle and sheep) under the age of 21 days harbored hungaroviruses, which were excreted in the feces. These important farm animals could be a reservoir for hungaroviruses. Members of the related genus Teschovirus are sometimes pathogenic to their hosts (domestic pig) and have been associated with a variety of clinical conditions, sometimes with high morbidity and mortality (teschovirus encephalomyelitis, or Teschen disease), although the majority of infections are asymptomatic (1). While hungaroviruses are proposed to form a novel picornavirus genus, the relationship of the modular genetic composition of these viruses to important picornaviruses in human and veterinary medicine, HPeV in humans and PTV in domestic pigs, raises important questions about virulence, pathogenicity, cellular tropism, and cross-species transmission. Attempts were made to cultivate both hungaroviruses (ovine and bovine) in Vero cells; however, neither CPE nor virus replication could be detected, even after serial passage.
Further epidemiological and molecular studies are also required regarding the incidence, diversity, biology, geographic distribution, pathogenesis, and clinical importance of these novel picornaviruses in cattle, sheep, and possibly other animal species.
ACKNOWLEDGMENTS
This work was supported by a grant from the Hungarian Scientific Research Fund (OTKA) (K83013). G.R. was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. N.J.K. was partially supported by Institute for Animal Health core funding provided by the Biotechnology and Biological Sciences Research Council, United Kingdom.
Footnotes
Published ahead of print 26 September 2012
REFERENCES
- 1. Alexandersen S, et al. 2012. Picornaviruses, p 587–620 In Zimmerman JJ, Karriker LA, Ramirez A, Schwartz KJ, Stevenson GW. (ed), Diseases of swine, 10th ed Wiley-Blackwell, Ames, IA [Google Scholar]
- 2. Benschop KS, et al. 2010. Comprehensive full-length sequence analyses of human parechoviruses: diversity and recombination. J. Gen. Virol. 91:145–154 [DOI] [PubMed] [Google Scholar]
- 3. Benschop KS, Williams CH, Wolthers KC, Stanway G, Simmonds P. 2008. Widespread recombination within human parechoviruses: analysis of temporal dynamics and constraints. J. Gen. Virol. 89:1030–1035 [DOI] [PubMed] [Google Scholar]
- 4. Blinkova O, et al. 2009. Frequent detection of highly diverse variants of cardiovirus, cosavirus, bocavirus, and circovirus in sewage samples collected in the United States. J. Clin. Microbiol. 47:3507–3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blom N, Hansen J, Blaas D, Brunak S. 1996. Cleavage site analysis in picornaviral polyproteins: discovering cellular targets by neural networks. Protein Sci. 5:2203–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boros Á, et al. 2012. Porcine teschovirus in wild boars in Hungary. Arch. Virol. 157:1573–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boros Á, Pankovics P, Simmonds P, Reuter G. 2011. Novel positive-sense, single-stranded RNA (+ssRNA) virus with di-cistronic genome from intestinal content of freshwater carp (Cyprinus carpio). PLoS One 6:e29145 doi:10.1371/journal.pone.0029145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen HH, Kong WP, Roos RP. 1995. The leader peptide of Theiler's murine encephalomyelitis virus is a zinc-binding protein. J. Virol. 69:8076–8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghazi F, Hughes PJ, Hyypiä T, Stanway G. 1998. Molecular analysis of human parechovirus type 2 (formerly echovirus 23). J. Gen. Virol. 79:2641–2650 [DOI] [PubMed] [Google Scholar]
- 10. Gorbalenya AE, Koonin EV, Lai MM. 1991. Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-, alpha- and coronaviruses. FEBS Lett. 288:201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harvala H, et al. 2009. Specific association of human parechovirus type 3 with sepsis and fever in young infants, as determined by direct typing of cerebrospinal fluid samples. J. Infect. Dis. 199:1753–1760 [DOI] [PubMed] [Google Scholar]
- 12. Hellen CUT, de Breyne S. 2007. A distinct group of Hepacivirus/Pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: evidence for modular exchange of functional noncoding RNA elements by recombination. J. Virol. 81:5850–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holtz LR, Finkbeiner SR, Kirkwood CD, Wang D. 2008. Identification of a novel picornavirus related to cosaviruses in a child with acute diarrhea. Virol. J. 5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honkavuori KS, et al. 2011. Novel picornavirus in turkey poults with hepatitis, California, USA. Emerg. Infect. Dis. 17:480–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kapoor A, et al. 2008. A highly prevalent and genetically diversified Picornaviridae genus in South Asian children. Proc. Natl. Acad. Sci. U. S. A. 105:20482–20487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knowles NJ, et al. 2012. Picornaviridae, p 855–880 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses: 9th report of the International Committee on Taxonomy of Viruses, Elsevier, San Diego, CA [Google Scholar]
- 17. Kofstad T, Jonassen CM. 2011. Screening of feral and wood pigeons for viruses harbouring a conserved mobile viral element: characterization of novel astroviruses and picornaviruses. PLoS One 6:e25964 doi:10.1371/journal.pone.0025964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kozak M. 1987. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 15:8125–8148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z, Yu M, Zhang H, Wang HY, Wang LF. 2005. Improved rapid amplification of cDNA ends (RACE) for mapping both the 5′ and 3′ terminal sequences of paramyxovirus genomes. J. Virol. Methods 130:154–156 [DOI] [PubMed] [Google Scholar]
- 20. Nicholas KB, Nicholas HB. 1997. GeneDoc: a tool for editing and annotating multiple sequence alignments. National Resource for Biomedical Supercomputing; http://www.psc.edu/biomed/genedoc Accessed 2 December 2011 [Google Scholar]
- 21. Palmquist JM, Munir S, Taku A, Kapu V, Goyal SM. 2002. Detection of porcine teschovirus and enterovirus type II by reverse transcription-polymerase chain reaction. J. Vet. Diagn. Invest. 14:476–480 [DOI] [PubMed] [Google Scholar]
- 22. Pankovics P, Boros Á, Reuter G. 2012. Novel picornavirus in domesticated common quail (Coturnix coturnix) in Hungary. Arch. Virol. 157:525–530 [DOI] [PubMed] [Google Scholar]
- 23. Phan TG, et al. 2011. The fecal viral flora of wild rodents. PLoS Pathog. 7:e1002218 doi:10.1371/journal.ppat.1002218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Racaniello VR. 2007. Picornaviridae: The viruses and their replication, p 795–838 In Knipe DM, Howley PM. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 25. Reuter G, Boldizsár Á, Kiss I, Pankovics P. 2008. Candidate new species of Kobuvirus in porcine hosts. Emerg. Infect. Dis. 14:1968–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reuter G, Boldizsár Á, Pankovics P. 2009. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, member of a new species in genus Kobuvirus, family Picornaviridae. Arch. Virol. 154:101–108 [DOI] [PubMed] [Google Scholar]
- 27. Reuter G, Boros Á, Pankovics P, Egyed L. 2010. Kobuvirus in domestic sheep, Hungary. Emerg. Infect. Dis. 16:869–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reuter G, Egyed L. 2009. Bovine kobuvirus in Europe. Emerg. Infect. Dis. 15:822–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shan TL, et al. 2010. Human parechovirus infections in monkeys with diarrhea, China. Emerg. Infect. Dis. 16:1168–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. 1997. The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whelan S, Goldman N. 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18:691–699 [DOI] [PubMed] [Google Scholar]
- 33. Woo PC, et al. 2010. Comparative analysis of six genome sequences of three novel picornaviruses, turdiviruses 1, 2 and 3, in dead wild birds and proposal of two novel genera, Orthoturdivirus and Paraturdivirus, in Picornaviridae. J. Gen. Virol. 91:2433–2448 [DOI] [PubMed] [Google Scholar]
- 34. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]