Abstract
Phosphoenolpyruvate carboxylase (PEPC) activity and corresponding mRNA levels were investigated in developing and germinating wheat (Triticum aestivum) grains. During grain development PEPC activity increased to reach a maximum 15 d postanthesis. Western-blot experiments detected two main PEPC polypeptides with apparent molecular masses of 108 and 103 kD. The most abundant 103-kD PEPC subunit remained almost constant throughout the process of grain development and in the scutellum and aleurone layer of germinating grains. The less-abundant 108-kD polypeptide progressively disappeared during the second half of grain development and was newly synthesized in the scutellum and aleurone layer of germinating grains. PEPC mRNA was detected throughout the process of grain development; however, in germinating grains PEPC mRNA accumulated transiently in the scutellum and aleurone layer, showing a sharp maximum 24 h after imbibition. Immunolocalization studies revealed the presence of the enzyme in tissues with a high metabolic activity, as well as in the vascular tissue of the crease area of developing grains. A clear increase in PEPC was observed in the scutellar epithelium of grains 24 h after imbibition. The data suggest that the transiently formed PEPC mRNA in the scutellar epithelium encodes the 108-kD PEPC subunit.
PEPC (EC 4.1.1.31) catalyzes the β-carboxylation of PEP using HCO3− as a substrate in a reaction that yields oxaloacetate and Pi (for review, see Lepiniec et al., 1994; Chollet et al., 1996; Vidal and Chollet, 1997). This enzyme has been extensively studied in C4 and CAM plants, where it plays an essential role in photosynthetic C metabolism. C3 plants contain less, yet appreciable, amounts of PEPC, which is found in virtually all organs and may play multiple physiological roles (Latzko and Kelly, 1983). One such role is the so-called anaplerotic function, which consists of the replenishment of oxaloacetate in the tricarboxylic acid cycle whenever the demand for C skeletons for amino acid biosynthesis is high (Huppe and Turpin, 1994).
The connection of PEPC to N metabolism and its contribution to N assimilation has been well established by previous studies. Feeding maize (Zea mays) leaves with NH4+ or NO3− increased the levels of C4 PEPC protein and mRNA via cytokinin-dependent stimulated transcription of the C4 PEPC gene (Sugiharto and Sugiyama, 1992; Suzuki et al., 1994). The supply of NO3− to illuminated, N-deficient wheat (Triticum aestivum) leaves markedly enhanced the PEPC kinase activity/PEPC phosphorylation status, thereby causing an activation of the enzyme and a decrease in the sensitivity to the feedback inhibitor l-malate (Van Quy et al., 1991; Van Quy and Champigny, 1992; Duff and Chollet, 1995). PEPC in soybean (Glycine max) nodules seems to be modulated by photosynthate availability, strongly supporting the view that PEPC phosphorylation might be contributing to control the C/N balance in the nodules (Zhang et al., 1995). PEPC in nodules, in algae, and in leaves of C3 plants is strongly inhibited by the amino acids aspartate and glutamate (Huppe and Turpin, 1994); a similar inhibitory effect of these amino acids on PEPC activity of germinating castor bean (Ricinus communis) cotyledons has also been reported (Podestá and Plaxton, 1994b).
There is increasing evidence for the presence of PEPC in seeds of different plant species (Blanke and Lenz, 1989). Two different full-length PEPC cDNA clones, gmppc16 and gmppc1, have been isolated from developing soybean seeds (Sugimoto et al., 1992; Vazquez-Tello et al., 1993). The deduced proteins resemble more closely C3-type PEPCs and possess the N-terminal phosphorylation domain found in other PEPCs sequenced so far. Furthermore, gmppc16 is expressed not only in seeds but also in leaves, stems, and roots. Therefore, soybean contains a small multigene family of PEPC sequences, some members of which (at least two) are expressed in seeds. Sangwan et al. (1992) proposed that the large increase in PEPC activity during maturation of castor seeds might be involved in generating malate for the synthesis of storage lipids. The increased malate flux would also be a source of additional C skeletons for N assimilation in cotyledons of germinating castor seeds (Podestá and Plaxton, 1994b).
The presence of PEPC in barley (Hordeum vulgare) kernels was reported as early as 1973 (Duffus and Rosie, 1973). Isolated aleurone layers from barley and wheat grains show capacity for endosperm acidification (Mikola and Virtanen, 1980; Hamabata et al., 1988) following the accumulation of organic and phosphoric acids (Drozdowicz and Jones, 1995). Concomitant increases in malate and PEPC activity during the last stages of barley grain development pointed to the involvement of PEPC in the process of acidification that takes place in the endosperm during development (Macnicol and Jacobsen, 1992).
Using an electron-microscopic immunolabeling technique, Araus et al. (1993) detected a substantial amount of PEPC in the protein bodies of immature durum wheat (Triticum durum) grains, where it might contribute to amino acid and protein biosynthesis during grain development. The presence of PEPC protein and mRNA has also been reported in germinating sorghum (Sorghum bicolor) seeds (Khayat et al., 1991).
Recently, we have showed that wheat grain PEPCs possess the characteristic N-terminal phosphorylation domain and that the predominant 103-kD form was susceptible to phosphorylation, both in desalted crude extracts and in situ, indicating that the machinery for PEPC phosphorylation is present and functional in these grains (Osuna et al., 1996). The present work was undertaken to investigate further the distribution and fate of the enzyme and its mRNA in the different parts of the grain during development and germination.
MATERIALS AND METHODS
Wheat (Triticum aestivum cv Chinese Spring) plants were cultivated under controlled-environment conditions in a greenhouse under a 16-h day/8-h night cycle at 22 to 25°C. Grains were harvested at different stages of development, frozen in liquid N2, and kept at −80°C until used. Mature grains were sterilized in 2% (v/v) NaOCl for 20 min and washed twice with sterile water, once with 0.1 m HCl, and then thoroughly with sterile water. Grains were allowed to germinate at room temperature on sterile filter paper soaked with water for periods of up to 4 d. The rabbit polyclonal antibodies against native sorghum (Sorghum bicolor) C4 PEPC were prepared as reported by Osuna et al. (1996). All reagents were of analytical grade and were purchased from Sigma.
PEPC Activity Assay
PEPC activity was determined from crude extracts of grains at different stages of development. Extracts were prepared from 5 to 10 grains/mL of buffer A containing 0.1 m Tris-HCl, pH 7.5, 20% (v/v) glycerol, 1 mm EDTA, 10 mm MgCl2, 10 μg/mL chymostatin, 10 μg/mL bestatin, 10 μg/mL leupeptin, 1 mm PMSF, 1 μg/mL microcystin-LR (L and R are the two variable amino acids in the structure of microcystin), and 14 mm 2-mercaptoethanol. To analyze PEPC activity from germinating grains, aleurone layers, scutellum, and starchy endosperm were carefully dissected from 10 grains at the indicated times after imbibition and washed thoroughly with 0.1 m Tris-HCl, pH 8.0. PEPC activity was assayed using the NADH-malate dehydrogenase coupled assay as previously reported (Osuna et al., 1996). Protein determinations were carried out by the method of Bradford (1976) using a kit (Bio-Rad).
PEPC Immunocharacterization
Extracts (75 μL containing 2.7 mg protein/mL) from aleurone layers of germinating grains were incubated overnight at 4°C in the presence of increasing amounts of preimmune serum or sorghum C4-PEPC IgG, which was added in a total volume of 50 μL of TBS. After this incubation, protein A (5 mg) was added, incubated for 15 min at 4°C, and centrifuged for 15 min at 13,000g, and PEPC activity was assayed in the supernatant.
Aliquots of the tissue extracts were subjected to SDS-PAGE (10% acrylamide) according to the method of Laemmli (1970). After electrophoresis, proteins were electroblotted to nitrocellulose membranes (N-8017 from Sigma) at 0.8 mA/cm2 for 2 h using an electrophoretic transfer kit (Novablot, LKB, Bromma, Sweden). Membranes were blocked in TBS (20 mm Tris-HCl, pH 7.5, and 150 mm NaCl) containing 5% (w/v) powdered milk, and PEPC bands were immunochemically labeled by overnight incubation of the membranes at 4°C in 20 mL of TBS and affinity-purified polyclonal sorghum C4 PEPC IgG (27 μg of protein). Control experiments were carried out by incubating the membranes under the same conditions with a preimmune serum. No bands were detected in these controls. Subsequent detection was performed by a peroxidase assay (affinity-purified goat anti-rabbit IgG horseradish peroxidase conjugate from Bio-Rad).
Wheat PEPC cDNA Cloning and Northern Analysis
A cDNA library constructed in λgt10 with poly(A+) RNA from roots of 7-d-old wheat seedlings was screened with 32P-labeled CP28 cDNA encoding a sorghum C3-type PEPC (Lepiniec et al., 1991). The library (approximately 40,000 plaque-forming units) was plated, transferred to Hybond-N filters (Amersham), and hybridized at 60°C according to manufacturer's instructions. Four positive plaques were selected and purified, and the size of their cDNA inserts was analyzed. None of them contained a full-length cDNA; the longest one (1.3 kb) was subcloned in pBluescript SK+ (Stratagene) and sequenced in both chains.
For northern analysis, total RNA samples were extracted from liquid N2-frozen tissue, fractionated on agarose-formaldehyde gels, and blotted onto Hybond-N filters according to the manufacturer's instructions. Hybridization was performed as described by Sambrook et al. (1989) with 32P-labeled probe (the wheat PEPC cDNA clone described above). After hybridization, filters were washed at 60°C in washing solution (0.5× SSC and 0.1% SDS). Signal intensities of the autoradiographs were quantitated with a densitometer (Millipore). Variations in RNA loading were corrected for by normalizing each value to the level of the corresponding radish 18S rRNA sequence (Grellet et al., 1989).
Immunohistochemistry
Grains harvested at different stages of development and germinating grains at the indicated times after imbibition were fixed in 4% paraformaldehyde, dehydrated in a graded series of aqueous ethanol solution, and embedded in Paraplast Plus (Sigma) as described by Cejudo et al. (1992). Sections (10 μm thick) were cut in a microtome (model RM2025, Leica) and placed on poly-l-Lys-coated microscope slides. After deparaffinizing in xylol and rehydrating in decreasing concentrations of ethanol, sections were blocked for 30 min in TBS buffer containing 1% (w/v) BSA. Then, 100 μL of the same solution containing affinity-purified anti-PEPC antibodies (1 μg of protein) or preimmune serum was placed on the samples and incubated overnight at 4°C. Unbound primary antibodies were removed by three 10-min washes in TBS. Tissue sections were then incubated with alkaline phosphatase-conjugated goat anti-rabbit IgG for 2 h at 37°C. The reaction of alkaline phosphatase was developed with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate.
RESULTS
PEPC in Developing Wheat Grains
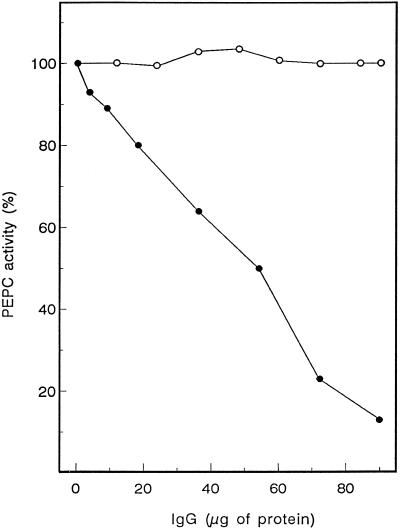
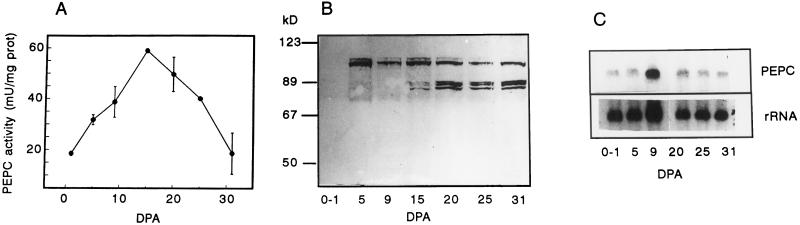
To study the amount of PEPC and its polymorphism during wheat grain development and germination, a polyclonal sorghum C4 PEPC IgG was used that effectively cross-reacted with PEPC from wheat grains, as shown by the immunoinhibition of PEPC activity (Fig. 1; see also Osuna et al., 1996). No inhibitory effect was observed in the presence of the preimmune serum. Extracts prepared from whole grains harvested at different stages of development showed PEPC activity (Fig. 2A). Activity increased at early stages of development to reach a maximum 15 DPA and then progressively decreased as grains approached maturity (up to 31 DPA).
Figure 1.
Immunotitration of wheat grain PEPC. Aliquots (75 μL) of an extract (2.7 mg protein/mL) from the dissected aleurone layer of germinating grains were incubated with increasing amounts of sorghum C4-PEPC IgG (•) or preimmune serum (○). PEPC activity remaining in the supernatants was measured as indicated in Methods. One hundred percent activity corresponds to 56 milliunits/mg protein.
Figure 2.
Analysis of PEPC during wheat grain development. A, Time course of PEPC activity during wheat grain development. Activity was assayed in crude extracts from grains at different stages of development. Results are means ± se of three independent determinations. mU, Milliunits; and prot, protein. B, Western-blot analysis of PEPC in developing grains. Crude extracts (75 μg of protein) from grains harvested at different stages of development were resolved in SDS-PAGE, transferred onto nitrocellulose, and probed with polyclonal PEPC IgGs (27 μg of protein/20 mL of incubation medium). Molecular masses of standard proteins are indicated on the left. C, Northern-blot analysis. Total RNA samples (10 μg) extracted from grains at the indicated DPA were fractionated in 1% agarose/formaldehyde gels, transferred onto Hybond-N filters, and hybridized to 32P-labeled PEPC cDNA. As a control of the amount of RNA loaded per lane, filters were stripped of radioactivity and rehybridized with 32P-labeled 18S rRNA.
When extracts of developing wheat grains were analyzed by western blot using the polyclonal sorghum C4 PEPC IgG as a probe (Fig. 2B), the antibodies detected two main polypeptides with molecular masses of 103 and 108 kD during the first part of the development process (5–15 DPA). During the second half of the development process (15–31 DPA), the 108-kD polypeptide progressively disappeared to become hardly detectable in grains 31 DPA. In these samples (15–31 DPA), three additional polypeptides of molecular mass ranging between 75 and 85 kD were recognized by the anti-PEPC antibody. These bands are very specific to these developmental stages, since they were not observed at the early stages (up to 9 DPA). These bands may correspond to PEPC-degradation products, although this possibility was not tested further.
To carry out studies on PEPC expression in wheat grains, a partial PEPC cDNA clone was isolated from a cDNA library made with poly(A+) RNA from roots of wheat seedlings (see Methods), which encoded the C-terminal region (331 amino acids) of wheat PEPC. The deduced amino acid sequence presented a high identity (more than 70%) to the C-terminal region of PEPCs from different sources, including tobacco, alfalfa, sorghum, and maize (Zea mays). Using the wheat partial cDNA as probe, we studied the accumulation of PEPC mRNA during grain development by northern-blot hybridization (Fig. 2C). When membranes were washed under low-stringency conditions (60°C, 0.5× SSC, and 0.1% SDS), a single band corresponding to an mRNA of approximately 3.4 kb was detected throughout the process of grain development. However, wheat grain maturation was accompanied by a decline in PEPC mRNA (Fig. 2C), in concert with reductions in PEPC activity and in the 108-kD subunit (Fig. 2, A and B).
PEPC in Germinating Wheat Grains
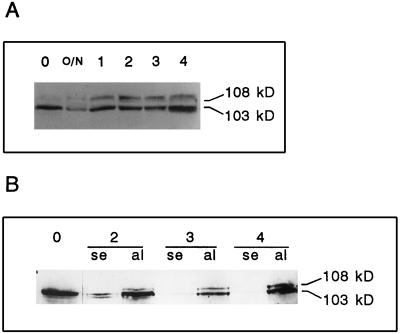
As mentioned above, mature wheat grains contain almost exclusively a single type of PEPC subunit with a molecular mass of 103 kD (Fig. 3, lanes 0). We analyzed by western blot the evolution of PEPC content and polymorphism in the different parts of germinating grains. In dissected scutellum the 103-kD polypeptide was detected throughout the 4 d after imbibition (Fig. 3A). The 108-kD polypeptide, barely detectable in mature grains, was found to increase during the 1st d of imbibition and then did not show any significant variation following grain germination. It should be mentioned that the higher amount of the 103-kD polypeptide observed at d 4 (Fig. 3A) is not significant and is most probably due to variation of the detection reaction.
Figure 3.
Western-blot analysis of PEPC in germinating wheat grains. Wheat grains were allowed to germinate for the indicated times and scutella (A) or aleurone layers and starchy endosperm (B) were carefully dissected. Crude extracts from scutella (70 μL, containing 140 μg of protein), aleurone layer (30 μL, containing 159 μg of protein), and starchy endosperm (30 μL, containing 160 μg of protein) were resolved in SDS-PAGE, transferred onto nitrocellulose, and probed with polyclonal PEPC IgGs (27 μg of protein/20 mL of incubation medium). 0, Dry grains; O/N, 14 h after imbibition; and 1 to 4, days after imbibition. al, Aleurone layers; and se, starchy endosperm.
The polymorphism of PEPC was also analyzed in the endosperm (the aleurone layer and starchy endosperm) of germinating grains (Fig. 3B). In mature grains the endosperm contained exclusively the 103-kD subunit (Fig. 3B). After the 2nd d of imbibition the aleurone layer could be easily dissected and therefore it was possible to analyze separately the PEPC pattern in the aleurone layer and the remaining starchy endosperm. In the aleurone layer the most abundant 103-kD polypeptide was constantly present up to 4 d after imbibition, whereas the 108-kD band was already detected 2 d after imbibition (Fig. 3B). In the starchy endosperm the level of PEPC enzyme decreased so that 4 d after imbibition it was no longer detectable in western blots (Fig. 3B), suggesting that PEPC, like other enzymes and storage proteins that accumulated in the starchy endosperm during grain development, was hydrolyzed during grain germination to serve as a nutrient for seedling growth.
PEPC activity was also analyzed in dissected tissues of germinating grains (Table I). In the scutellum PEPC activity increased after 1 d of imbibition and then remained invariable. In the aleurone layer a progressive increase in PEPC activity was observed (Table I), whereas in the starchy endosperm activity was almost undetectable 2 to 3 d after imbibition (not shown), in agreement with the disappearance of PEPC polypeptides described above (Fig. 3B). Therefore, the overall increase of PEPC activity observed in the scutellum and the aleurone layer of germinating grains is concomitant with the appearance of the 108-kD subunit, whereas the 103-kD subunit shows no significant variation.
Table I.
PEPC activity in dissected scutellum and aleurone layer of wheat germinating grains
| Dissected Tissue | PEPC Activity
|
||||
|---|---|---|---|---|---|
| 0 daia | 1 dai | 2 dai | 3 dai | 4 dai | |
| milliunits mg−1 protein | |||||
| Aleurone | n.d.b | n.d. | 28.6 ± 2.2 | 41.5 ± 7.9 | 57.8 ± 11 |
| Scutellum | 25.24 ± 1.27 | 48.74 ± 0.09 | 51.76 ± 6.3 | 50.5 ± 2.4 | 53.5 ± 2.9 |
Values are means ± se of at least three replicate experiments.
dai, Days after imbibition.
n.d., Not determined.
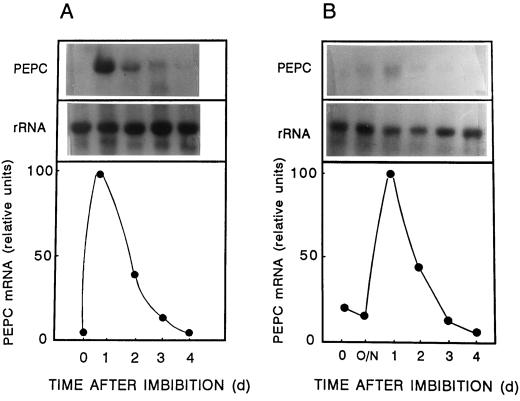
Northern-blot analysis revealed that PEPC mRNA in the scutellum of germinating grains was sharply and strongly enhanced by the 1st d of imbibition and then quickly declined (Fig. 4A). A similar pattern was observed in the aleurone layer (Fig. 4B).
Figure 4.
Northern-blot analysis of PEPC in germinating wheat grains. Grains were allowed to germinate, and at the indicated times scutellum (A) and aleurone layers (B; de-embryonated grains) were dissected. Total RNA (10 μg) extracted from dissected tissues was fractionated in 1% agarose/formaldehyde gels, transferred onto Hybond-N filters, and hybridized to 32P-labeled PEPC cDNA. mRNA levels were quantified and normalized with the corresponding rRNA sample. The results were plotted as a percentage of the maximum level of PEPC mRNA. 0, Dry grains; O/N, 14 h after imbibition; and 1 to 4, days after imbibition.
Immunolocalization of PEPC in Developing and Germinating Wheat Grains
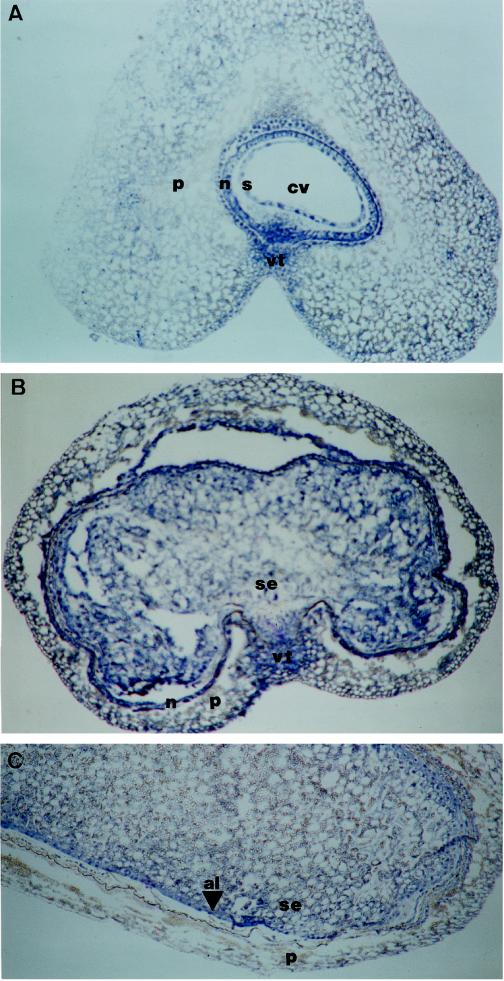
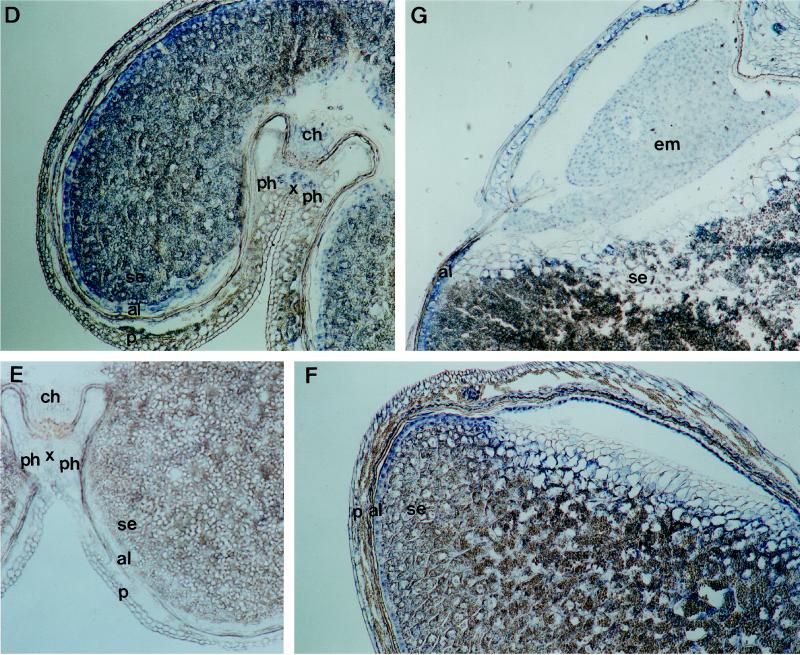
The distribution and possible variations of PEPC in wheat grain tissues were studied by immunolocalization. At the early stages of development (5 DPA), PEPC was abundant in the nucellus, the multinucleate syncytium, and the vascular tissue over the crease area of the grain, whereas no signal was detected in the pericarp (Fig. 5A). As grain development proceeds (10 DPA), the endosperm is formed by the cellularization of the syncytium. At this stage, PEPC was mostly present in the endosperm and the vascular tissue, as was shown in transverse sections of the grain (Fig. 5B). The differentiation of the aleurone cells was already initiated and was immunodecorated, as observed in longitudinal sections (Fig. 5C). At 16 DPA the aleurone cells, which show a high mitotic activity, were fully differentiated and contained a high level of PEPC (Fig. 5D). Remarkably, well-differentiated phloem and xylem in the vascular tissue of the crease area were immunodecorated by the PEPC antibody (Fig. 5D). A similar section probed with preimmune antiserum showed no signal (Fig. 5E). At 22 DPA the pattern of PEPC localization in the grain was similar to that of the preceding stage, PEPC being very abundant in the aleurone layer (Fig. 5F) and also detected in developing embryo (Fig. 5G). No significant differences were observed at 31 DPA, when the maturation process was almost complete (results not shown).
Figure 5.
(Figure continues on facing page.)
Immunological localization of PEPC in developing wheat grains. Immunolocalizations were carried out as described in Methods. Sections of grains (10 μm thick) 5 DPA (A), 10 DPA (B and C), 16 DPA (D and F), and 22 DPA (G) were probed with polyclonal PEPC IgGs (1 μg of protein per slide). A control section from 16-DPA grains was probed with preimmune serum (E). al, Aleurone layer; ch, chalaza; cv, central vacuole; em, embryo, n, nucellus; p, pericarp; ph, phloem; s, syncytium; se, starchy endosperm; vt, vascular tissue; and x, xylem. Magnification ×50.
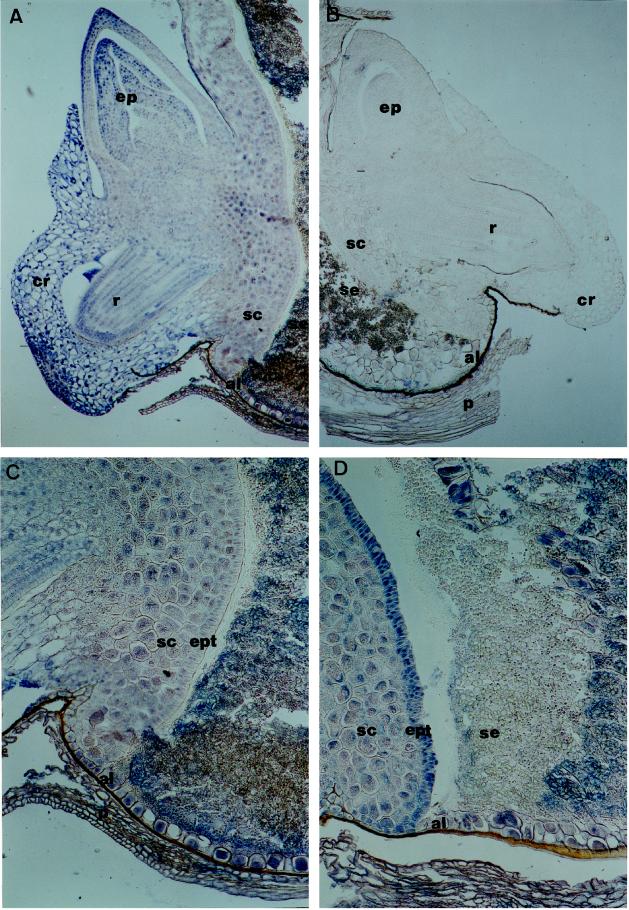
In a second set of experiments, the localization of PEPC was studied in germinating grains. Early after imbibition (14 h), a wide distribution of the enzyme was observed in the embryo (Fig. 6A) in the epicotyl, radicle, scutellum, and aleurone layer. No signal above background was detected in sections probed with the preimmune antiserum (Fig. 6B). Following germination (24 h after imbibition) several differences were noted in the pattern of PEPC localization in the scutellum and the part of the starchy endosperm facing it (Fig. 6, compare C and D). On a magnified image it can be seen that as germination proceeded (24 h after imbibition) the signal increased considerably in the scutellar epithelium, a fact that may reflect the changes in the 108-kD PEPC seen in western blots (Fig. 3A). However, the PEPC signal started to decrease in the part of the starchy endosperm facing the scutellar epithelium (Fig. 6D), most probably because of the effect of secreted proteases from the scutellum and the aleurone layer to the starchy endosperm, in agreement with the previously described disappearance of PEPC polypeptides observed in the starchy endosperm of germinating grains (Fig. 3B).
Figure 6.
Immunological localization of PEPC in germinating wheat grains. Grains were soaked for 14 h (A–C) or 24 h (D). Sections (10 μm) were probed with polyclonal PEPC IgGs (1 μg per slide; A, C, and D) or preimmune serum (B). al, Aleurone layer; cr, coleorhiza; ep, epicotyl; ept, epithelium; sc, scutellum; se, starchy endosperm; and r, radicle. Magnifications: A and B, ×50; and C and D, ×100.
DISCUSSION
The results presented in this paper establish the presence and wide distribution of PEPC in developing and germinating wheat grains. They confirm and extend a previous study of the regulatory phosphorylation of the enzyme in this organ (Osuna et al., 1996). The study of PEPC polymorphism in wheat grains was carried out with polyclonal antibodies raised against a C4 (sorghum) enzyme, which effectively cross-reacts with wheat PEPC, indicating a high similarity between these enzymes.
Several lines of evidence show that wheat PEPC is very homologous to PEPCs from other sources, including C4 plants. The high degree of homology was confirmed by the sequence comparisons of the partial cDNA clone from wheat, which shows more than 70% identity at the amino acid level in the C-terminal region to PEPC from different sources, including both C3 and C4 plants. Although no sequence of the N-terminal domain of wheat PEPC is yet available, we have shown (Osuna et al., 1996) that the wheat 103-kD enzyme undergoes a true phosphorylation that is prevented by an antibody raised against a 21-mer synthetic peptide representing the phosphorylation site of the sorghum enzyme at the N-terminal region (Pacquit et al., 1995). This result suggests that the high degree of identity may also be extended to the N-terminal domain of plant PEPCs.
The polyclonal sorghum C4 PEPC IgG used in this study recognized two polypeptides in developing and germinating wheat grains with molecular masses of 103 and 108 kD. A similar PEPC polymorphism has been described in castor bean (Ricinus communis) seeds using anti-PEPC antibody raised against maize C4 PEPC (Sangwan et al., 1992). In developing wheat grains, the 108-kD subunit progressively disappears after 15 DPA; therefore, most of the PEPC in mature grains has the 103-kD subunit. The disappearance of the 108-kD subunit coincides with the detection of three lower-molecular-mass polypeptides (ranging from 75–85 kD) in western blots, which were very efficiently immunodecorated by the PEPC antibodies (Fig. 2B). The possibility that these bands correspond to unspecific detection may be ruled out, since the antibody shows a high specificity in the detection of wheat PEPC polypeptides either at the early stages of grain development or in germinating grains. Because no PEPC subunit from any source has been described with a molecular mass as low as 75 to 85 kD, we conclude that these bands probably correspond to PEPC-degradation products. The strong intensity of these bands could have been due to the fact that buried epitopes on the entire PEPC are accessible to corresponding antibodies on these PEPC fragments. Provided this assumption is valid, the result suggests that the 108-kD subunit is degraded during the second half of the development process.
One attractive explanation for these collective results could be that the upper band corresponds to ubiquitinated enzyme prior to its degradation via the ubiquitin pathway, as has been reported in leaves of broad bean (Schulz et al., 1993). However, using commercially available anti-ubiquitin antibodies, we could not detect any recognition of the PEPC molecule (data not shown). Another possibility is that the 103-kD PEPC is the stable, housekeeping enzyme of the grain, whereas the 108-kD form is inducible in nature. This view is supported by the transient accumulation of mRNA in the scutellum and the aleurone layer of germinating grains (see below).
These findings underscore the fact that the 103-kD PEPC subunit is very stable throughout the entire process of development and germination of wheat grains. In addition, they suggest that the newly synthesized mRNA encodes the inducible 108-kD subunit in the scutellum and the aleurone layer of germinating grains, but more information about the PEPC gene family in wheat is needed before a firm conclusion can be drawn. Since both PEPC subunits are found together in different tissues of the grain, the question is raised whether the tetrameric enzyme has a chimeric nature.
Such a hypothesis has been proposed by Sangwan et al. (1992) and Podestá and Plaxton (1994a) in the case of PEPC in germinating but not developing broad bean seeds. In the wheat grain, it might be that the quaternary structure of PEPC changes from mixed 103- and 108-kD subunits during early development and following germination to mainly 103-kD subunits when maturation is being reached in the mature grain and early after imbibition. Such a chimeric enzyme with modified subunit composition could have specific regulatory and functional properties as required by its cytosolic environment and developmental stage of the grain. This interpretation awaits further work before a conclusion is drawn.
The immunolocalization experiments (Figs. 5 and 6) showed that PEPC is localized in tissues with high metabolic activity in developing and germinating grains; it is present in developing endosperm (5–10 DPA) when cellularization is taking place (Bosnes et al., 1992). The high PEPC content of the aleurone layer during differentiation also coincides with mitotic activity in these cells, as is the case in the developing embryo. The presence of PEPC in the aleurone layer of developing grains may also be related to the accumulation of malate in the starchy endosperm, as has been described by Macnicol and Jacobsen (1992) for late stages of barley grain development. A remarkable result described in this study is the presence of high amounts of PEPC in the vascular tissue of developing grains. The role played by PEPC in this tissue is not clear yet; one possible explanation is that it produces dicarboxylic acids, which act as counterions for the translocation of cations (such as K+) to the starchy endosperm.
Following imbibition, the enzyme is abundant in most tissues of the grain. This localization pattern probably reflects the crucial role of PEPC activity in maintaining the C content of the grain by refixation of the CO2 evolved by actively respiring tissues. This activity may generate C skeletons to help supply the demand of amino acid biosynthesis in these tissues. This is particularly important for the scutellum and the aleurone layer of germinating grains, since both tissues are very active in the synthesis and secretion of hydrolytic enzymes that mobilize the storage compounds of the starchy endosperm during germination (Cejudo et al., 1995; Domínguez and Cejudo, 1995). It is also important for the developing starchy endosperm, the PEPC of which is associated with protein bodies during protein filling of the grain (Araus et al., 1993).
Especially significant is the high and transient accumulation of PEPC mRNA in the scutellum and the concurrent increase in the 108-kD PEPC subunit after the first 24 h of imbibition (Fig. 4). The immunological study reveals a parallel PEPC enrichment in the scutellar epithelium at this time after imbibition. Collectively, these results lend support to the hypothesis that the corresponding PEPC gene is stimulated to produce PEPC mRNA encoding the 108-kD PEPC, whereas the 103-kD PEPC subunit would already be present to form the housekeeping enzyme. A specific physiological task for the inducible 108-kD PEPC could be linked to the fact that in germinating grains the scutellar epithelium is deeply committed to the synthesis and secretion of hydrolytic enzymes and the transport of nutrients (such as sugars and amino acids) from the starchy endosperm (Drozdowicz and Jones, 1995).
The expression pattern of PEPC and α-amylase-1 (α-Amy1) genes in wheat grains have several features in common. Both mRNAs accumulate transiently in the scutellum of germinating grains, showing a maximum 1 d after imbibition (Cejudo et al., 1995). In addition, both genes are predominantly expressed in the scutellar epithelium. It has been shown that α-Amy1 expression is down-regulated by the availability of sugars in the scutellum of rice grains (Yu et al., 1991; Karrer and Rodriguez, 1992; Chen et al., 1994; Thomas and Rodriguez, 1994). Furthermore, PEPC expression is metabolically repressed by sugars in maize mesophyll protoplasts (Sheen, 1990). A similar repression might be taking place in the scutellum of germinating wheat grains due to sugars that are produced in the starchy endosperm by the action of α-amylase activity and taken up by the scutellum. The data presented in this study suggest a coordination of PEPC and α-Amy1 expression in embryos of germinating wheat grains. The possibility that PEPC expression is under metabolic control in wheat grains is an open question. In this regard, it is worth mentioning the presence of common elements in the promoter region of PEPC and α-Amy1 genes (Karrer and Rodriguez, 1992). Whether or not these elements are relevant in the control of PEPC expression in response to sugars remains to be determined.
ACKNOWLEDGMENTS
Thanks are due to Dr. C. Hartman and E. Bismuth for the generous gift of the wheat PEPC probe. The photography work of M.J. Cubas is deeply appreciated.
Abbreviations:
- DPA
days postanthesis
- PEPC
PEP carboxylase
Footnotes
This work was supported by grant no. PB92-0675 from Dirección General de Investigación Científica y Técnica, Ministerio de Educación y Ciencia, and grant no. CVI 0118 from Junta de Andalucía, Spain.
The nucleotide sequence data reported in this paper appear in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession number Y15897.
LITERATURE CITED
- Araus JL, Bort J, Brown RH, Bassett CL, Cortadellas Immunocytochemical localization of phosphoenolpyruvate carboxylase and photosynthesis gas-exchange characteristics in ears of Triticum durum Desf. Planta. 1993;191:507–514. [Google Scholar]
- Blanke MM, Lenz F. Fruit photosynthesis. Plant Cell Environ. 1989;12:31–46. [Google Scholar]
- Bosnes M, Weideman F, Olsen O-A. Endosperm differentiation in barley wild-type and sex mutants. Plant J. 1992;2:661–674. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cejudo FJ, Cubo MT, Baulcombe DC. Amy1 expression during wheat seed germination. Plant Sci. 1995;106:207–213. [Google Scholar]
- Cejudo FJ, Murphy G, Chinoy C, Baulcombe DC. A gibberellin-regulated gene from wheat with sequence homology to cathepsin B of mammalian cells. Plant J. 1992;2:937–948. [PubMed] [Google Scholar]
- Chen M-H, Liu L-F, Chen Y-R, Wu H-K, Yu S-M. Expression of α-amylases, carbohydrate metabolism, and autophagy in cultured rice cells is coordinately regulated by sugar nutrient. Plant J. 1994;6:625–636. doi: 10.1046/j.1365-313x.1994.6050625.x. [DOI] [PubMed] [Google Scholar]
- Chollet R, Vidal J, Oleary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Domínguez F, Cejudo FJ. Pattern of endoproteolysis following wheat grain germination. Physiol Plant. 1995;95:253–259. [Google Scholar]
- Drozdowicz YM, Jones RL. Hormonal regulation of organic and phosphoric acid release by barley aleurone layers and scutella. Plant Physiol. 1995;108:769–776. doi: 10.1104/pp.108.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SMG, Chollet R. In vivo regulation of wheat-leaf phosphoenolpyruvate carboxylase by reversible phosphorylation. Plant Physiol. 1995;107:775–782. doi: 10.1104/pp.107.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffus CM, Rosie R. Some enzyme activities associated with the chlorophyll containing layers of the immature barley pericarp. Planta. 1973;114:219–226. doi: 10.1007/BF00389037. [DOI] [PubMed] [Google Scholar]
- Grellet F, Delcasso-Tremousaygue D, Delseny M. Isolation and characterization of unusual repeated sequence from the ribosomal intergenic spacer of the crucifer Sisimbrium irio. Plant Mol Biol. 1989;12:695–706. doi: 10.1007/BF00044160. [DOI] [PubMed] [Google Scholar]
- Hamabata A, García-Maya M, Romero T, Bernal-Lugo I. Kinetics of the acidification capacity of aleurone layer and its effect upon solubilization of reserve substances from starchy endosperm of wheat. Plant Physiol. 1988;86:643–644. doi: 10.1104/pp.86.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppe HC, Turpin DH. Integration of carbon and nitrogen metabolism in plant and algal cells. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:577–607. [Google Scholar]
- Karrer EE, Rodriguez RL. Metabolic regulation of α-amylase and sucrose synthase genes in planta. Plant J. 1992;2:517–523. [PubMed] [Google Scholar]
- Khayat E, Dumbroff EB, Glick BR. The synthesis of phosphoenolpyruvate carboxylase in imbibing sorghum seeds. Biochem Cell Biol. 1991;69:141–145. doi: 10.1139/o91-021. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latzko E, Kelly GJ. The many-faceted function of phosphoenolpyruvate carboxylase in C3 plants. Physiol Veg. 1983;21:805–815. [Google Scholar]
- Lepiniec L, Santi S, Keryer E, Amiet V, Vidal J, Gadal P, Crétin C. Complete nucleotide sequence of one member of the Sorghum phosphoenolpyruvate carboxylase gene family. Plant Mol Biol. 1991;17:1077–1079. doi: 10.1007/BF00037146. [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Vidal J, Chollet R, Gadal P, Cretin C. Phosphoenolpyruvate carboxylase: structure, regulation and evolution. Plant Sci. 1994;99:111–124. [Google Scholar]
- Macnicol PK, Jacobsen JV. Endosperm acidification and related metabolic changes in the developing barley grain. Plant Physiol. 1992;98:1098–1104. doi: 10.1104/pp.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikola J, Virtanen M. Secretion of l-malic acid by barley aleurone layers (abstract no. 783) Plant Physiol. 1980;65:S-142. [Google Scholar]
- Osuna L, González MC, Cejudo FJ, Vidal J, Echevarría C. In vivo and in vitro phosphorylation of the phosphoenolpyruvate carboxylase from wheat seeds during germination. Plant Physiol. 1996;111:551–558. doi: 10.1104/pp.111.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacquit V, Giglioli N, Crétin C, Pierre JN, Vidal J, Echevarria C. Regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase from Sorghum: an immunological study using specific anti-phosphorylation site antibodies. Photosynth Res. 1995;43:283–288. doi: 10.1007/BF00029941. [DOI] [PubMed] [Google Scholar]
- Podestá FE, Plaxton WC. Regulation of cytosolic carbon metabolism in germinating Ricinus communis cotyledons. I. Developmental profiles for the activity, concentration, and molecular structure of the pyrophosphate- and ATP-dependent phosphofructokinases, phosphoenolpyruvate carboxylase and pyruvate kinase. Planta. 1994a;194:374–380. [Google Scholar]
- Podestá FE, Plaxton WC. Regulation of cytosolic carbon metabolism in germinating Ricinus communis cotyledons. II. Properties of phosphoenolpyruvate carboxylase and cytosolic pyruvate kinase associated with the regulation of glycolysis and nitrogen assimilation. Planta. 1994b;194:381–387. [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sangwan RS, Singh N, Plaxton WC. Phosphoenolpyruvate carboxylase activity and concentration in the endosperm of developing and germinating castor oil seeds. Plant Physiol. 1992;99:445–449. doi: 10.1104/pp.99.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M, Klockenbring T, Hunte C, Schnabl H. Involvement of ubiquitin in phosphoenolpyruvate carboxylase degradation. Bot Acta. 1993;106:143–145. [Google Scholar]
- Sheen J. Metabolic repression of transcription in higher plants. Plant Cell. 1990;2:1027–1038. doi: 10.1105/tpc.2.10.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiharto B, Sugiyama T. Effects of nitrate and ammonium on gene expression of phosphoenolpyruvate carboxylase and nitrogen metabolism in maize leaf tissue during recovery from nitrogen stress. Plant Physiol. 1992;98:1403–1408. doi: 10.1104/pp.98.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Kawasaki T, Kato T, Whittier RF, Shibata D, Kawamura Y. cDNA sequence and expression of a phosphoenolpyruvate carboxylase gene from soybean. Plant Mol Biol. 1992;20:743–747. doi: 10.1007/BF00046459. [DOI] [PubMed] [Google Scholar]
- Suzuki I, Crétin C, Omata T, Sugiyama T. Transcriptional and posttranscriptional regulation of nitrogen-responding expression of phosphoenolpyruvate carboxylase gene in maize. Plant Physiol. 1994;105:1223–1229. doi: 10.1104/pp.105.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas BR, Rodriguez RL. Metabolite signals regulate gene expression and source/sink relations in cereal seedlings. Plant Physiol. 1994;106:1235–1239. doi: 10.1104/pp.106.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Quy L, Champigny ML. Nitrate enhances the kinase activity for phosphorylation of phosphoenolpyruvate carboxylase and sucrose phosphate synthase proteins in wheat leaves. Plant Physiol. 1992;99:344–347. doi: 10.1104/pp.99.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Quy L, Foyer C, Champigny ML. Effect of light and nitrate on wheat leaf phosphoenolpyruvate carboxylase activity. Evidence for covalent modulation of C3 enzyme. Plant Physiol. 1991;97:1476–1482. doi: 10.1104/pp.97.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez–Tello A, Whittier RF, Kawasaki T, Sugimoto T, Kawamura Y, Shibata D. Sequence of a soybean (Glycine max L.) phosphoenolpyruvate carboxylase cDNA. Plant Physiol. 1993;103:1025–1026. doi: 10.1104/pp.103.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Chollet R. Regulatory phosphorylation of C4 PEP carboxylase. Trends Plant Sci. 1997;2:203–241. [Google Scholar]
- Yu S-M, Kuo Y-H, Sheu G, Sheu Y-J, Liu L-F. Metabolic derepression of α-amylase gene expression in suspension-cultured cells of rice. J Biol Chem. 1991;266:21131–21137. [PubMed] [Google Scholar]
- Zhang X-Q, Bin L, Chollet R (1995) In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol 108: 1561–1568 [DOI] [PMC free article] [PubMed]