Abstract
Human noroviruses (HuNoV) are the major cause of epidemic, nonbacterial gastroenteritis in the world. The short course of HuNoV-induced symptoms has implicated innate immunity in control of norovirus (NoV) infection. Studies using murine norovirus (MNV) confirm the importance of innate immune responses during NoV infection. Type I alpha and beta interferons (IFN-α/β) limit HuNoV replicon function, restrict MNV replication in cultured cells, and control MNV replication in vivo. Therefore, the cell types and transcription factors involved in antiviral immune responses and IFN-α/β-mediated control of NoV infection are important to define. We used mice with floxed alleles of the IFNAR1 chain of the IFN-α/β receptor to identify cells expressing lysozyme M or CD11c as cells that respond to IFN-α/β to restrict MNV replication in vivo. Furthermore, we show that the transcription factors IRF-3 and IRF-7 work in concert to initiate unique and overlapping antiviral responses to restrict MNV replication in vivo. IRF-3 and IRF-7 restrict MNV replication in both cultured macrophages and dendritic cells, are required for induction of IFN-α/β in macrophages but not dendritic cells, and are dispensable for the antiviral effects of IFN-α/β that block MNV replication. These studies suggest that expression of the IFN-α/β receptor on macrophages/neutrophils and dendritic cells, as well as of IRF-3 and IRF-7, is critical for innate immune responses to NoV infection.
INTRODUCTION
Viruses within the genus Norovirus of the family Caliciviridae are the major etiologic agents of epidemic, nonbacterial gastroenteritis, causing ∼90% of viral gastroenteritis and ∼50% of all-cause outbreaks worldwide (22, 52). Human noroviruses (HuNoV) are also an important cause of sporadic gastroenteritis (53). HuNoV infection has been associated with necrotizing enterocolitis in infants and postinfectious irritable bowel syndrome in adults (44, 67, 71). Murine noroviruses (MNVs) are enteric viruses that share many molecular and biological properties with HuNoV (reviewed in references 25 and 77). MNV infection has been reported to trigger Crohn's disease-like intestinal pathology (7, 8) and atherosclerosis progression (51) and to exacerbate enteric bacterial infection (32, 37). The capacity of MNVs to grow in culture and infect mice permits the dissection of immune responses during NoV infection in vitro and in vivo.
The short course of HuNoV disease (often only 2 to 3 days) suggests that innate immune responses play a key role in control of HuNoV infection. Elevated interferon (IFN) levels are observed in ex vivo-stimulated peripheral blood mononuclear cells and in serum from human volunteers and gnotobiotic pigs inoculated with HuNoV (39, 63). Studies using MNV provide strong support for the role of innate immune responses, including both type I and type II IFN (IFN-α/β and IFN-γ), in controlling norovirus (NoV) replication both in vivo and in vitro (13, 28, 31, 32, 49, 76). The relevance of these observations is supported by studies showing a reduction of HuNoV RNA and protein expression in cells expressing replicons after IFN treatment (11, 12). IFN-α/β responses during NoV infection are likely initiated by the pattern recognition receptors melanoma differentiation-associated protein 5 (MDA-5) and Toll-like receptor 3 (TLR-3), since mice lacking MDA-5 or TLR-3 have elevated MNV replication compared to wild-type mice (46). However, the molecular pathways downstream of virus recognition which induce IFN-α/β responses during NoV infection are unknown. In addition, the role of specific cells and tissues in mediating IFN-α/β responses during NoV infection has not been defined.
Despite the importance of noroviruses with regard to public health, no drug or vaccine is currently available to treat or prevent HuNoV infection. The potential for long-term homologous protection against NoV infection has been shown by previous studies using the murine and chimpanzee model systems (5, 9). Importantly, a recent study showed that it is possible to vaccinate against homologous challenge with a HuNoV strain, providing support for the idea that vaccination of the population may be possible (2, 21). Several recent studies show that IFN-α/β is also important for generating effective antiviral CD8 T-cell responses (41, 54, 70, 72–74). Therefore, it is likely that IFN-α/β responses participate in both innate and adaptive immune responses to NoV infection.
IFN regulatory factor 3 (IRF-3) and IRF-7 are key mediators of transcriptional responses upon virus infection and are therefore likely candidates for initiating IFN-α/β responses to NoV infection. IRF-3 is constitutively expressed in most cell types and activated by phosphorylation upon virus infection (60, 78). IRF-7 expression is induced by IFN in most cell types; IRF-7 is constitutively expressed in plasmacytoid dendritic cells and also activated by phosphorylation upon virus infection (4, 29, 56). Mice lacking IRF-3 (IRF-3−/−) are susceptible to lethal infection with encephalomyocarditis virus (EMCV) and West Nile virus (WNV), but their systemic IFN-α/β response upon virus infection remains relatively intact (17, 57). In contrast, the systemic IFN-α response upon virus infection is decreased in mice lacking IRF-7 (IRF-7−/−), and these mice are more susceptible than IRF-3−/− mice to infection with EMCV, WNV, and herpes simplex virus-1 (18, 26).
Here, we used mice with a targeted knockout (Ifnar1tm1Agt) or floxed allele (Ifnar1tm1Uka) of the IFNAR1 chain of the IFN-α/β receptor (IFN-α/βR) to show that signaling through the IFN-α/βR and cells expressing lysozyme M (macrophages/neutrophils) or CD11c (dendritic cells) restrict MNV replication in vivo. Further, we found that IRF-3 and IRF-7 are key initiators of antiviral immune responses that restrict MNV replication in multiple tissues and in primary macrophages and dendritic cells. Lastly, we found that IRF-3 and IRF-7 are essential for induction of IFN-α/β in primary macrophages, but not primary dendritic cells, and are not required for IFN-α/β-dependent generation of an antiviral state. These data suggest a key role for macrophages/neutrophils, dendritic cells, IRF-3, and IRF-7 in innate immunity to NoV infection.
MATERIALS AND METHODS
Mice and infections.
The original Ifnar1tm1Uka (herein called IFN-α/βRf/f), Ifnar1tm1Agt (herein called IFN-α/βR−/−), and IRF3−/− and IRF7−/− mice were generously provided by Ulrich Kalinke (Paul-Ehrlich-Institut, Langen, Germany), Michel Aguet (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland), and Tadatsugu Taniguchi (University of Tokyo, Tokyo, Japan), respectively. Wild-type C57BL/6 (B6) mice (catalog no. 000664) were purchased from the Jackson Laboratory (Bar Harbor, ME). IFN-α/βR−/−, IRF-3−/−, IRF-7−/−, IRF-3−/− × IRF-7−/−, IFN-α/βRf/f, CD11c-Cre, and LysM-Cre mice (15, 19, 20, 26, 48, 57, 65), as well as IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice, were bred and housed at Washington University, St. Louis, MO, under specific-pathogen-free conditions (8), in accordance with federal and university guidelines. All of these mice were fully back-crossed to the C57BL/6 background.
Seven- to eight-week-old, sex-matched mice were orally inoculated with 25 μl of MNV diluted in phosphate-buffered saline (PBS) or mock inoculated with 25 μl of PBS. Mice were sacrificed 3 days after inoculation, and tissue and serum were harvested (10).
Virus stocks and plaque assays.
A concentrated stock of MNV-1.CW3 (69), herein referred to as MNV, was generated using RAW 264.7 cells (American Type Culture Collection [ATCC], Manassas, VA) as described previously (10) and used in all experiments. MNV titer was determined by plaque assay (10, 76) with the following modifications: suspension-adapted RAW 264.7 cells were plated in DMEM supplemented with 10% fetal bovine serum (FBS), 100 μg/ml penicillin and streptomycin (P/S), 2 mM l-glutamine, and 10 mM N-2-hydroxyethylpiperazine-N9-2-ethanesulfonic acid (HEPES) (pH 7.3). Cells were inoculated with MNV and overlaid with 2 ml/well of overlay medium [MEM supplemented with 10% FBS, 100 μg/ml P/S, 2 mM l-glutamine, and 10 mM HEPES and containing 1% methylcellulose (viscosity, 1,500 cP) (Sigma, St. Louis, MO)]. After 2 to 4 days of incubation at 37°C in 5% CO2, the overlay medium was removed and plaques were visualized with a 1% crystal violet–20% ethanol solution.
Cell culture, interferon treatment, and infection.
RAW 264.7 cells were grown as described previously (76). Suspension-adapted RAW 264.7 cells were grown at 35°C and maintained in MEM supplemented with 10% bovine serum, a 1% final concentration of nonessential amino acids, 100 μg/ml P/S, 2 mM l-glutamine, 10 mM HEPES, and 0.225% (wt/vol) sodium bicarbonate. Primary bone marrow-derived macrophages (herein referred to as macrophages) were generated as described previously (79) with the following modification: bone marrow cells were incubated in culture medium for 7 days, after which adherent cells were harvested and replated in fresh culture medium. Primary bone marrow-derived dendritic cells (herein referred to as dendritic cells) were generated as described previously (36) with the following modification: bone marrow cells were incubated in culture medium for 7 days, after which nonadherent cells were harvested and replated in fresh culture medium. For in vitro infections, macrophages and dendritic cells were plated on day 7 at a density of 2 × 105 cells/well in 24-well plates or 1 × 106 cells/well in 6-well plates. Macrophages and dendritic cells were either untreated or treated with 100 U/ml of recombinant IFN-α4 or recombinant IFN-β 12 to 16 h before inoculation (PBL InterferonSource, Piscataway, NJ). Macrophages were inoculated on day 10 of in vitro culture with MNV at a multiplicity of infection (MOI) of 0.05 (76) and either left untreated or treated with 100 U/ml of IFN-α/β after inoculation. Dendritic cells were inoculated on day 7 of in vitro culture with MNV at an MOI of 0.05 (76) and either left untreated or treated with 100 U/ml of IFN-α/β after inoculation. Cells and supernatant were harvested at 48 hours postinfection (hpi) and then stored frozen at −80°C.
Quantification of IFN-α/β activity.
Levels of biologically active IFN-α/β were determined using an EMCV cytopathic effect bioassay performed in L929 cells as described previously (55) with the following modification: prior to the assay, serum samples were treated with citrate buffer (40 mM citric acid, 10 ml KCl, 135 mM NaCl [pH 3.0]) for 5 min and neutralized with medium containing 45 mM HEPES. The amount of IFN-α/β per ml of serum was calculated from a standard curve using IFN-β (PBL InterferonSource) and adjusted for the background inhibitory activity of naïve serum (approximately 0.1 IU/ml). The inhibitory activity of naïve serum was IFN-α/β independent because it was acid labile but was resistant to treatment with heat (56°C) and the IFN-α/βR-blocking antibody MAR1-5A3 (61).
Quantitative RT-PCR.
Macrophages and dendritic cells were either mock inoculated or inoculated with MNV at an MOI of 10 for 8 or 12 h as described above. Total RNA was isolated and cDNA was synthesized as described previously (23). Quantitative reverse transcription-PCR (qRT-PCR) was performed with SYBR green (Invitrogen, Carlsbad, CA) using primers for IFN-α4 (5′-TGT GTG ATG CAG GAA CCT CCT-3′ and 5′-GGT ACA CAG TGA TCC TGT GG-3′), IFN-β (5′-ATA AGC AGC TCC AGC TCC AAG-3′ and 5′GTCTCATTCCACCCAGTGCTG-3′) and 18S (5′-CGC CGC TAG AGG TGA AAT TCT-3′ and 5′-CGA ACC TCC GAC TTT CGT TCT-3′). Viral RNA transcript levels were normalized to the level of transcription of 18S rRNA within each sample using the ΔΔCT method (where CT is the threshold cycle) (40).
Statistical analyses.
All data were analyzed using Prism 5 software (Graph-Pad Software, San Diego, CA). Mean values were used to calculate fold change.
RESULTS
IFN-α/β responses control MNV infection in vivo.
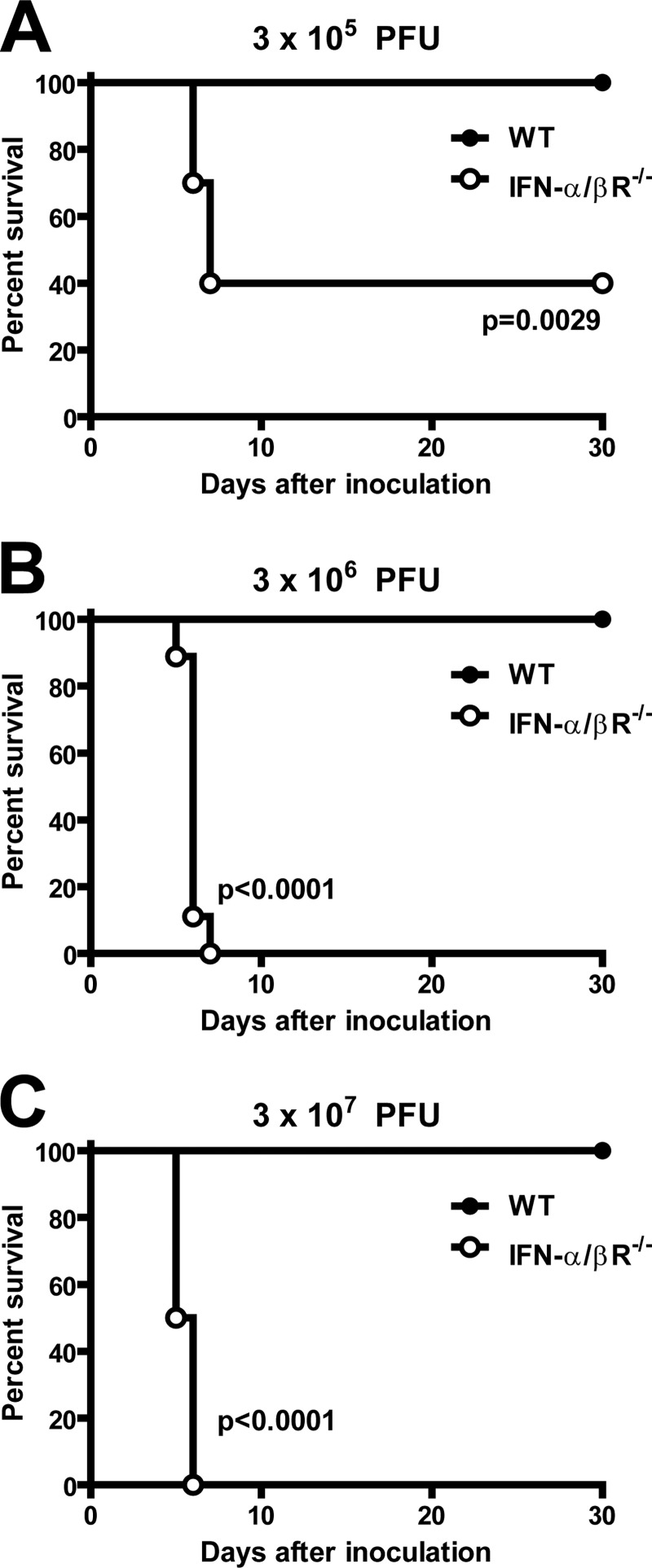
A combination of IFN-α/β and IFN-γ responses mediate control of MNV infection in vivo (28, 31, 49). To further elucidate the role of IFN-α/β in control of MNV infection in vivo, we examined the specific contribution of IFN-α/β responses in preventing lethal MNV infection. Consistent with previous studies (28, 31), significant lethality was not observed in IFN-α/βR−/− mice inoculated with 3 × 104 PFU of MNV (data not shown). However, when mice were inoculated with higher doses of MNV (3 × 105, 3 × 106, or 3 × 107 PFU), we observed statistically significant lethality in IFN-α/βR−/− mice compared to C57BL/6 mice (wild-type control) (Fig. 1). No lethality was observed in infected wild-type control mice at any of the tested doses of MNV. These data demonstrate a key role for IFN-α/β responses in preventing lethal MNV infection in vivo.
Fig 1.
IFN-α/β responses prevent lethal MNV infection. Wild-type (WT) and IFN-α/βR−/− mice were inoculated with the indicated doses of MNV, and survival was recorded for two independent experiments of five mice each. Statistical significance was determined using the log-rank test.
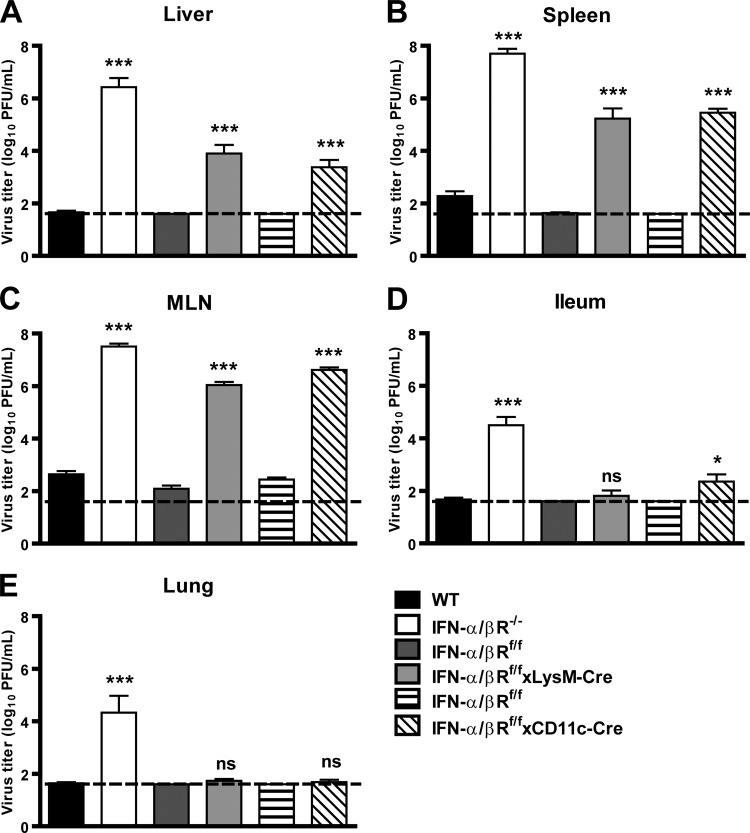
Since we observed lethality in IFN-α/βR−/− mice inoculated with 3 × 105 PFU of MNV (Fig. 1A), we assessed the contribution of IFN-α/β responses in restricting MNV replication in tissues after inoculation of mice with this dose of virus. Three days after inoculation, we observed a significant increase in MNV replication in the livers (300,000-fold), spleen (200,000-fold), mesenteric lymph nodes (70,000-fold), ilea (2,000-fold), and lungs (70,000-fold) of IFN-α/βR−/− mice compared to wild-type controls (Fig. 2). These data demonstrate that IFN-α/β responses are critical for host restriction of MNV replication in multiple tissues.
Fig 2.
IFN-α/β responses restrict MNV replication in vivo. WT, IFN-α/βR−/−, IFN-α/βRf/f, IFN-α/βRf/f × LysM-Cre, and IFN-α/βRf/f × CD11c-Cre mice were inoculated with 3 × 105 PFU of MNV and tissues were harvested 3 days postinfection (dpi). MLN, mesenteric lymph node. Data are the means and standard errors of the means from at least two independent experiments of at least four mice each. The dashed line represents the limit of detection. Titers from IFN-α/βR−/− mice were compared with those from wild-type controls; titers from IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice were compared with those from IFN-α/βRf/f controls. Statistical significance was determined using the nonparametric Mann-Whitney test. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.005.
IFN-α/β responses in lysozyme M- and CD11c-expressing cells restrict MNV replication in vivo.
MNV replicates in macrophages and dendritic cells in vivo (75, 76), and IFN-α/β and IFN-γ responses restrict MNV replication in these cells in vitro (13, 28, 76). To assess the role of IFN-α/β responses in macrophages and dendritic cells in restricting MNV replication in vivo, we examined acute MNV replication in mice with floxed alleles of the IFNAR1 chain of the IFN-α/βR in cells expressing either lysozyme M (IFN-α/βRf/f × LysM-Cre cells; macrophages/neutrophils) or CD11c (IFN-aBRf/fxCD11c-Cre cells; dendritic cells) (20, 30).
Three days after inoculation, we observed a significant increase in MNV replication in the livers (900-fold), spleens (20,000-fold), and mesenteric lymph nodes (8,000-fold) of IFN-α/βRf/f × LysM-Cre mice compared to control IFN-α/βRf/f mice, which carry floxed alleles of the IFNAR1 chain of the IFN-α/βR (Fig. 2A to C). We also observed a significant increase in MNV replication in the livers (223-fold), spleens (10,000-fold), and mesenteric lymph nodes (15,000-fold) of IFN-α/βRf/f × CD11c-Cre mice compared to levels in IFN-α/βRf/f controls. In contrast to the elevated titers observed in IFN-α/βR−/− mice, a significant increase in MNV replication was not observed in the lungs of IFN-α/βRf/f × LysM-Cre or IFN-α/βRf/f × CD11c-Cre mice compared to levels in IFN-α/βRf/f controls (Fig. 2E). These data demonstrate that IFN-α/β responses in lysozyme M- and CD11c-expressing cells restrict MNV replication in vivo and suggest that further analysis of the mechanisms of IFN-α/β-dependent resistance to NoV should include examination of the role of macrophages and dendritic cells, for which MNV has a tropism (75, 76).
Since we observed elevated titers in several tissues of IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice, we assessed the contribution of IFN-α/β responses in lysozyme M- and CD11c-expressing cells in preventing lethal MNV infection. In contrast to the lethality observed in IFN-α/βR−/− mice, no lethality was observed in IFN-α/βRf/f × LysM-Cre (n = 10), IFN-α/βRf/f × CD11c-Cre (n = 6), or IFN-α/βRf/f (n = 16) mice inoculated with 3 × 107 PFU (data not shown). Thus, the extent of IFN-α/βR deletion in IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice was not sufficient to result in lethality, potentially because of residual IFN-α/β signaling due to incomplete deletion of the IFN-α/βR in specific lysozyme M or CD11c cells or subsets (20, 30) or to a role for IFN-α/β signaling in other cell types.
IRF-3 and IRF-7 responses restrict MNV replication in vivo but are dispensable for preventing lethal MNV infection.
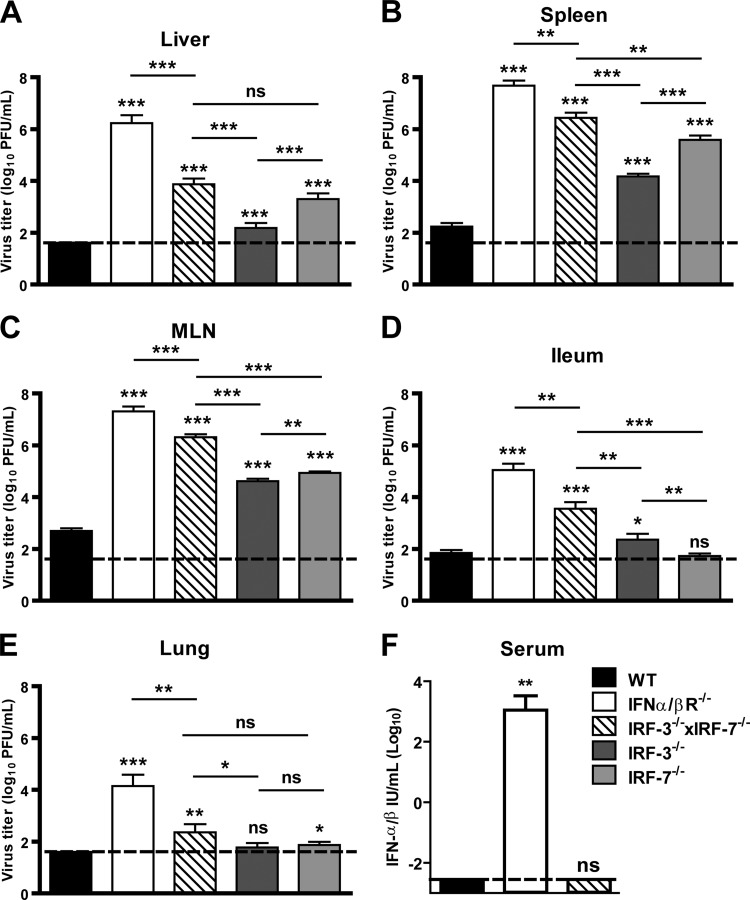
IRF-3 and IRF-7 are key transcription factors that regulate IFN-α/β responses following virus infection (26, 57). To assess their role in control of MNV infection in vivo, we examined acute MNV replication in mice lacking both IRF-3 and IRF-7 (IRF-3−/− × IRF-7−/−) compared to wild-type and IFN-α/βR−/− mice. Three days after inoculation, we observed a significant increase in MNV replication in the livers (370-fold), spleens (14,000-fold), mesenteric lymph nodes (3,400-fold), ilea (56-fold), and lungs (200-fold) of IRF-3−/− × IRF-7−/− mice compared to wild-type controls (Fig. 3A to E). Of note, we also observed a further increase in MNV replication in the livers (487-fold), spleen (14-fold), mesenteric lymph node (13-fold), ilea (20-fold), and lungs (14-fold) of IFN-α/βR−/− mice compared to levels in IRF-3−/− IRF-7−/− mice. These data demonstrate that IRF-3 and IRF-7 function to restrict MNV replication in vivo but also suggest that additional factors are involved in IFN-α/β responses during MNV infection in vivo.
Fig 3.
IRF-3 and IRF-7 restrict MNV replication in vivo. (A to E) WT, IFN-α/βR−/−, IRF-3−/− × IRF-7−/−, IRF-3−/− and IRF-7−/− mice were inoculated with 3 × 105 PFU of MNV, and tissues were harvested 3 dpi. Virus titers shown are from three independent experiments of at least three mice each. Titers were compared with those from wild-type controls. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.005. (F) WT, IRF-3−/− × IRF-7−/−, and IFN-α/βR−/− mice were mock inoculated or inoculated with 3 × 105 PFU of MNV, and serum was harvested 3 dpi. Data are from two independent experiments of three mice each. Serum concentrations were compared with those from wild-type controls. Statistical significance was determined using the Mann-Whitney test. ns, not significant; **, P = 0.0043.
IRF-3 is constitutively expressed in most cell types, whereas IRF-7 expression is induced by IFN-α/β in most cell types (56, 60). Since we observed elevated MNV titers in IRF-3−/− × IRF-7−/− mice, we examined acute MNV replication in IRF-3−/− mice and in IRF-7−/− mice to establish the relative hierarchy of these transcription factors in the innate immune responses to MNV. Three days after inoculation, we observed an increase in MNV replication in the livers (20-fold), spleens (42-fold), and mesenteric lymph nodes (60-fold) of IRF-3−/− mice and in the livers (163-fold), spleens (1,500-fold), and mesenteric lymph nodes (105-fold) of IRF-7−/− mice compared to wild-type controls (Fig. 3A to C). We also observed a significant increase in MNV replication in the ilea (4-fold) of IRF-3−/− mice and in the lungs (3-fold) of IRF-7−/− mice compared to wild-type controls (Fig. 3D and E). These data suggest that while IRF-3 and IRF-7 independently restrict MNV replication in vivo, IRF-7 is a greater participant in innate immune responses during MNV infection.
Since we observed roles for IRF-3 and IRF-7 in restricting MNV replication in the ilea and lungs, we examined the relative requirements for IRF-3 and IRF-7 in restricting MNV replication in vivo by comparing titers in IRF-3−/−, IRF-7−/−, and IRF-3−/− ×IRF-7−/− mice. We observed increased MNV replication in the livers (8-fold), spleens (36-fold), and mesenteric lymph nodes (2-fold) of IRF-7−/− compared to IRF-3−/− mice (Fig. 3A to C). Furthermore, enhanced MNV replication was observed in the livers (19-fold), spleens (330-fold), mesenteric lymph nodes (56-fold), ilea (13-fold), and lungs (19-fold) of IRF-3−/− × IRF-7−/− mice compared to IRF-3−/− mice and in the spleens (9-fold), mesenteric lymph nodes (326-fold), and ilea (152-fold) of IRF-3−/− × IRF-7−/− mice compared to IRF-7−/− mice (Fig. 3A to E). These data are consistent with the idea that IRF-3 and IRF-7 work together to induce both unique and overlapping tissue-specific antiviral responses, as observed for other viruses (19, 58), to restrict MNV replication in vivo.
Because elevated MNV titers were observed in IRF-3−/− × IRF-7−/− mice, we evaluated whether IRF-3 and IRF-7 are required to prevent lethal MNV infection. In contrast to results obtained with IFN-α/βR−/− mice, no lethality was observed in IRF-3−/− × IRF-7−/− mice inoculated with 3 × 107 PFU of MNV (n = 10). Thus, IRF-3 and IRF-7 are dispensable for preventing lethal MNV infection. When combined with the observation that IRF-3−/− × IRF-7−/− mice had reduced MNV titers compared to IFN-α/βR−/− mice, these data suggest that IRF-3, IRF-7, and additional factors control MNV infection in vivo through the induction of IFN-α/β responses.
Since we observed decreased MNV titers and no lethality in IRF-3−/− × IRF-7−/− mice compared to IFN-α/βR−/− mice, we investigated the production of IFN-α/β during MNV infection in vivo. High levels of IFN-α/β were observed in IFN-α/βR−/− mice 3 days after MNV infection, likely due to elevated virus titers and the lack of the IFN-α/βR to bind and internalize IFN-α/β (1, 34) (Fig. 3F). In contrast, circulating IFN-α/β was not detected in either wild-type or IRF-3−/− × IRF-7−/− mice despite the elevated MNV titers observed in IRF-3−/− × IRF-7−/− mice. Of note, circulating IFN-α/β was not detected in mock-inoculated wild-type, IRF-3−/− × IRF-7−/− and IFN-α/βR−/− mice (data not shown). Our inability to detect serum IFN-α/β in wild-type mice is unexpected, as IFN-α/βR−/− mice are profoundly susceptible to MNV infection, and suggests that limited IFN-α/β production at the site of virus infection may be sufficient to control MNV infection.
IRF-3 and IRF-7 restrict MNV replication in macrophages and dendritic cells.
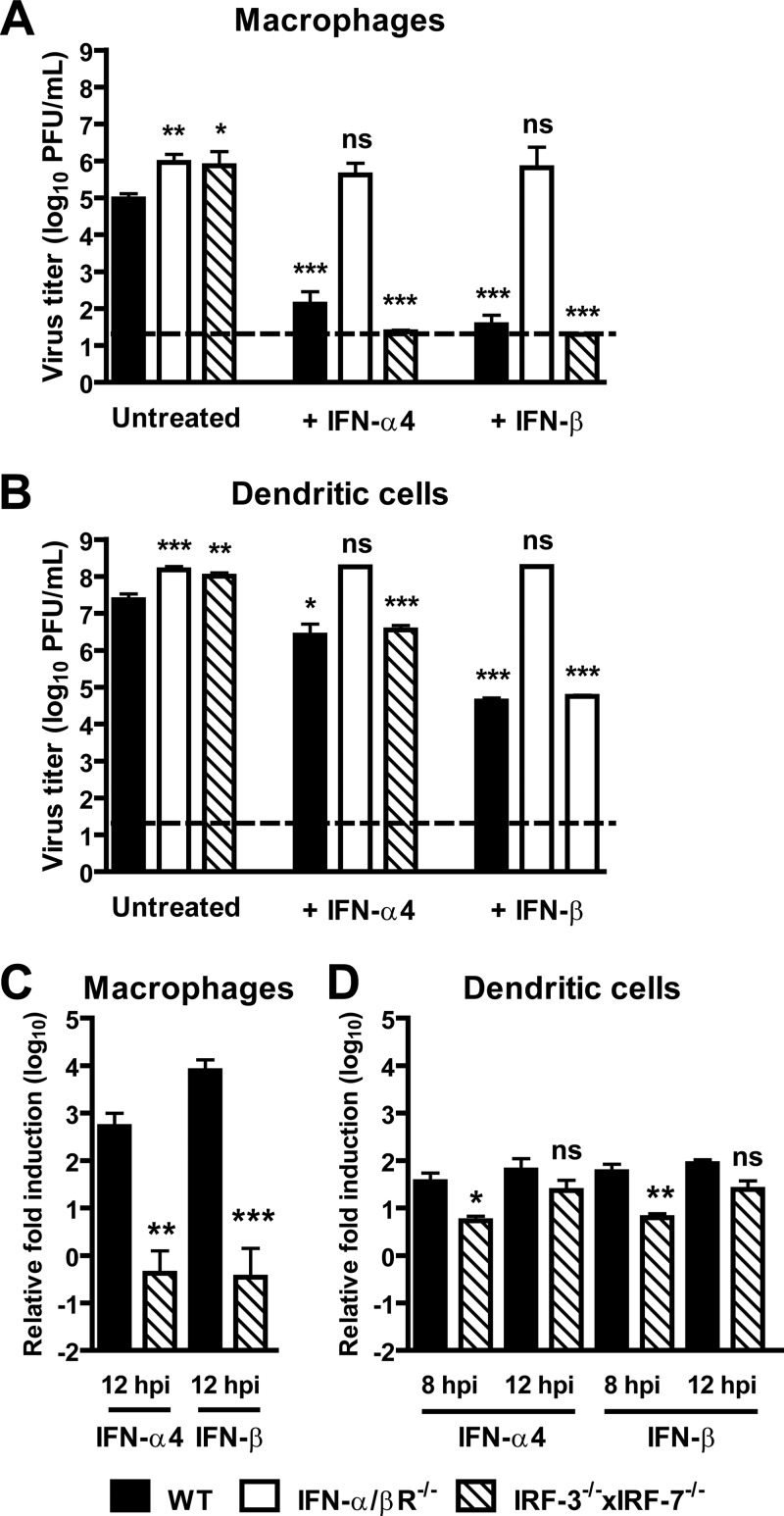
To directly assess the role of IRF-3 and IRF-7 in restricting MNV replication, we generated primary macrophages and dendritic cells from wild-type, IFN-α/βR−/−, and IRF-3−/− × IRF-7−/− mice. Consistent with a prior study (76), we observed enhanced MNV replication in IFN-α/βR−/− macrophages (12-fold) and dendritic cells (4-fold) compared to wild-type controls (Fig. 4A and B). We also observed similarly increased MNV replication in IRF-3−/− × IRF-7−/− macrophages (19-fold) and dendritic cells (3-fold) compared to wild-type controls (Fig. 4A and B). These data demonstrate that IRF-3 and IRF-7 restrict MNV replication in primary macrophages and dendritic cells.
Fig 4.
IRF-3 and IRF-7 are required for IFN-α/β induction during MNV infection but are dispensable for the antiviral activity of IFN-α/β. (A) Primary bone marrow-derived macrophages (macrophages) from WT, IRF-3−/− ×IRF-7−/−, and IFN-α/βR−/− mice were untreated or treated with recombinant IFN-α4 or recombinant IFN-β and inoculated with MNV (MOI of 0.05). Titers at 48 hpi are the mean and the standard error of the mean from four independent experiments. Titers from untreated IRF-3−/− × IRF-7−/− and IFN-α/βR−/− macrophages were compared with those from wild-type controls; titers from IFN-α4 or IFN-β treated wild-type, IRF-3−/− × IRF-7−/−, and IFN-α/βR−/− macrophages were compared with those from untreated controls. Statistical significance was determined using the unpaired t test. ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.005. (B) Primary bone-derived dendritic cells from WT, IRF-3−/− × IRF-7−/−, and IFN-α/βR−/− mice were untreated or treated with recombinant IFN-α4 or recombinant IFN-β and inoculated with MNV (MOI of 0.05). Virus titers at 24 hpi are from at least three independent experiments. Titers from untreated IRF-3−/− × IRF-7−/− and IFN-α/βR−/− dendritic cells were compared with those from wild-type controls; titers from IFN-α4 or IFN-β-treated wild-type, IRF-3−/− × IRF-7−/−, and IFN-α/βR−/− dendritic cells were compared with those from untreated controls. (C) Macrophages from WT and IRF-3−/− × IRF-7−/− mice were mock inoculated or inoculatedwith MNV (MOI of 10). Induction of IFN-α4 and IFN-β mRNA levels at 12 hpi was normalized to 18S RNA levels and calculated using the ΔΔCT method. Relative induction was determined from four independent experiments and compared to induction in wild-type controls. Statistical significance was determined using the unpaired t test. (D) Dendritic cells from WT and IRF-3−/− × IRF-7−/− mice were mock inoculated or inoculated with MNV (MOI of 10). Induction of IFN-α4 and IFN-β mRNA levels at 8 and 12 hpi was normalized to 18S RNA levels and calculated using the ΔΔCT method. Relative induction was determined from three independent experiments and compared to induction in wild-type controls.
IRF-3 and IRF-7 are required for IFN-α/β induction but are dispensable for the downstream antiviral activity mediated by IFN-α/β.
To further elucidate the role of IRF-3 and IRF-7 during MNV infection, we examined MNV replication in macrophages and dendritic cells from wild-type, IFN-α/βR−/−, and IRF-3−/− ×IRF-7−/− mice after treatment with IFN-α4 or IFN-β. We elected to examine the ability of IFN-α4 to inhibit replication of MNV in vitro, as virus infection in the mouse can induce both IFN-α4 and IFN-β with immediate-early kinetics (38, 42). Treatment with IFN-α4 and IFN-β significantly decreased MNV replication in wild-type and IRF-3−/− × IRF-7−/− macrophages and dendritic cells compared to untreated controls but had no significant effect, as expected, in IFN-α4- and IFN-β-treated IFN-α/βR−/− macrophages and dendritic cells compared to untreated controls (Fig. 4A and B). Thus, IRF-3 and IRF-7 are dispensable for the antiviral activity mediated by IFN-α4 or IFN-β in macrophages and dendritic cells.
To assess whether IRF-3 or IRF-7 is important for induction of IFN-α/β in cells infected with MNV, we measured IFN-α/β mRNA during MNV infection of wild-type and IRF-3−/− × IRF-7−/− macrophages and dendritic cells. Notably, we observed a significant decrease in IFN-α4 (850-fold) and IFN-β (3,600-fold) mRNA induction in IRF-3−/− × IRF-7−/− macrophages compared to wild-type controls during MNV infection, with virtually no induction of either cytokine seen in cells lacking both IRF-3 and IRF-7 (Fig. 4C). In striking contrast, we observed a limited decrease in IFN-α4 (7-fold) and IFN-β (11-fold) mRNA induction in IRF-3−/− × IRF-7−/− dendritic cells compared to wild-type controls at 8 hpi with MNV, with near-wild-type levels of induction by MNV of both cytokines in cells lacking both IRF-3 and IRF-7 at 12 hpi (Fig. 4D). These data demonstrate that IRF-3 and IRF-7 are essential for induction of IFN-α/β during MNV infection of primary macrophages and participate in IFN-α/β induction during MNV infection of primary dendritic cells. These results also indicate that there is a cell type-specific role for these transcription factors in IFN-α/β induction during MNV infection.
DISCUSSION
IFN-α/β likely plays an important role in the induction of antiviral immune mechanisms during HuNoV infection and has been shown to be important, when combined with IFN-γ, for control of MNV replication. However, the contribution of IFN-α/β responses to host defense during NoV infection, as well as the upstream initiators of these responses, is not well defined. Previous studies using the MNV model system identified a requirement for MDA-5 in IFN-α/β production during MNV infection in vitro, as well as a role for MDA-5 in restricting MNV infection both in vitro and in vivo (46). In this report, we demonstrate an important role for IFN-α/β responses in control of MNV replication and pathogenesis in vivo. Using mice with floxed alleles of the IFNAR1 chain of the IFN-α/βR, we showed that IFN-α/β responses in lysozyme M- and CD11c-expressing cells, likely macrophages/neutrophils and dendritic cells, restrict MNV replication in vivo. Moreover, by analyzing cultured macrophages and dendritic cells, we demonstrated that IRF-3 and IRF-7 are key mediators of the antiviral immune response during MNV infection. The role for IRF-3 and IRF-7 is prominently in the induction of IFN-α/β rather than in the induction of an antiviral state by IFN-α/β.
Macrophages and dendritic cells participate in IFN-α/β immune responses against MNV.
The tropism of HuNoV is not completely understood. The binding of NV-like particles to enterocytes, as well as histopathology in the intestines, of human volunteers inoculated with NV or recombinant virus-like particles (43, 59) suggests that HuNoV replicates in intestinal epithelial cells. However, the detection of NV capsid antigen in mononuclear cells in the intestinal lamina propria of an infected person, as well as in experimentally infected chimpanzees (5, 35), suggests that HuNoV may also infect macrophages and/or dendritic cells in gut lymphoid tissues.
MNV infects mononuclear cells in the intestinal lamina and has a tropism for macrophages and dendritic cells in vitro (49, 75, 76). Furthermore, IFN-α/β responses are important for restricting MNV replication in macrophages and dendritic cells in vitro (13, 76). Our data suggest that IFN-α/β responses in CD8α+ dendritic cells, which lack the IFN-α/βR (20), likely play a role in restricting MNV replication in vivo. Data obtained from IFN-α/βRf/f × LysM-Cre mice is more complicated in that the IFNAR1 allele is floxed in macrophage-lineage cells, in particular resident macrophages, and in neutrophils (20). To date, the role of neutrophils in NoV infection has not been explored. The lack of MNV titers observed in the lung of IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice is consistent with the idea that IFN-α/β responses in additional as-yet-uncharacterized cell types may also prevent MNV replication. Alternatively, incomplete deletion of the IFN-α/βR in macrophages or neutrophils and dendritic cells may occur in certain tissues of IFN-α/βRf/f × LysM-Cre and IFN-α/βRf/f × CD11c-Cre mice (20, 30). Given that IFN-α/βR levels were not significantly decreased in plasmacytoid dendritic cells in IFN-α/βRf/f × CD11c-Cre mice in a prior study (20), it is interesting to speculate that plasmacytoid DC that constitutively express IRF-7, which was critical for restriction of MNV replication in livers, spleens, and mesenteric lymph nodes, may regulate IFN-α/β responses at distal sites during MNV infection. Additional studies will be needed to elucidate the role of neutrophils, and subsets of macrophages and dendritic cells, during NoV infection.
IRF-3 and IRF-7 are key components of the transcriptional antiviral response upon MNV infection.
Our studies with primary macrophages revealed a requirement for IRF-3 and IRF-7 in the induction of IFN-α4 and IFN-β during MNV infection. In addition, we demonstrated that both IFN-α4 and IFN-β can inhibit MNV replication in macrophages and dendritic cells in the absence of IRF-3 and IRF-7. Consistent with these observations, we observed elevated MNV titers in all tissues of IRF-3−/− × IRF-7−/− mice compared to wild-type controls. We previously showed that MDA-5 and TLR-3 are involved in IFN-α/β release during MNV infection (46). These new data, in combination with prior findings, are consistent with a recent paradigm suggesting that upon virus infection, cells utilize a subset of both pattern recognition receptors and IRF transcription factors to induce cell-specific IFN-α/β responses (reviewed in reference 45).
Although IFN-α4 and IFN-β mRNA induction was abolished in IRF-3−/− × IRF-7−/− macrophages, we observed IFN-α4 and IFN-β mRNA induction in IRF-3−/− × IRF-7−/− dendritic cells, especially at later time points. Consistent with these observations, despite elevated MNV titers in all tissues examined, IRF-3−/− ×IRF-7−/− mice failed to succumb to lethal MNV infection, in contrast to IFN-α/βR−/− mice, which were highly vulnerable. These observations show that expression of the IFN-α/βR is more important for control of MNV infection than the combination of IRF-3 and IRF-7. These observations are reminiscent of recent studies that uncovered an IRF-3- and IRF-7-independent antiviral mechanism during WNV and chikungunya infection in vivo (6, 16, 19), and they suggest that one or more transcription factors in addition to IRF-3 and IRF-7 are involved in IFN-α/β induction or IFN-α/β antiviral effects during MNV infection in vivo. To date, these additional transcription factor(s) remain unknown; a significant decrease in IFN-α4 and IFN-β mRNA induction during MNV infection was not observed in macrophages lacking IRF-1 compared to wild-type controls (L. Thackray, unpublished observations), but this may reflect compensatory effects of IRF-3 or IRF-7. Alternatively, IRF-3−/− × IRF-7−/− mice may be protected against lethal MNV infection by compensatory immune mechanisms, such as enhanced IFN-γ production, in a manner similar to that of murine cytomegalovirus infection, or by enhanced IFN-λ production (33, 62, 64). Future studies will be needed to identify other transcription factors involved in initiating antiviral host responses during NoV infection.
IRF-3 and IRF-7 prevent disseminated MNV infection.
HuNoV infection is typically associated with gastrointestinal symptoms, including nausea, vomiting, and watery diarrhea (3, 24), all consistent with the current paradigm that the gastrointestinal tract is the primary site of HuNoV replication. However, the more recent association of HuNoV infection with extraintestinal symptoms such as benign infantile seizures and encephalopathy, as well as the detection of HuNoV RNA in serum samples from patients (14, 47, 50, 68), suggests that HuNoV may accumulate in other tissues of infected patients. Of note, while HuNoV RNA was not detected in serum of experimentally infected chimpanzees, it was detected in the liver (5). The mechanisms that restrict HuNoV replication to the intestine or limit the replication of virus that is released into the lymphatic or somatic organ systems remain unknown.
MNV replicates in the livers, spleens, mesenteric lymph nodes, and intestines of immunocompetent mice (27, 49, 69) and thus can spread to extraintestinal sites after peroral inoculation. Mice lacking B or T cells, STAT-1, or the IFN-α/βR and IFN-γ receptor (IFN-γR) sustain high levels of disseminated MNV infection, including in the brain and lung (31, 49, 66). Our data show that IRF-3 and IRF-7, as well as signaling through the IFN-α/βR, induce an antiviral response that limits dissemination of MNV and/or the replication of the virus in extraintestinal organs. These effects could be due to increased MNV replication at primary sites of replication such as the intestine, changes in the release of the virus into the circulation, or uncontrolled MNV replication in other organs. In this study, we observed that MNV titers in mice lacking IRF-3 and IRF-7 or the IFN-α/βR increased more dramatically in extraintestinal sites than in the intestine. Combined with previous observations of high MNV titers in the intestine of mice lacking components of both the IFN-α/βR and IFN-γR pathways (28, 49, 66), the data presented in this study suggest a key role for IFN-α/β responses in controlling norovirus replication at extraintestinal sites and a role for additional antiviral responses, such as those mediated by IFN-γ, in controlling norovirus replication in the intestine. Although the idea is speculative, impaired IFN-α/β production during HuNoV infection in a subset of individuals, such as children or immunocompromised adults, could result in disseminated HuNoV infection and more severe disease.
IFN-β limits expression of HuNoV RNA and protein expression in cells expressing an NV replicon (12), indicating that the cytokine is important for control of both human and murine norovirus replication. It is interesting to speculate that the findings here regarding mechanisms of IFN-α/β control of MNV reflect similar antiviral mechanisms that control other NoV. Future studies that elucidate the molecular pathways that induce IFN-α/β and initiate IFN-α/β responses in specific cells and tissues during MNV infection may identify novel targets for vaccination or therapeutic strategies to prevent or treat NoV infection.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH) grant AI054483, U54 AI057160 and AI084887 to H.W.V. and U19 AI083019 to M.S.D. Speed congenics was performed by the Rheumatic Diseases Core Center for Speed Congenics at Washington University School of Medicine which is supported by NIH grant P30 AR48335.
Washington University and H.W.V. receive income based on licenses for MNV technology.
We thank Sarah C. Vick for her technical assistance, Darren Kreamalmeyer for managing mouse colonies, and members of the Virgin lab for their comments on the manuscript.
Footnotes
Published ahead of print 3 October 2012
REFERENCES
- 1. Abraham AK, Kagan L, Kumar S, Mager DE. 2010. Type I interferon receptor is a primary regulator of target-mediated drug disposition of interferon-beta in mice. J. Pharmacol. Exp. Ther. 334:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atmar RL, et al. 2011. Norovirus vaccine against experimental human Norwalk virus illness. N. Engl. J. Med. 365:2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atmar RL, et al. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Au WC, Moore PA, Lafleur DW, Tombal B, Pitha PM. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210–29217 [DOI] [PubMed] [Google Scholar]
- 5. Bok K, et al. 2011. Chimpanzees as an animal model for human norovirus infection and vaccine development. Proc. Natl. Acad. Sci. U. S. A. 108:325–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brien JD, et al. 2011. Interferon regulatory factor-1 (IRF-1) shapes both innate and CD8 T cell immune responses against West Nile virus infection. PLoS Pathog. 7:e1002230 doi:10.1371/journal.ppat.1002230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cadwell K, et al. 2008. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456:259–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cadwell K, et al. 2010. Virus-plus-susceptibility gene interaction determines Crohn's disease gene Atg16L1 phenotypes in intestine. Cell 141:1135–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chachu KA, LoBue AD, Strong DW, Baric RS, Virgin HW. 2008. Immune mechanisms responsible for vaccination against and clearance of mucosal and lymphatic norovirus infection. PLoS Pathog. 4:e1000236 doi:10.1371/journal.ppat.1000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chachu KA, et al. 2008. Antibody is critical for the clearance of murine norovirus infection. J. Virol. 82:6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang KO, George DW. 2007. Interferons and ribavirin effectively inhibit Norwalk virus replication in replicon-bearing cells. J. Virol. 81:12111–12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. 2006. Stable expression of a Norwalk virus RNA replicon in a human hepatoma cell line. Virology 353:463–473 [DOI] [PubMed] [Google Scholar]
- 13. Changotra H, et al. 2009. Type I and type II interferons inhibit the translation of murine norovirus proteins. J. Virol. 83:5683–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen SY, et al. 2009. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin. Infect. Dis. 48:849–855 [DOI] [PubMed] [Google Scholar]
- 15. Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277 [DOI] [PubMed] [Google Scholar]
- 16. Couderc T, et al. 2008. A mouse model for chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4:e29 doi:10.1371/journal.ppat.0040029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daffis S, Samuel MA, Keller BC, Gale M, Jr, Diamond MS. 2007. Cell-specific IRF-3 responses protect against West Nile virus infection by interferon-dependent and -independent mechanisms. PLoS Pathog. 3:e106 doi:10.1371/journal.ppat.0030106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Daffis S, et al. 2008. Interferon regulatory factor IRF-7 induces the antiviral alpha interferon response and protects against lethal West Nile virus infection. J. Virol. 82:8465–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. 2009. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS Pathog. 5:e1000607 doi:10.1371/journal.ppat.1000607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diamond MS, et al. 2011. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208:1989–2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. El-Kamary SS, et al. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodwin MM, Canny S, Steed A, Virgin HW. 2010. Murine gammaherpesvirus 68 has evolved gamma interferon and stat1-repressible promoters for the lytic switch gene 50. J. Virol. 84:3711–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Graham DY, et al. 1994. Norwalk virus infection of volunteers: new insights based on improved assays. J. Infect. Dis. 170:34–43 [DOI] [PubMed] [Google Scholar]
- 25. Henderson KS. 2008. Murine norovirus, a recently discovered and highly prevalent viral agent of mice. Lab Anim. (NY) 37:314–320 [DOI] [PubMed] [Google Scholar]
- 26. Honda K, et al. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772–777 [DOI] [PubMed] [Google Scholar]
- 27. Hsu CC, Riley LK, Wills HM, Livingston RS. 2006. Persistent infection with and serologic cross-reactivity of three novel murine noroviruses. Comp. Med. 56:247–251 [PubMed] [Google Scholar]
- 28. Hwang S, et al. 2012. Nondegradative role of Atg5-Atg12/ Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe 11:397–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Izaguirre A, et al. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125–1138 [DOI] [PubMed] [Google Scholar]
- 30. Jakubzick C, et al. 2008. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J. Exp. Med. 205:2839–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW. 2003. STAT1-dependent innate immunity to a Norwalk-like virus. Science 299:1575–1578 [DOI] [PubMed] [Google Scholar]
- 32. Kim YG, et al. 2011. Viral infection augments nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe 9:496–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotenko SV, et al. 2003. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 4:69–77 [DOI] [PubMed] [Google Scholar]
- 34. Kushnaryov VM, MacDonald HS, Sedmak JJ, Grossberg SE. 1985. Murine interferon-beta receptor-mediated endocytosis and nuclear membrane binding. Proc. Natl. Acad. Sci. U. S. A. 82:3281–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lay MK, et al. 2010. Norwalk virus does not replicate in human macrophages or dendritic cells derived from the peripheral blood of susceptible humans. Virol. 406:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. 2011. Beta interferon controls West Nile virus infection and pathogenesis in mice. J. Virol. 85:7186–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lencioni KC, Seamons A, Treuting PM, Maggio-Price L, Brabb T. 2008. Murine norovirus: an intercurrent variable in a mouse model of bacteria-induced inflammatory bowel disease 1. Comp. Med. 58:522–533 [PMC free article] [PubMed] [Google Scholar]
- 38. Levy DE, Marie I, Smith E, Prakash A. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon. Cytokine. Res. 22:87–93 [DOI] [PubMed] [Google Scholar]
- 39. Lindesmith LC, et al. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J. Virol. 84:1800–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 41. Macal M, et al. 2012. Plasmacytoid dendritic cells are productively infected and activated through TLR-7 early after arenavirus infection. Cell Host Microbe 11:617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maniatis T, Goodbourn S, Fischer JA. 1987. Regulation of inducible and tissue-specific gene expression. Science 236:1237–1245 [DOI] [PubMed] [Google Scholar]
- 43. Marionneau S, et al. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastro 122:1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marshall JK, Thabane M, Borgaonkar MR, James C. 2007. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin. Gastroenterol. Hepatol. 5:457–460 [DOI] [PubMed] [Google Scholar]
- 45. McCartney SA, Colonna M. 2009. Viral sensors: diversity in pathogen recognition. Immunol. Rev. 227:87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McCartney SA, et al. 2008. MDA-5 recognition of a murine norovirus. PLoS Pathog. 4:e1000108 doi:10.1371/journal.ppat.1000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Medici MC, Abelli LA, Dodi I, Dettori G, Chezzi C. 2010. Norovirus RNA in the blood of a child with gastroenteritis and convulsions—a case report. J. Clin. Virol. 48:147–149 [DOI] [PubMed] [Google Scholar]
- 48. Muller U, et al. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918–1921 [DOI] [PubMed] [Google Scholar]
- 49. Mumphrey SM, et al. 2007. Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 81:3251–3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Obinata K, et al. 2010. Norovirus encephalopathy in a previously healthy child. Pediatr. Infect. Dis. J. 29:1057–1059 [DOI] [PubMed] [Google Scholar]
- 51. Paik J, et al. 2011. Murine norovirus increases atherosclerotic lesion size and macrophages in Ldlr(-/-) mice. Comp. Med. 61:330–338 [PMC free article] [PubMed] [Google Scholar]
- 52. Patel MM, Hall AJ, Vinje J, Parashar UD. 2009. Noroviruses: a comprehensive review. J. Clin. Virol. 44:1–8 [DOI] [PubMed] [Google Scholar]
- 53. Patel MM, et al. 2008. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 14:1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pinto AK, et al. 2011. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 7:e1002407 doi:10.1371/journal.ppat.1002407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Samuel MA, et al. 2006. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009–7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sato M, et al. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106–110 [DOI] [PubMed] [Google Scholar]
- 57. Sato M, et al. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539–548 [DOI] [PubMed] [Google Scholar]
- 58. Schilte C, et al. 2012. Cutting edge: independent roles for IRF-3 and IRF-7 in hematopoietic and nonhematopoietic cells during host response to Chikungunya infection. J. Immunol. 188:2967–2971 [DOI] [PubMed] [Google Scholar]
- 59. Schreiber DS, Blacklow NR, Trier JS. 1973. The mucosal lesion of the proximal small intestine in acute infectious nonbacterial gastroenteritis. N. Engl. J. Med. 288:1318–1323 [DOI] [PubMed] [Google Scholar]
- 60. Servant MJ, et al. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355–363 [DOI] [PubMed] [Google Scholar]
- 61. Sheehan KC, et al. 2006. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection 1. J. Interferon Cytokine Res. 26:804–819 [DOI] [PubMed] [Google Scholar]
- 62. Sheppard P, et al. 2003. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 4:63–68 [DOI] [PubMed] [Google Scholar]
- 63. Souza M, Cheetham SM, Azevedo MS, Costantini V, Saif LJ. 2007. Cytokine and antibody responses in gnotobiotic pigs after infection with human norovirus genogroup II. 4 (HS66 strain). J. Virol. 81:9183–9192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steinberg C, et al. 2009. The IFN regulatory factor 7-dependent type I IFN response is not essential for early resistance against murine cytomegalovirus infection. Eur. J. Immunol. 39:1007–1018 [DOI] [PubMed] [Google Scholar]
- 65. Stranges PB, et al. 2007. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26:629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Strong DW, Thackray LB, Smith TJ, Virgin HW. 2012. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J. Virol. 86:2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stuart RL, et al. 2010. An outbreak of necrotizing enterocolitis associated with norovirus genotype GII. 3. Pediatr. Infect. Dis. J. 29:644–647 [DOI] [PubMed] [Google Scholar]
- 68. Takanashi S, et al. 2009. Detection, genetic characterization, and quantification of norovirus RNA from sera of children with gastroenteritis. J. Clin. Virol. 44:161–163 [DOI] [PubMed] [Google Scholar]
- 69. Thackray LB, et al. 2007. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 81:10460–10473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Trinchieri G. 2012. Lymphocyte choriomeningitis virus plays hide-and-seek with type 1 interferon. Cell Host Microbe 11:553–555 [DOI] [PubMed] [Google Scholar]
- 71. Turcios-Ruiz RM, et al. 2008. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J. Pediatr. 153:339–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Walsh KB, et al. 2012. Toll-like receptor 7 is required for effective adaptive immune responses that prevent persistent virus infection. Cell Host Microbe 11:643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang Y, Cella M, Gilfillan S, Colonna M. 2010. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J. Immunol. 184:2751–2755 [DOI] [PubMed] [Google Scholar]
- 74. Wang Y, et al. 2012. Timing and magnitude of type I interferon responses by distinct sensors impact CD8 T cell exhaustion and chronic viral infection. Cell Host Microbe 11:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ward JM, et al. 2006. Pathology of immunodeficient mice with naturally occurring murine norovirus infection. Toxicol. Pathol. 34:708–715 [DOI] [PubMed] [Google Scholar]
- 76. Wobus CE, et al. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:e432 doi:10.1371/journal.pbio.0020432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wobus CE, Thackray LB, Virgin HW. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 80:5104–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yoneyama M, Suhara W, Fujita T. 2002. Control of IRF-3 activation by phosphorylation. J. Interferon Cytokine Res. 22:73–76 [DOI] [PubMed] [Google Scholar]
- 79. Zhao Z, et al. 2007. Coronavirus replication does not require the autophagy gene ATG5. Autophagy 3:581–585 [DOI] [PubMed] [Google Scholar]