Abstract
Tipranavir (TPV), a protease inhibitor (PI) inhibiting the enzymatic activity and dimerization of HIV-1 protease, exerts potent activity against multi-PI-resistant HIV-1 isolates. When a mixture of 11 multi-PI-resistant (but TPV-sensitive) clinical isolates (HIV11MIX), which included HIVB and HIVC, was selected against TPV, HIV11MIX rapidly (by 10 passages [HIV11MIXP10]) acquired high-level TPV resistance and replicated at high concentrations of TPV. HIV11MIXP10 contained various amino acid substitutions, including I54V and V82T. The intermolecular FRET-based HIV-1 expression assay revealed that TPV's dimerization inhibition activity against cloned HIVB (cHIVB) was substantially compromised. The introduction of I54V/V82T into wild-type cHIVNL4-3 (cHIVNL4-3I54V/V82T) did not block TPV's dimerization inhibition or confer TPV resistance. However, the introduction of I54V/V82T into cHIVB (cHIVBI54V/V82T) compromised TPV's dimerization inhibition and cHIVBI54V/V82T proved to be significantly TPV resistant. L24M was responsible for TPV resistance with the cHIVC genetic background. The introduction of L24M into cHIVNL4-3 (cHIVNL4-3L24M) interfered with TPV's dimerization inhibition, while L24M increased HIV-1's susceptibility to TPV with the HIVNL4-3 genetic background. When selected with TPV, cHIVNL4-3I54V/V82T most readily developed TPV resistance and acquired E34D, which compromised TPV's dimerization inhibition with the HIVNL4-3 genetic background. The present data demonstrate that certain amino acid substitutions compromise TPV's dimerization inhibition and confer TPV resistance, although the loss of TPV's dimerization inhibition is not always associated with significantly increased TPV resistance. The findings that TPV's dimerization inhibition is compromised with one or two amino acid substitutions may explain at least in part why the genetic barrier of TPV against HIV-1's development of TPV resistance is relatively low compared to that of darunavir.
INTRODUCTION
Tipranavir (TPV) is a nonpeptidyl protease (PR) inhibitor (PI) which is administered in combination antiretroviral therapy to treat HIV-1 infection and AIDS. TPV has been shown to inhibit the replication of HIV-1 variants that are resistant to other PIs and is recommended for patients who harbor multi-PI-resistant HIV-1 variants and do not respond to therapeutic regimens, including PIs (34). HIV-1 resistance to TPV has been reported to require multiple amino acid substitutions in protease (9); however, we have witnessed that HIV-1 often acquires significant levels of resistance to TPV among HIV-1-infected individuals receiving long-term combination chemotherapy (4, 17, 31). TPV is currently not recommended in the initial therapy mainly because of the higher rate at which patients previously experienced hepatic abnormalities (5, 29, 37).
Dimerization of HIV-1 PR subunits is an essential process for the acquisition of the proteolytic activity of HIV-1 PR (23, 39). Thus, inhibition of PR dimerization likely abolishes proteolytic activity and intervenes in the replication of HIV-1. We have recently developed an intermolecular fluorescence resonance energy transfer (FRET)-based HIV-1 expression assay employing cyan fluorescent protein (CFP)- and yellow fluorescent protein (YFP)-tagged HIV-1 PR monomers to detect and quantify PR dimerization (21). Using this assay, we have recently identified a group of nonpeptidyl small-molecule inhibitors of HIV-1 PR dimerization, including TPV, darunavir (DRV), and a series of experimental antiretroviral agents (21). DRV (12, 13, 22) potently inhibits the enzymatic activity and dimerization of HIV-1 PR (20, 21), and a high genetic barrier against HIV-1 development of DRV resistance exists (7, 8). However, various amino acid substitutions that are potentially related to HIV-1 resistance to DRV have been reported (7, 19, 20, 27, 38). We have recently demonstrated, using the FRET-based HIV-1 expression assay, that the loss of DRV activity to inhibit protease dimerization requires at least four combined amino acid substitutions in protease and that the loss of DRV's protease dimerization inhibition activity is associated with HIV-1 acquisition of DRV resistance (20). In the present work, we specifically asked whether TPV's protease dimerization inhibition activity contributes to the antiretroviral activity of TPV against a series of multi-PI-resistant HIV-1 variants and whether the loss of such protease dimerization inhibition activity of TPV is associated with the development of HIV-1 resistance to TPV.
MATERIALS AND METHODS
Cells and viruses.
MT-4 cells were grown in RPMI 1640-based culture medium, while 293T and COS7 cells were propagated in Dulbecco's modified Eagle's medium. These media were supplemented with 10% fetal calf serum (FCS; PAA Laboratories GmbH, Linz, Austria) plus 50 U of penicillin and 50 μg of kanamycin per ml. Peripheral blood mononuclear cells (PBMCs) were isolated from the buffy coat of HIV-1-seronegative individuals using Ficoll-Hypaque density gradient centrifugation and cultured in RPMI 1640-based culture medium containing 10% FCS and antibiotics with 10 μg of phytohemagglutinin (PHA-PBMCs) for 3 days prior to use. The following HIV-1 strains were used for the drug susceptibility assay and selection experiments: a clinical HIV-1 strain isolated from an antiretroviral therapy-naïve patients with AIDS (HIV104pre) (41) and 11 HIV-1 clinical strains (HIVA, HIVB, HIVC, HIVG, HIVTM, HIVMM, HIVJSL, HIVSS, HIVES, HIVEV, and HIV13-52) which were originally isolated from patients with AIDS who were enrolled in an open-label clinical study of amprenavir (APV) and abacavir (ABC) at the Clinical Center, National Institutes of Health, and were randomly chosen from among the enrollees who had failed the APV-plus-ABC therapy (36, 41) or a phase I/II study of tenofovir disoproxil fumarate (TDF) (15). Such patients had failed existing anti-HIV-1 regimens after receiving 7 to 11 anti-HIV-1 drugs over the previous 24 to 83 months in the 1990s (15, 36, 41). The clinical strains used in the present study contained 8 to 16 amino acid substitutions in the protease-encoding region which have reportedly been associated with HIV-1 resistance to various PIs and have been genotypically and phenotypically characterized as multi-PI-resistant HIV-1. The amino acid sequences of the protease of the 11 clinical isolates are illustrated in Fig. S1 in the supplemental material.
Antiviral agents.
DRV was synthesized as described previously (14). Saquinavir (SQV) was kindly provided by Roche Products Ltd. (Welwyn Garden City, United Kingdom) and Abbott Laboratories (Abbott Park, IL). APV was a kind gift from GlaxoSmithKline (Research Triangle Park, N.C). Indinavir (IDV) and lopinavir (LPV) were kindly provided by Japan Energy Inc., Tokyo, Japan. Atazanavir (ATV) was a kind gift from Bristol-Myers Squibb (New York, NY). TPV was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, National Institutes of Health.
Generation of highly TPV-resistant HIV-1 in vitro.
Thirty 50% tissue culture infectious doses (TCID50s) of each of the 11 highly multi-PI-resistant HIV-1 isolates was mixed and propagated in a mixture of an equal number of PHA-PBMCs (5 × 105) and MT-4 cells (5 × 105), in an attempt to adapt them for replication in MT-4 cells. The cell-free supernatant was harvested on day 7 of coculture (PHA-PBMCs and MT-4 cells), and the viruses (designated HIV11MIXP0, where P0 represents passage 0) were further propagated in fresh MT-4 cells for the selection experiment (19). On the first passage, MT-4 cells (5 × 105) were exposed to culture supernatant of mixed viruses or 500 TCID50s of each infectious molecular HIV-1 clone and cultured in the presence of TPV at initial concentrations of 0.1 to 0.4 μM. On the last day of each passage (approximately day 7), 1 ml of the cell-free supernatant was harvested and transferred to a culture of fresh uninfected MT-4 cells in the presence of increased concentrations of the drug for the following round of culture. In this round of culture, three drug concentrations (increased by 1-, 2-, and 3-fold compared to the previous concentration) were employed. When the replication of HIV-1 in the culture was confirmed by substantial Gag protein production (greater than 200 ng/ml), the highest drug concentration among the three concentrations was used to continue the selection (for the next round of culture). This protocol was repetitively used until the drug concentration reached the targeted concentration (2, 19). Proviral DNA samples obtained from the lysates of infected cells were subjected to nucleotide sequencing.
Determination of nucleotide sequences.
Molecular cloning and determination of the nucleotide sequences of HIV-1 strains passaged in the presence of TPV were performed as previously described (2, 40). In brief, high-molecular-weight DNA was extracted from HIV-1-infected MT-4 cells by using the InstaGene matrix (Bio-Rad Laboratories, Hercules, CA) and was subjected to molecular cloning, followed by sequence determination. The primers used for the first round of PCR with the entire Gag- and protease-encoding regions of the HIV-1 genome were LTR F1 (5′-GAT GCT ACA TAT AAG CAG CTG C-3′) and PR12 (5′-CTC GTG ACA AAT TTC TAC TAA TGC-3′). The first-round PCR mixture consisted of 1 μl of proviral DNA solution, 10 μl of Premix Taq (ExTaq version; TaKaRa Bio Inc., Otsu, Japan), and 10 pmol of each of the first PCR primers in a total volume of 20 μl. The PCR conditions used were an initial 3 min at 95°C, followed by 30 cycles of 40 s at 95°C, 20 s at 55°C, and 2 min at 72°C, with a final 10 min of extension at 72°C. The first-round PCR products (1 μl) were used directly in the second round of PCR with primers LTR F2 (5′-GAG ACT CTG GTA ACT AGA GAT C-3′) and Ksma2.1 (5′-CCA TCC CGG GCT TTA ATT TTA CTG GTA C-3′) under the PCR conditions of an initial 3 min at 95°C, followed by 30 cycles of 30 s at 95°C, 20 s at 55°C, and 2 min at 72°C, with a final 10 min of extension at 72°C. The second-round PCR products were purified with spin columns (MicroSpin S-400 HR columns; Amersham Biosciences Corp., Piscataway, NJ), cloned directly, and subjected to sequencing with a model 3130 automated DNA sequencer (Applied Biosystems, Foster City, CA).
Drug susceptibility assay.
To determine the susceptibility of HIV104pre and clinical multi-PI-resistant HIV-1 isolates, PHA-PBMCs (106/ml) were exposed to 50 TCID50s of each HIV-1 isolate and cultured in the presence or absence of various concentrations of drugs in 10-fold serial dilutions in 96-well microtiter culture plates. PHA-PBMCs were derived from a single donor in each independent experiment. For obtaining the data, three different donors were recruited. To determine the drug susceptibility of infectious molecular HIV-1 clones and TPV-selected HIV-1 variants, MT-4 cells were used as target cells. MT-4 cells (105/ml) were exposed to 50 TCID50s of infectious molecular HIV-1 clones and TPV-selected HIV-1 variants in the presence or absence of various concentrations of drugs and were incubated at 37°C. On day 7 of culture, the supernatant was harvested and the amount of p24 Gag protein was determined by using a fully automated chemiluminescent enzyme immunoassay system (Lumipulse F; Fujirebio Inc., Tokyo, Japan) (22). The drug concentrations that suppressed the production of p24 Gag protein by 50% (50% inhibitory concentrations [IC50s]) were determined by comparison with the level of p24 production in drug-free control cell cultures. All assays were performed in duplicate or triplicate (1, 18, 22).
Generation of recombinant HIV-1 clones.
The PCR products obtained as described above were digested with two of the three enzymes BssHII, ApaI, and XmaI, and the obtained fragments were introduced into pHIV-1NLSma, designed to have a SmaI site by changing two nucleotides (2590 and 2593) of pHIV-1NL4-3 (2, 11). To generate HIV-1 clones carrying the mutations, site-directed mutagenesis was performed using a QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), and the mutation-containing genomic fragments were introduced into pHIV-1NLSma. Determination of the nucleotide sequences of plasmids confirmed that each clone had the desired mutations but no unintended mutations. 293T cells were transfected with each recombinant plasmid by using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA), and the infectious virions thus obtained were harvested 48 h after transfection and stored at −80°C until use.
Generation of FRET-based HIV-1 expression system.
The intermolecular fluorescence resonance energy transfer-based HIV-1 expression assay employing cyan and yellow fluorescent protein (CFP and YFP, respectively)-tagged protease monomers was generated as previously described (Fig. 1) (21). In brief, CFP- and YFP-tagged wild-type HIV-1 protease constructs (pHIV-PRWTCFP and pHIV-PRWTYFP, respectively) were generated using BD Creator DNA cloning kits (BD Biosciences, San Jose, CA). For the generation of full-length molecular infectious clones containing CFP- or YFP-tagged protease, the PCR-mediated recombination method was used (10). A linker consisting of five alanines was inserted between protease and fluorescent proteins. The phenylalanine-proline site that HIV-1 protease cleaves was also introduced between the fluorescent protein and reverse transcriptase (RT). DNA fragments thus obtained were subsequently joined by using the PCR-mediated recombination reaction performed under the standard conditions for ExTaq polymerase (TaKaRa Bio Inc., Otsu, Japan). The amplified PCR products were cloned into the pCR-XL-TOPO vector according to the manufacturer's instructions (Gateway cloning system; Invitrogen, Carlsbad, CA). PCR products were generated with the pCR-XL-TOPO vector as the templates, followed by digestion by both ApaI and XmaI, and the ApaI-XmaI fragment was introduced into pHIV-1NLSma (11), generating pHIV-PRWTCFP and pHIV-PRWTYFP, respectively.
Fig 1.
FRET-based HIV expression system. Plasmids encoding full-length molecular infectious HIV (HIVNL4-3) clones that produce CFP- or YFP-tagged PR were prepared using the PCR-mediated recombination method, as described in Materials and Methods. A linker consisting of five alanines was inserted between HIV PR and the fluorescent protein. A phenylalanine-proline site (F/P) that HIV PR cleaves was introduced between the fluorescent protein and RT. Shown are structural representations of PR monomers and dimer in association with the linker atoms and fluorescent proteins. FRET occurs when the two fluorescent proteins become 1 to 10 nm apart. If an agent that is capable of inhibiting the dimerization of PR monomer subunits is present when the CFP- and YFP-tagged PR monomers are produced within the cell upon cotransfection, no FRET occurs.
FRET procedure.
COS7 cells plated on an EZ view cover-glass-bottom culture plate (Iwaki, Tokyo, Japan) were transfected with pHIV-PRWTCFP and pHIV-PRWTYFP using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions in the presence of various concentrations of each compound, cultured for 72 h, and analyzed under a Fluoview FV500 confocal laser scanning microscope (Olympus Optical Corp., Tokyo, Japan) at room temperature as previously described (21). When the effect of each compound was analyzed by FRET, test compounds were added to the culture medium simultaneously with plasmid transfection. Results of FRET were determined by quenching of CFP (donor) fluorescence and an increase in YFP (acceptor) fluorescence (sensitized emission), since part of the energy of CFP is transferred to YFP instead of being emitted. The changes in the CFP and YFP fluorescence intensity in the images of selected regions were examined and quantified using the Olympus FV500 Image software system (Olympus Optical Corp). Background values were obtained from the regions where no cells were present and were subtracted from the values for the cells examined in all calculations. Ratios of intensities of CFP fluorescence after photobleaching to CFP fluorescence prior to photobleaching (CFPA/B ratios) were determined. It is well established that CFPA/B ratios of greater than 1.0 indicate that an association of CFP- and YFP-tagged proteins occurred, and it was interpreted that the dimerization of protease subunits occurred. CFPA/B ratios of less than 1 indicated that the association of the two subunits did not occur, and it was interpreted that protease dimerization was inhibited (21).
RESULTS
Generation of TPV-resistant HIV-1 using a mixture of multi-PI-resistant clinical HIV-1 isolates.
TPV and DRV inhibit the enzymatic activity of HIV-1 protease (21, 30), block the dimerization of protease subunits (21), and exert potent activity against a wide spectrum of wild-type and multi-PI-resistant HIV-1 variants (3, 22, 30). Although the emergence of DRV-resistant HIV-1 is substantially delayed in vitro (6, 19) and in vivo (32), we recently reported that the use of a mixture of 8 multiple-PI-resistant clinical HIV-1 isolates as a starting HIV-1 population makes it possible to relatively rapidly select highly DRV-resistant HIV-1 variants (19). We therefore attempted to generate TPV-resistant HIV-1 variants, employing as a starting HIV-1 population a mixture of 11 highly multiple-PI-resistant clinical HIV-1 strains (HIV11MIX) isolated from patients with AIDS, who had failed multiple antiretroviral regimens containing various PIs. All such clinical isolates contained a variety of PI resistance-associated amino acid substitutions in their protease and showed, in general, high levels of resistance to five approved PIs (SQV, IDV, APV, LPV, and ATV), as examined in PHA-PBMCs as target cells using p24 production inhibition as an endpoint (Table 1). However, it was noted that TPV retained its anti-HIV-1 activity against each of the virus strains in HIV11MIX, with 0.4- to 3-fold-differences in its IC50 against a wild-type clinical strain HIV104pre (35) relative to those against multiple-PI-resistant clinical HIV-1 strains (Table 1).
TABLE 1.
Sensitivities of multidrug-resistant clinical isolates to various PIsa
| Virus | IC50, mean ± SD (μM) (fold change) |
|||||
|---|---|---|---|---|---|---|
| SQV | IDV | APV | LPV | ATV | TPV | |
| HIV104pre | 0.0073 ± 0.0014 | 0.042 ± 0.003 | 0.029 ± 0.002 | 0.034 ± 0.002 | 0.002 ± 0.0004 | 0.16 ± 0.03 |
| HIVA | 0.075 ± 0.001 (10) | 0.59 ± 0.04 (14) | 0.074 ± 0.013 (3) | 0.26 ± 0.014 (8) | 0.024 ± 0.003 (12) | 0.36 ± 0.007 (2) |
| HIVB | 0.36 ± 0.001 (49) | >1 (>24) | >1 (>34) | >1 (>29) | 0.28 ± 0.01 (140) | 0.18 ± 0.01 (1) |
| HIVC | 0.032 ± 0.002 (4) | >1 (>24) | 0.37 ± 0.01 (11) | >1 (>29) | 0.034 ± 0.004 (17) | 0.17 ± 0.04 (1) |
| HIVG | 0.030 ± 0.001 (4) | 0.36 ± 0.1 (9) | 0.43 ± 0.04 (15) | 0.26 ± 0.04 (8) | 0.043 ± 0.022 (22) | 0.24 ± 0.08 (2) |
| HIVTM | 0.26 ± 0.04 (36) | >1 (>24) | 0.32 ± 0.007 (11) | >1 (>29) | 0.065 ± 0.008 (33) | 0.38 ± 0.05 (2) |
| HIVMM | 0.22 ± 0.01 (29) | >1 (>24) | 0.32 ± 0.03 (11) | 0.59 ± 0.004 (17) | 0.21 ± 0.02 (105) | 0.35 ± 0.007 (2) |
| HIVSS | 0.17 ± 0.004 (23) | >1 (>24) | 0.13 ± 0.01 (4) | 0.21 ± 0.04 (6) | 0.032 ± 0.006 (16) | 0.41 ± 0.14 (3) |
| HIVJSL | 0.30 ± 0.02 (41) | >1 (>24) | 0.78 ± 0.1 (27) | >1 (>29) | 0.43 ± 0.04 (215) | 0.23 ± 0.05 (1) |
| HIVEV | 0.36 ± 0.05 (49) | 0.25 ± 0.02 (6) | >1 (>34) | >1 (>29) | 0.43 ± 0.04 (215) | 0.066 ± 0.016 (0.4) |
| HIVES | 0.45 ± 0.01 (62) | >1 (>24) | >1 (>34) | 0.41 ± 0.01 (12) | 0.032 ± 0.004 (16) | 0.34 ± 0.01 (2) |
| HIV13-52 | 0.029 ± 0.003 (4) | 0.32 ± 0.03 (7) | 0.030 ± 0.003 (1) | 0.22 ± 0.1 (6) | 0.021 ± 0.007 (11) | 0.10 ± 0.01 (0.6) |
IC50s were determined by using PHA-PBMCs as target cells, and the inhibition of p24 Gag protein production by each drug was used as the endpoint. Numbers in parentheses represent the n-fold change of the IC50 for each isolate compared to the IC50 for wild-type strain HIV104pre. All assays were conducted in duplicate or triplicate, and the data shown represent mean values ±1 standard deviation derived from the results of three independent experiments. PHA-PBMCs were derived from a single donor in each independent experiment.
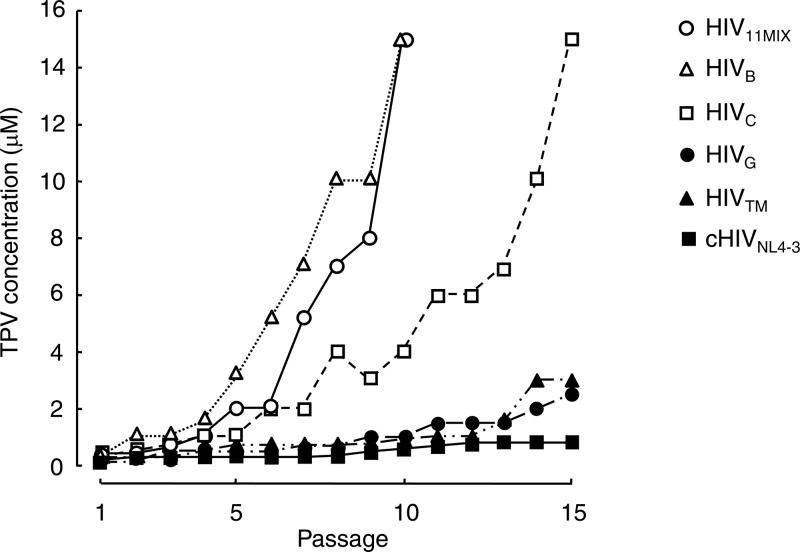
Each of four selected primary strains (HIVB, HIVC, HIVG, and HIVTM) and HIV11MIX were propagated in a mixture of an equal number of PHA-PBMCs and MT-4 cells, in an attempt to adapt each virus population for replication in MT-4 cells in the presence of 0.4 μM TPV as a starting concentration. The four primary HIV-1 strains were chosen since they were all highly resistant to the majority of nucleoside reverse transcriptase inhibitors (35, 41) and a variety of PIs (Table 1). The cell-free supernatant was harvested at the conclusion of each passage (approximately 7 days) of culture, and the viral preparation was further propagated in fresh MT-4 cells. HIV11MIX at passage 10 (HIV11MIXP10) was capable of replicating in the presence of TPV at a concentration as high as 15 μM (Fig. 2). Two (HIVB and HIVC) of the four primary HIV strains also became replicative in the presence of 15 μM TPV by passages 10 and 15, respectively, while HIVG and HIVTM failed to replicate in the presence of 2 and 3 μM TPV, respectively (Fig. 2). However, the HIVNL4-3 clone (cHIVNL4-3) did not develop a high level of resistance to TPV, and its replication was found to be significantly compromised in the presence of >1.5 μM TPV (Fig. 2).
Fig 2.
In vitro selection of TPV-resistant variants using multidrug-resistant clinical HIV-1 isolates. A mixture of 11 multi-PI-resistant HIV-1 isolates (HIV11MIX), four multidrug-resistant clinical HIV-1 isolates (HIVB, HIVC, HIVG, and HIVTM), and an infectious molecular HIV-1 clone (cHIVNL4-3) were propagated in the presence of increasing concentrations of TPV in MT-4 cells. The selection was carried out in a cell-free manner for a total of 10 to 15 passages with drug concentrations escalating from the IC50 for each virus up to 15 μM. The nucleotide sequences of proviral DNA were determined using the cell lysates of HIV-1-infected MT-4 cells at the termination of each indicated passage.
Susceptibility of TPV-selected HIV11MIX populations to various PIs, including TPV.
In order to determine the susceptibility of the above-described TPV-selected HIV-1 variants to seven FDA-approved PIs, we harvested HIV11MIXP0, HIV11MIXP5, and HIV11MIXP10 at passages 0, 5, and 10, respectively, and tested them using the drug susceptibility assay. As shown in Table 2, HIV11MIXP0 showed considerable resistance to 5 PIs (SQV, IDV, APV, LPV, and ATV), with fold changes of 8 to 29 in their IC50s against the variants over the IC50 of each PI against wild-type HIV104pre. HIV11MIXP5 was more resistant to each of the 5 PIs, while DRV and TPV remained fairly active against both HIV11MIXP0 and HIV11MIXP5, with fold changes of 1 to 8 (Table 2). HIV11MIXP10 showed high levels of resistance to the 5 PIs (IC50s for all drugs, >1 μM). HIV11MIXP10 was resistant to TPV with a fold change of >34, while it remained relatively susceptible to DRV with a fold change of 10. Of note, the absolute IC50 of DRV against HIV11MIXP10 was 0.034 ± 0.004 μM, while that of TPV was >10 μM (Table 2), indicating that the TPV-selected HIV-1 variant was yet fairly susceptible to DRV.
TABLE 2.
Sensitivities of TPV-resistant variants to various PIsa
| Virus | IC50, mean ± SD (μM) (fold change) |
||||||
|---|---|---|---|---|---|---|---|
| SQV | IDV | APV | LPV | ATV | DRV | TPV | |
| HIV104pre | 0.0043 ± 0.0004 | 0.033 ± 0.002 | 0.031 ± 0.001 | 0.031 ± 0.001 | 0.0036 ± 0.0001 | 0.0035 ± 0.0002 | 0.29 ± 0.03 |
| HIV11MIXP0 | 0.036 ± 0.002 (8) | 0.97 ± 0.007 (29) | 0.31 ± 0.003 (10) | 0.34 ± 0.04 (11) | 0.043 ± 0.005 (12) | 0.015 ± 0.001 (4) | 0.32 ± 0.01 (1) |
| HIV11MIXP5 | 0.21 ± 0.08 (48) | >1 (>30) | 0.32 ± 0.01 (10) | >1 (>32) | 0.38 ± 0.04 (105) | 0.021 ± 0.008 (6) | 2.5 ± 0.01 (8) |
| HIV11MIXP10 | >1 (>233) | >1 (>30) | >1 (>32) | >1 (>32) | >1 (>280) | 0.034 ± 0.004 (10) | >10 (>34) |
IC50s were determined by employing MT-4 cells exposed to each virus (50 TCID50s) in the presence of each PI, and the inhibition of p24 Gag protein production was used as the endpoint. All values were determined in triplicate, and the data are shown as mean values ± 1 standard deviation of the results from two or three independent experiments. The numbers in parentheses are changes (n-fold) in the IC50 for each isolate compared to the IC50 of each PI for HIV104pre.
I54V and V82T are major substitutions associated with HIV11MIX acquisition of TPV resistance.
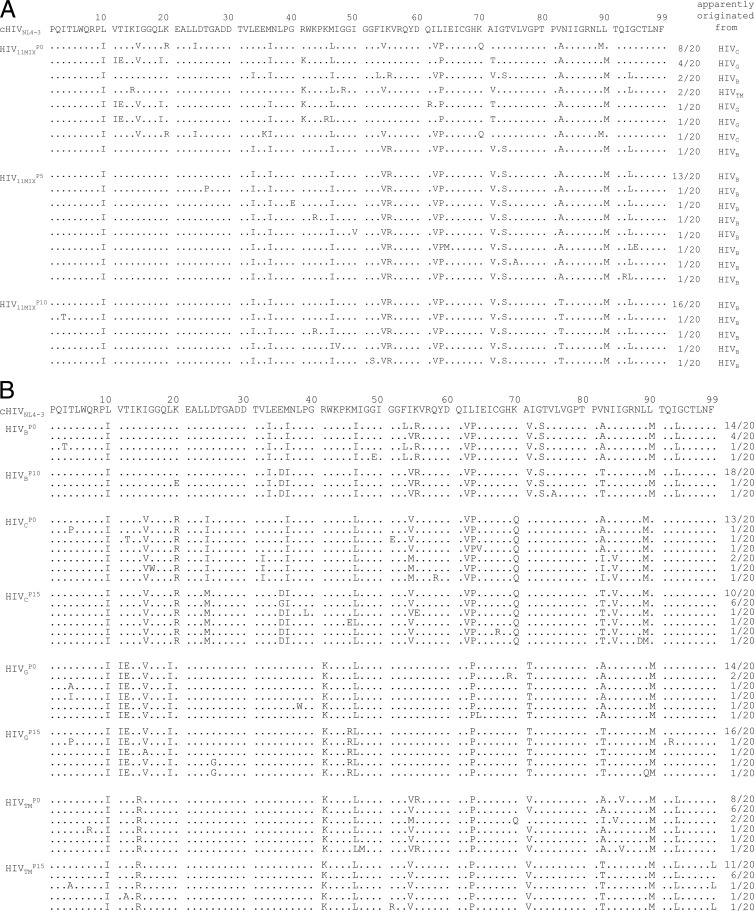
We determined the amino acid sequences of the protease in HIV-1 clones prepared at various selection passages of each viral population. In HIV11MIXP0, the original protease sequences of the four isolates HIVB, HIVC, HIVG, and HIVTM (see Fig. S1 in the supplemental material for each sequence) were predominantly identified (3 of 20 clones, 9 of 20 clones, 6 of 20 clones, and 2 of 20 clones, respectively), while the original sequence of HIVB protease appeared to be the most predominant in HIV11MIXP5and HIV11MIXP10, as shown in Fig. 3A. Of note, I54V was present in the protease of 12 of 20 HIV11MIXP0 clones, while it was present in all clones generated from HIV11MIXP5 and HIV11MIXP10. V82A was present in all clones of HIV11MIXP0 and HIV11MIXP5 (20 of 20 clones), while it had been converted to V82T in all clones of HIV11MIXP10 (20 of 20 clones) (Fig. 3A), in line with the results previously reported by Baxter et al. (4).
Fig 3.
Amino acid sequences of protease-encoding regions of HIV11MIX, HIVB, HIVC, HIVG, and HIVTM on TPV selection. Shown are the amino acid sequences deduced from the nucleotide sequences of the protease-encoding region of proviral DNA isolated from HIV11MIX at passages 1, 5, and 10 (A) and HIVB, HIVC, HIVG, and HIVTM at passages 1 and 10 or 15 (B) in the TPV selection. The consensus sequence of cHIVNL4-3 is illustrated at the top as a reference. Identity with the consensus sequence at individual amino acid positions is indicated by dots. The fractions of the virus from which each clone is presumed to have originated over the number of clones examined are shown on the right.
We also analyzed the amino acid sequences of the protease of the four TPV-selected primary HIV-1 strains. I54V was present in 4 of 20 clones of HIVBP0, while it was found in all clones of HIVBP10. Similarly, I54V was seen in 16 of 20 clones and 18 of 20 clones of HIVCP0 and HIVTMP0, respectively, while it was seen in all clones of HIVCP15 and HIVTMP15. As seen in the case of HIV11MIX, V82A was seen in 20, 16, 20, and 18 of 20 clones of HIVBP0, HIVCP0, HIVGP0, and HIVTMP0, respectively, and V82T was seen in all clones of HIVBP10, HIVCP15, HIVGP15, and HIVTMP15, as illustrated in Fig. 3B. The results that I54V and V82T were seen in all clones by passage 10 in HIV11MIX and 3 of the 4 primary HIV-1 strains at passage 10 and/or passage 15 suggested that these two amino acid substitutions may represent major amino acid substitutions presumably responsible for the initiation and/or progression of the development of TPV resistance.
I54V and V82T substitutions do not significantly compromise TPV activity to block protease dimerization.
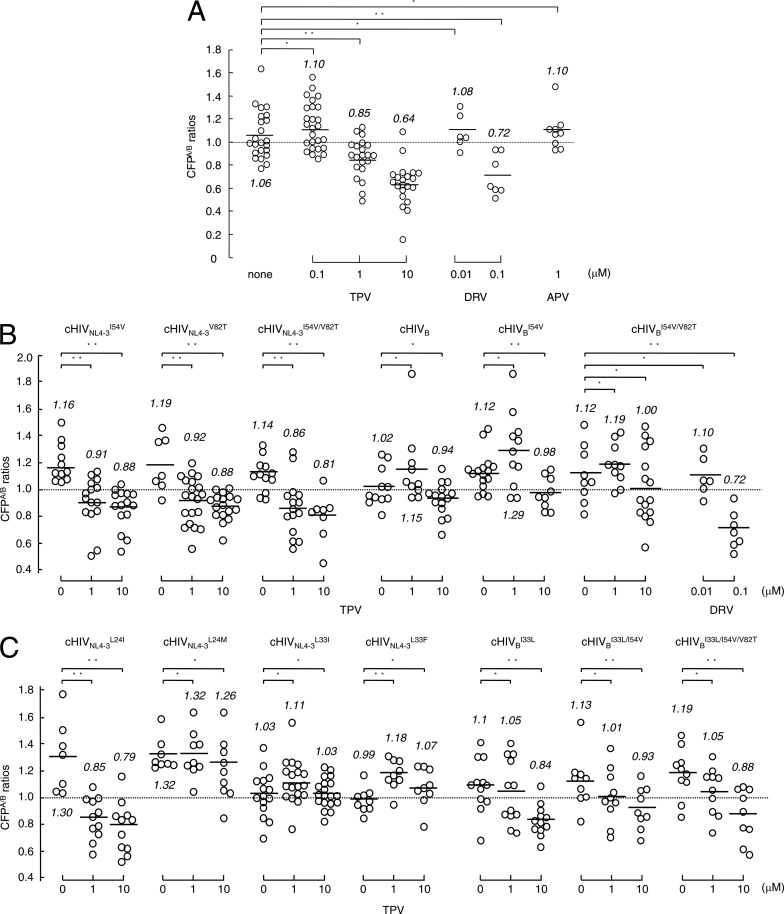
We previously reported that TPV inhibits the dimerization of wild-type HIV-1 protease, as examined with the FRET-based HIV-1 expression system (21). Figure 4A demonstrates the dimerization inhibition profiles of TPV and DRV against wild-type HIV-1 protease. In this assay, COS7 cells were exposed to various concentrations of TPV, DRV, or APV and subsequently transfected with pHIV-PRWTCFP and pHIV-PRWTYFP, and the CFPA/B ratios were determined at the end of the 72-h period of culture. In the absence of drug, the average CFPA/B ratio was 1.06, indicating that protease dimerization occurred in the absence of TPV in the system. In the presence of 0.1 μM TPV, the average CFPA/B ratio was 1.10, indicating that the dimerization still occurred. However, in the presence of 1 and 10 μM TPV, the average CFPA/B ratios were 0.85 and 0.64, respectively, indicating that TPV at both concentrations clearly blocked the dimerization of HIV-1 protease. In contrast, DRV was active in blocking the dimerization at 0.1 μM, with the average ratio being 0.72, while APV failed to block the dimerization at 1 μM (Fig. 4A), as previously reported (21).
Fig 4.
Impact of amino acid substitutions on TPV's dimerization inhibition. (A) COS7 cells were exposed to each of the drugs (TPV, DRV, and APV) at various concentrations and subsequently cotransfected with plasmids encoding each full-length molecular infectious HIV-1 (HIVNL4-3) clone producing CFP- or YFP-tagged wild-type protease. After 72 h, cultured cells were examined in the FRET-HIV-1 assay system and the CFPA/B ratios were determined. The mean values of the ratios obtained are shown as bars. A CFPA/B ratio that is greater than 1 signifies that protease dimerization occurred, whereas a ratio that is less than 1 signifies the disruption of protease dimerization. All the experiments were conducted in a blind fashion. *, not significant; **, P < 0.05. (B) COS7 cells were cotransfected with a pair of clones, pHIVNL4-3CFP and pHIVNL4-3YFP, carrying I54V (cHIVNL4-3I54V), V82T (cHIVNL4-3V82T), or I54V/V82T (cHIVNL4-3I54V/V82T) or a pair of clones, pHIVBCFP and pHIVBYFP, carrying no additional substitution (cHIVB), I54V (cHIVBI54V), or I54V/V82T (cHIVBI54V/V82T) in the absence or presence of 1 or 10 μM TPV or 0.01 or 0.1 μM DRV, and CFPA/B ratios were determined. *, not significant; **, P < 0.05. The statistical differences (P values) between the CFPA/B ratios in the absence of drug (CFPA/Bno drug) and the CFPA/B ratios in the presence of 1 μM TPV (CFPA/B1 TPV) and those between the CFPA/B ratios in the absence of drug (CFPA/Bno drug) and the CFPA/B ratios in the presence of 10 μM TPV (CFPA/B10 TPV) were, respectively, <0.0001 and <0.0001 for cHIVNL4-3I54V, 0.0067 and 0.0005 for cHIVNL4-3V82T, 0.0009 and 0.0005 for cHIVNL4-3I54V/V82T, 0.359 and 0.2865 for cHIVB, 0.132 and 0.0234 for cHIVBI54V, and 0.3689 and 0.1653 for cHIVBI54V/V82T. The statistical differences (P values) between the CFPA/B ratios in the absence of drug (CFPA/Bno drug) and the CFPA/B ratios in the presence of 0.01 μM DRV (CFPA/B0.01 DRV) and those between the CFPA/B ratios in the absence of drug (CFPA/Bno drug) and the CFPA/B ratios in the presence of 0.1 μM DRV (CFPA/B0.1 DRV) were 0.5956 and 0.0021, respectively, for cHIVBI54V/V82T. (C) COS7 cells were cotransfected with a pair of clones, pHIVNL4-3CFP and pHIVNL4-3YFP, carrying L24I, L24M, L33I, or L33F or a pair of clones, pHIVBCFP and pHIVBYFP, with Leu-33 (wild-type) reverted from Ile-33 (cHIVBI33L) with an additional I54V substitution (cHIVBI33L/I54V) or with an additional I54V/V82T substitution (cHIVBI33L/I54V/V82T) in the absence or presence of 1 or 10 μM TPV, and CFPA/B ratios were determined. *, not significant; **, P < 0.05.
We next asked whether amino acid substitutions identified under the selection with TPV affected the dimerization inhibition activity by TPV. As shown in Fig. 3A and B, I54V was seen in all HIV11MIX clones by passage 5 and beyond in all clones of HIV11MIXP10, HIVBP10, HIVCP15, and HIVTMP15. It was noted that all the clones of passage 0 virus populations (HIVBP0, HIVCP0, HIVGP0, and HIVTMP0) contained V82A or V82I; however, by passage 10 or 15, all clones contained V82T, strongly suggesting that another base change produced V82A (bases for V and A were GTC and GCC, respectively) and V82I (bases for I were ATC), resulting in acquisition of V82T (bases for T were ACC) (Fig. 3B).
We therefore examined the effects of the I54V and V82T substitutions on the susceptibility of cloned HIVNL4-3I54V (cHIVNL4-3I54V), cHIVNL4-3V82T, and cHIVNL4-3I54V/V82T to the dimerization inhibition activity of TPV. As shown in Fig. 4B, in the presence of 1 and 10 μM TPV, the respective CFPA/B ratios for cHIVNL4-3I54V were 0.91 and 0.88, those for cHIVNL4-3V82T were 0.92 and 0.88, and those for cHIVNL4-3I54V/V82T were 0.86 and 0.81 (Fig. 4B), signifying that I54V and V82T do not significantly affect the dimerization process of protease with the genetic background of cHIVNL4-3.
We also examined the effects of the I54V and V82T substitutions on the susceptibility of protease with the genetic background of HIVB to TPV's dimerization inhibition activity by generating and testing pHIV-PRWTCFP- and pHIV-PRWTYFP-tagged cHIVBI54V and cHIVBI54V/V82T in the FRET-based HIV-1 expression assay (Fig. 4B). In the absence of TPV, the CFPA/B ratio for cHIVB was 1.02, and the ratios in the presence of 1 and 10 μM TPV were 1.15 and 0.94, respectively. The ratios seen with cHIVBI54V and cHIVBI54V/V82T in the absence of TPV were both 1.12, signifying that the presence of the I54V and V82T substitutions per se does not compromise the dimerization process of protease with the genetic background of HIVB. In the presence of 1 and 10 μM TPV, the CFPA/B ratios for cHIVBI54V were 1.29 and 0.98, respectively (Fig. 4B). These data suggested that 1 μM TPV failed to block the dimerization of cHIVBI54V protease, while 10 μM TPV barely blocked the dimerization. The ratios with cHIVBI54V/V82T in the presence of 1 and 10 μM TPV were 1.19 and 1.0, respectively, indicating that 10 μM TPV did not significantly block the dimerization of cHIVBI54V/V82T protease. However, DRV at 0.1 μM effectively blocked the dimerization of cHIVBI54V/V82T protease, suggesting that DRV is more potent in blocking protease dimerization than TPV and/or that the way in which DRV blocks the dimerization of protease monomers differs from that of TPV.
L24M, L33I, and L33F are associated with the loss of TPV's dimerization inhibition.
HIVC originally contained the L24I substitution in its protease (see Fig. S1 in the supplemental material), and this substitution converted to L24M in HIVCP15 in the presence of the selection pressure with TPV (Fig. 3B). In order to examine the impact of L24M on the susceptibility of HIVC to TPV's dimerization inhibition activity, we introduced L24I and L24M into the protease in the FRET-based HIV-1 expression assay, and the clones were designated cHIVNL4-3L24I and cHIVNL4-3L24M, respectively. TPV at 1 and 10 μM effectively inhibited the dimerization of the protease of cHIVNL4-3L24I, giving average ratios of 0.85 and 0.79, respectively (Fig. 4C). In contrast, upon the introduction of L24M (cHIVNL4-3L24M), both 1 and 10 μM TPV failed to block the dimerization, giving ratios of 1.32 and 1.26, respectively.
L33I was also identified in all HIV11MIXP5and HIV11MIXP10 clones examined, although 3 of 20 HIV11MIXP0 clones had L33I. Reportedly, the L33F substitution in protease is frequently seen in HIV-1 isolates from patients failing TPV-containing regimens (24, 28). Thus, we introduced L33I and L33F into the protease in the FRET-based HIV-1 expression assay, and the clones were designated cHIVNL4-3L33I and cHIVNL4-3L33F, respectively. TPV at both 1 and 10 μM failed to block the dimerization of the protease of cHIVNL4-3L33I, giving average ratios of 1.11 and 1.03, respectively (Fig. 4C). TPV also failed to block the dimerization of the cHIVNL4-3L33F protease at both 1 and 10 μM.
Moreover, to confirm the responsibility of L33I for compromising TPV's dimerization inhibition, we reverted L33I to the wild-type amino acid (Leu-33) in cHIVB, cHIVBI54V, and cHIVBI54V/V82T, generating cHIVBI33L, cHIVBI33L/I54V, and cHIVBI33L/I54V/V82T, respectively, and determined the CFPA/B ratios. TPV failed to inhibit the dimerization of cHIVBI33L, cHIVBI33L/I54V, and cHIVBI33L/I54V/V82T at 1 μM, while the CFPA/B values were barely over 1.0. In fact, TPV at 10 μM blocked the dimerization in all three revertants (Fig. 4C). The observed moderately increased susceptibility of the three revertants to TPV's dimerization inhibition appears to confirm that L33I and L33F play a role in compromising TPV's dimerization inhibition activity.
Impact of L33I, L33F, I54V, and V82T substitutions on susceptibility of HIVNL4-3 and HIVB to TPV's antiviral activity.
We also examined the impact of the L33I, L33F, I54V, and V82T substitutions on the activity of TPV to block HIV-1 replication. When the L33I substitution was introduced into cHIVNL4-3 (designated cHIVNL4-3L33I), cHIVNL4-3L33I failed to replicate, so the IC50 of TPV against cHIVNL4-3L33I could not be determined. However, cHIVNL4-3L33I/M36I propagated fairly well. All of the other 4 newly generated infectious clones, cHIVNL4-3L33F, cHIVNL4-3I54V, cHIVNL4-3V82T, and cHIVNL4-3I54V/V82T, also replicated well, and the IC50s of TPV against these four recombinant clones were readily determined. The IC50s obtained were virtually identical to each other, with fold differences being ∼1 in comparison with the IC50 of TPV against cHIVNL4-3 (Table 3).
TABLE 3.
Effects of L33I, L33F, I54V, and V82T substitutions on the susceptibility of cHIVNL4-3 and cHIVB to TPVa
| Infectious clone | Amino acid substitution(s) in PR | Mean ± SD IC50 (μM) | Fold resistance |
|---|---|---|---|
| cHIVNL4-3 | Wild type | 0.27 ± 0.003 | 1 |
| cHIVNL4-3L33I | L33I | Does not replicate | |
| cHIVNL4-3L33F | L33F | 0.27 ± 0.001 | 1 |
| cHIVNL4-3I54V | I54V | 0.37 ± 0.005 | 1 |
| cHIVNL4-3V82T | V82T | 0.31 ± 0.025 | 1 |
| cHIVNL4-3V82L | V82L | 0.33 ± 0.023 | 1 |
| cHIVNL4-3L33I/M36I | L33I, M36I | 0.33 ± 0.004 | 1 |
| cHIVNL4-3I54V/V82T | I54V, V82T | 0.28 ± 0.004 | 1 |
| cHIVB | L10I, L33I, M36I, M46I, F53L, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L | 0.30 ± 0.02 | 1 |
| cHIVBI33L | L10I, M36I, M46I, F53L, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L | 0.26 ± 0.03 | 1 |
| cHIVBI54V | L10I, L33I, M36I, M46I, I54V, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L | 2.9 ± 0.11 | 11 |
| cHIVBI54V/V82T | L10I, L33I, M36I, M46I, I54V, K55R, I62V, L63P, A71V, G73S, V82T, L90M, I93L | 3.2 ± 0.1 | 12 |
| cHIVBI33L/154V | L10I, M36I, M46I, I54V, K55R, I62V, L63P, A71V, G73S, V82A, L90M, I93L | 1.2 ± 0.19 | 4 |
| cHIVBI33L/154V/V82T | L10I, M36I, M46I, I54V, K55R, I62V, L63P, A71V, G73S, V82T, L90M, I93L | 2.5 ± 0.26 | 9 |
Data shown represent mean values ± 1 standard deviation derived from the results of three independent experiments conducted in triplicate. The IC50s were determined by employing MT-4 cells exposed to each infectious HIV-1 clone (50 TCID50s) in the presence of each PI and using the inhibition of p24 Gag protein production as the endpoint.
When HIVB was propagated in the presence of increasing concentrations of TPV, all the clones (20/20) derived from the virus at passage 10 (HIVBP10) had I54V and V82T substitutions, both of which were also seen in all clones (20/20) derived from HIVCP15 and HIVTMP15 (Fig. 3B). It is of note that HIVBP10 and HIVCP15 were propagating in the presence of ∼15 μM TPV, while HIVTMP15 was barely propagating in the presence of 3 μM TPV. We therefore examined the impact of both I54V and V82T substitutions on the susceptibility of HIVB to TPV by introducing I54V alone and I54V/V82T into HIVB, generating cHIVBI54V and cHIVBI54V/V82T, respectively. cHIVBI54V and cHIVBI54V/V82T were significantly resistant to TPV, with IC50s of 2.9 and 3.2 μM, respectively, which were 11- and 12-fold increases in comparison to the IC50 against cHIVB, respectively (Table 3). Prior to the TPV selection, HIVB had contained the L33I substitution (see Fig. S1 in the supplemental material) and the HIV11MIXP0 population had the L33I substitution in 3 of 20 clones; however, L33I became predominant by passage 5 (20 of 20 clones; Fig. 3A). Thus, as mentioned above, we reverted L33I to the wild-type amino acid (Leu-33) in cHIVB, cHIVBI54V, and cHIVBI54V/V82T, generating cHIVBI33L, cHIVBI33L/I54V, and cHIVBI33L/I54V/V82T, respectively, and determined their susceptibility to TPV. The susceptibility of cHIVBI33L to TPV was similar to that of cHIVNL4-3; however, both cHIVBI33L/I54V and cHIVBI33L/I54V/V82T were less resistant to TPV (with fold differences in IC50s of 4 and 9, respectively) than their counterparts, cHIVBI54V and cHIVBI54V/V82T, respectively (which had fold differences of 11 and 12, respectively) (Table 3). These data suggest that (i) the L33I substitution alone is detrimental to the viral fitness of cHIVNL4-3, (ii) the L33I substitution renders cHIVNL4-3 resistant to TPV inhibition of protease dimerization, (iii) the L33I substitution alone does not significantly change the antiviral susceptibility of cHIVB to TPV, and (iv) with the genetic background of cHIVB, L33I in the presence of I54V and I54V/V82T contributes to the acquisition of relatively greater resistance to TPV, probably through compromising TPV's dimerization inhibition activity.
Impact of L24I, L24M, E35D, V82T, and I84V substitutions on the susceptibility of HIVNL4-3 and HIVB to TPV's antiviral activity.
Upon the selection of HIVC with TPV, by passage 15, L24I had been converted to L24M (20 of 20 clones) and E35D (15 of 20 clones), V82T (20 of 20), and I84V (20 of 20) had newly emerged (Fig. 3B). Thus, we assessed the effects of such amino acid substitutions on the susceptibility of HIVC to TPV's antiviral activity. Interestingly, the introduction of L24I and L24M substitutions rendered cHIVNL4-3 (cHIVNL4-3L24I and cHIVNL4-3L24M, respectively) more susceptible to TPV, with IC50s of 0.02 and 0.029 μM, respectively, compared with an IC50 of 0.27 μM against cHIVNL4-3 (Table 4). However, when L24I and L24M were present with the genetic background of HIVC, the IC50s of TPV were 0.24 μM (0.9-fold difference) and 0.6 μM (2.2-fold difference), respectively, suggesting that L24M is associated with the moderate resistance of HIVC to TPV. The introduction of V82T into HIVC made the virus slightly resistant to TPV, with an IC50 of 0.38 μM (1.4-fold difference). However, HIVC with the combination of three amino acid substitutions, cHIVCL24M/E35D/V82T, was significantly more resistant to TPV. HIVC with four substitutions, cHIVCL24M/E35D/V82T/I84V, was further more resistant to the drug, with an IC50 of 2.25 μM (8.3-fold difference) (Table 4). These data suggest that all five amino acid substitutions, L24I, L24M, E35D, V82T, and I84V, contribute to the decreased susceptibility of HIVC, in particular, when combined.
TABLE 4.
Effects of L24I, L24M, E35D, V82T, and I84V substitutions on the susceptibility of cHIVNL4-3 and cHIVC to TPVa
| Infectious clone | Amino acid substitution(s) in PR | Mean ± SD IC50 (μM) | Fold resistance |
|---|---|---|---|
| cHIVNL4-3 | Wild type | 0.27 ± 0.003 | 1 |
| cHIVNL4-3L241 | L24I | 0.02 ± 0.003 | 0.07 |
| cHIVNL4-3L24M | L24M | 0.029 ± 0.003 | 0.1 |
| cHIVC | L10I, I15V, K20R, L24I, M36I, M46L, I54V, I62V, L63P, K70Q, V82A, L89M | 0.24 ± 0.06 | 0.9 |
| cHIVCL24M | L10I, I15V, K20R, L24M, M36I, M46L, I54V, I62V, L63P, K70Q, V82A, L89M | 0.6 ± 0.02 | 2.2 |
| cHIVCV82T | L10I, I15V, K20R, L24I, M36I, M46L, I54V, I62V, L63P, K70Q, V82T, L89M | 0.38 ± 0.01 | 1.4 |
| cHIVCL24M/E35D/V82T | L10I, I15V, K20R, L24M, E35D, M36I, M46L, I54V, I62V, L63P, K70Q, V82T, L89M | 1.95 ± 0.04 | 7.2 |
| cHIVCL24M/E35D/V82T/I84V | L10I, I15V, K20R, L24M, E35D, M36I, M46L, I54V, I62V, L63P, K70Q, V82T, I84V, L89M | 2.25 ± 0.13 | 8.3 |
Antiviral data shown represent mean values ± 1 standard deviation derived from the results of three independent experiments conducted in triplicate. The IC50s were determined by employing MT-4 cells exposed to each infectious HIV-1 clone (50 TCID50s) in the presence of each PI and using the inhibition of p24 Gag protein production as the endpoint.
The presence of I54V/V82T expedites HIVNL4-3 acquisition of TPV resistance compared to that of L33F.
We further examined how the presence of L33F or I54V/V82T affected the acquisition of cHIVNL4-3 resistance to TPV by propagating cHIVNL4-3L33F or cHIVNL4-3I54V/V82T in the presence of increasing concentrations of TPV. As shown in Fig. 5, cHIVNL4-3I54V/V82T, followed by cHIVNL4-3L33F, readily started replicating robustly in the presence of up to 5 μM TPV. cHIVNL4-3 was most delayed in its acquisition of replicative ability in the presence of TPV. These data suggest that I54V/V82T substitutions are associated with TPV resistance with the genetic background of cHIVNL4-3 and prompt HIV-1's development of TPV resistance, while these two substitutions do not affect the susceptibility of protease to TPV's dimerization inhibition activity with the genetic background of cHIVNL4-3 (Fig. 4B).
Fig 5.
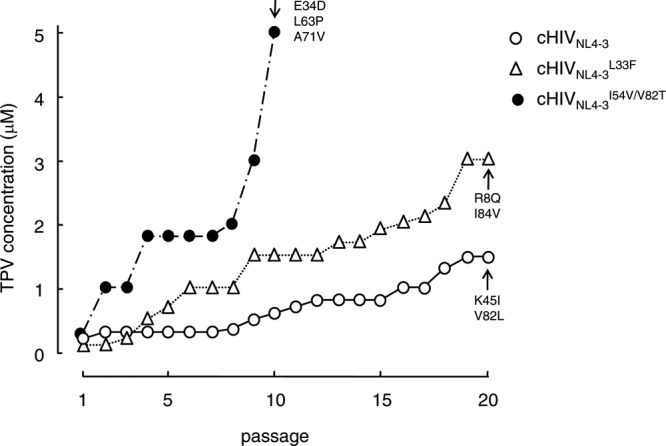
In vitro selection of TPV-resistant variants using HIV-1 carrying L33F or I54V/V82T. cHIVNL4-3 (○), cHIVNL4-3L33F (△), and cHIVNL4-3I54V/V82T (●) were propagated in the presence of increasing concentrations of TPV (starting at 0.3 μM) in MT-4 cells. The selection was carried out in a cell-free manner for a total of 10 or 20 passages. Amino acid substitutions identified in the protease of each HIV-1 strain at the conclusion of each passage of the selection are shown with arrows.
Determination of the amino acid sequences of cHIVNL4-3I54V/V82T at passage 10 revealed that the virus population had additionally acquired E34D, L63P, and A71V, while cHIVNL4-3L33F had additionally acquired R8Q and E35G (Fig. 5).
E34D renders HIVNL4-3 protease resistant to TPV's dimerization inhibition, while other substitutions (L63P, A71V, K45I, or V82L) do not.
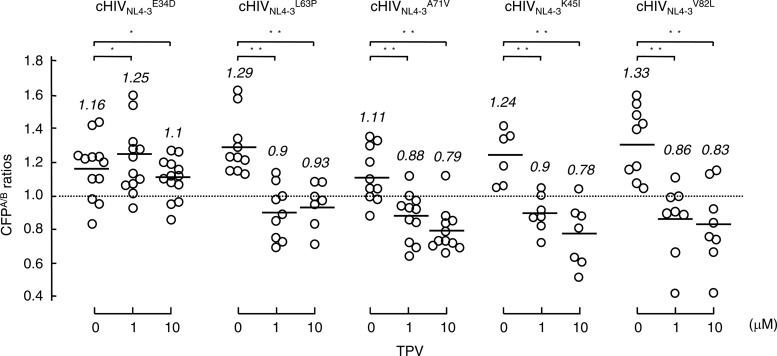
We finally examined whether each of the amino acid substitutions that emerged during the selection of cHIVNL4-3 with TPV altered the susceptibility of cHIVNL4-3 protease to TPV's dimerization inhibition (Fig. 6). As examined in the FRET-based HIV-1 expression assay, E34D rendered the cHIVNL4-3 protease resistant to TPV's dimerization inhibition, while none of the four other substitutions (L63P, A71V, K45I, or V82L) changed the susceptibility of cHIVNL4-3 protease to TPV's dimerization inhibition. These data suggest that certain amino acid substitutions that emerge under selection with TPV are associated with the loss of TPV's dimerization inhibition activity, although other substitutions do not affect the susceptibility of protease to TPV's dimerization inhibition activity but contribute to the acquisition of HIV-1's TPV resistance. Hence, there are two distinct types of amino acid substitutions contributing to the acquisition of HIV-1's TPV resistance with the genetic background of cHIVNL4-3: (i) amino acid substitutions conferring the loss of TPV's dimerization inhibition on protease and (ii) those contributing to the acquisition of HIV resistance to TPV without affecting its susceptibility to TPV's dimerization inhibition activity.
Fig 6.
Impact of amino acid substitutions that appeared in TPV selection on TPV's dimerization inhibition. COS7 cells were cotransfected with a pair of clones, HIVNL4-3CFP and pHIVNL4-3YFP, carrying E34D (cHIVNL4-3E34D), L63P (cHIVNL4-3L63P), A71V (cHIVNL4-3A71V), K45I (cHIVNL4-3K45I), or V82L (cHIVNL4-3V82L). COS7 cells were further cultured in the continuous presence of 1 or 10 μM TPV, and CFPA/B ratios were determined at the conclusion of the 3-day period of culture. *, not significant; **, P < 0.05.
DISCUSSION
TPV is a Food and Drug Administration (FDA)-approved, nonpeptidic PI (34). Doyon and her colleagues have reported that the development of HIV-1 resistance to TPV is slow and requires 9 months of sequential passage as well as multiple amino acid substitutions in culture (9). Indeed, when cHIVNL4-3 was used as a starting HIV-1 strain in the present study, cHIVNL4-3 failed to start replicating in the presence of TPV (Fig. 2), which is in line with the data presented by Doyon et al. (9). However, as shown in Fig. 2, three HIV-1 populations (HIV11MIX, HIVB, and HIVC) readily started propagating in the presence of increasing concentrations of TPV. This observation confirms that the use of a mixture of multiple-drug-resistant clinical strains and certain highly multi-PI-resistant clinical strains makes it possible to substantially more readily obtain otherwise hard-to-select drug-resistant HIV-1 variants, as previously described (19). Of note, all the clinical strains employed as a source of the mixed HIV-1 population in the present study were as sensitive to TPV as the wild-type clinical HIV-1 isolate (HIV104pre) (Table 1), although they were moderately to highly resistant to 5 other approved PIs, including SQV, IDV, APV, LPV, and ATV (Table 1). However, the TPV-selected HIV-1 variants became highly resistant to TPV and were much more resistant to all 5 PIs without specific resistance mutations to each PI, as previously reported (9). It is noteworthy that HIV11MIXP10 remained relatively susceptible to DRV with an IC50 of 0.034 μM (Table 2).
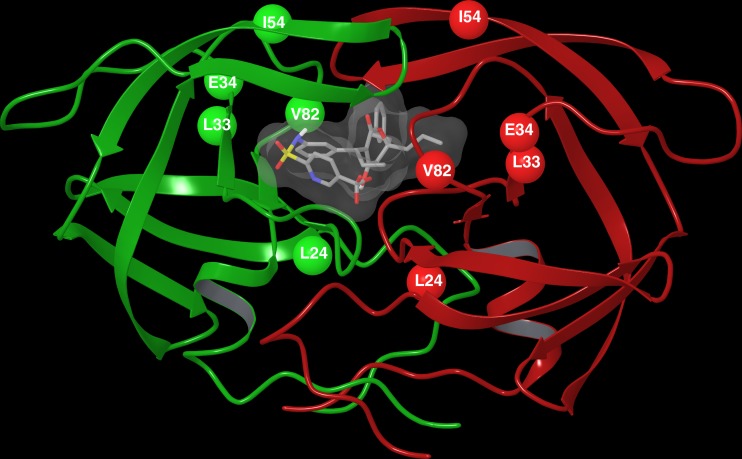
In HIV11MIXP10 and 3 of the 4 TPV-selected clinical strains (HIVBP10, HIVCP15, and HIVTMP15), two amino acid substitutions, I54V and V82T, which reportedly contribute to decreased TPV susceptibility (33) were identified to be major TPV resistance-associated amino acid substitutions (Fig. 3A and B). Since HIVB readily started propagating under TPV selection (Fig. 2) and acquired V82T by passage 10 (HIVBP10), we also examined the impact of I54V and I54V/V82T upon TPV's protease dimerization inhibition activity with the genetic background of cHIVB (Fig. 4B). Neither cHIVBI54V nor cHIVBI54V/V82T had any further impact on TPV's dimerization inhibition activity (Fig. 4B). Nevertheless, the susceptibility of cHIVBI54V and cHIVBI54V/V82T was significantly decreased (Table 3), even though the I54V and V82T mutations did not confer resistance to TPV in HIVNL4-3 (Table 3). These findings are perhaps in line with multiple reports that single amino acid substitutions in HIV-1 protease do not significantly change viral sensitivity to PIs (16). It is presumed that these two amino acid substitutions ultimately affect HIV-1's susceptibility to TPV only when they are present with other subsequently acquired amino acid substitutions. Figure 7 shows the mature dimerized HIV-1 protease in complex with TPV and the locations of the amino acid substitutions (L24M, L33I/F, E34D, I54V, and V82T) associated with HIV TPV resistance.
Fig 7.
The mature dimerized HIV-1 protease in complex with TPV. The locations of amino acid substitutions L24M, L33I/F, E34D, I54V, and V82T associated with HIV TPV resistance are shown.
Rhee et al. reported that the F53L mutation is slightly related to HIV's increased susceptibility to TPV (33). In the present study, HIVEV contained F53L (see Fig. S1 in the supplemental material) and had increased sensitivity to TPV (IC50, 0.066 μM) compared to the wild-type HIV (HIV104pre; IC50, 0.16 μM). Most of HIVBP0 clones (16/20) contained F53L but had a sensitivity (IC50, 0.18 μM) comparable to that of HIV104pre; however, by passage 10 under TPV selection, all the clones of HIVBP10 had lost F53L (Fig. 3B). These data suggest that F53L contributed to the increased sensitivity to TPV (in the case of HIVEV) and sensitivity comparable to that of HIV104pre (in the case of HIVB).
HIVC, which initially contained L24I, acquired L24M by passage 15 under TPV selection (Fig. 3B), and the L24M mutation conferred resistance to TPV on HIVC (IC50s of cHIVC and cHIVCL24M, 0.9 and 2.2 μM, respectively; Table 4). Moreover, TPV lost its protease dimerization inhibition activity when the L24M mutation was introduced into HIVNL4-3 (cHIVNL4-3L24M) (Fig. 4C). Nevertheless, the same cHIVNL4-3L24M clone had an increased sensitivity to TPV (0.1-fold difference), as shown in Table 4. It is possible that the TPV resistance may not always be caused by the PR mutations that interfere with TPV's inhibition of dimerization and that the genetic barrier to TPV resistance via the loss of dimerization inhibition could be much lower than that for DRV. However, it is also possible that the loss of TPV's dimerization inhibition activity associated with L24M acquisition might have offset an otherwise highly increased susceptibility of HIV-1 to TPV caused by the same effect of L24M on the catalytic activity of protease. In other words, if L24M does not confer TPV resistance by reducing TPV's dimerization inhibition activity, HIVNL4-3L24M could be extremely more susceptible to TPV. Thus, while L24M contributes to both the acquisition of TPV resistance of HIVC and the loss of dimerization inhibition activity of TPV, the effects of L24M apparently depend on the genetic background of the HIV-1 species. The present data suggest that the conformation of the active site in the proximity of amino acid position 24 is altered by additional amino acid substitutions, although such conformational changes remain to be elucidated.
The L33I substitution seen in HIV11MIXP5, HIV11MIXP10, HIVBP0, and HIVBP10 compromised the activity of TPV to block protease dimerization with the genetic background of cHIVNL4-3 (Fig. 4C). Nevertheless, cHIVB that contained L33I was as susceptible to TPV's activity against replication as cHIVNL4-3 (Table 3). Moreover, neither L33I in cHIVNL4-3L33I/M36I nor L33F in cHIVNL4-3L33F caused significant changes in the susceptibility of cHIVNL4-3 to TPV's antiviral activity (Table 3). Therefore, in order to further examine the effect of L33I in the genetic background of cHIVB, L33I was reverted to the wild-type amino acid, Ile-33, generating cHIVBI33L, which was also as susceptible to TPV's anti-HIV activity as cHIVNL4-3. However, when L33I was reverted to Ile-33 in cHIVBI54V and cHIVBI54V/V82T, generating cHIVBI33L/I54V and cHIVBI33L/I54V/V82T, respectively, they proved to be less resistant to TPV's anti-HIV activity (Table 3). These data suggest that although L33I is not significantly associated with the acquisition of TPV resistance in cHIVB, L33I does increase the level of resistance of cHIVBI54V and cHIVBI54V/V82T to TPV. The reason why the conversion of Ile-33 to Leu-33 did not alter the IC50 between cHIVB and cHIVBI33L (Table 3) could be that the p24 Gag protein production system was not sensitive enough to detect the potential difference in the IC50s. It is also possible that the activity of TPV to block protease dimerization may not significantly contribute to the overall anti-HIV activity of TPV.
In the present study, when L33I was introduced into cHIVNL4-3 (generating cHIVNL4-3L33I), cHIVNL4-3L33I had no replicative activity, while further addition of M36I (generating cHIVNL4-3L33I/M36I) restored the replicative activity (Table 3). In this regard, we have previously reported that HIV-1 can acquire substantial drug resistance with initial amino acid substitutions, sacrificing some replication ability; however, the same virus population subsequently acquires additional substitutions and becomes optimally replication competent (26). On the other hand, the introduction of L24I and L24M into cHIVNL4-3 (generating cHIVNL4-3L24I and cHIVNL4-3L24M) rendered cHIVNL4-3 more susceptible to TPV, with the IC50 difference being 13.5- and 9.3-fold, respectively, relative to the IC50 of cHIVNL4-3 (Table 4). Such a case has also been well-known. For example, the M184V substitution alone renders HIV-1 highly resistant to lamivudine, but on the contrary, M184V makes HIV-1 highly susceptible to zidovudine (25). It is presumed that such an amino acid substitution(s) alters viral enzyme structures critical for the interactions of the enzyme with substrates and drugs; however, the mechanisms by which such profound changes in HIV replicability and drug sensitivity occur largely remain to be elucidated.
Since the introduction of L33I completely abrogated the replication of HIVNL4-3 (Table 3), we introduced L33F (generating cHIVNL4-3L33F), which has been identified in HIV-1 clinically exposed to TPV (24, 28). In cHIVNL4-3L33F, TPV failed to block protease dimerization (Fig. 4C); however, cHIVNL4-3L33F was as TPV sensitive as cHIVNL4-3 (Table 3). Thus, we presumed that the loss of dimerization inhibition by TPV did not significantly contribute to cHIVNL4-3L33F's overall sensitivity to TPV. It is possible that in cHIVNL4-3L33F, L33F confers an increased sensitivity to TPV, overriding the loss of TPV's dimerization inhibition and rendering cHIVNL4-3L33F as sensitive to TPV as wild-type cHIVNL4-3. Therefore, we finally propagated three recombinant clones, cHIVNL4-3, cHIVNL4-3L33F, and cHIVNL4-3I54V/V82T, in the presence of increasing concentrations of TPV, in an attempt to examine whether these substitutions help the virus rapidly acquire resistance to TPV. The acquisition of TPV resistance of cHIVNL4-3L33F was fairly delayed compared to that of cHIVNL4-3I54V/V82T, followed by cHIVNL4-3, suggesting that the presence of I54V/V82T not only renders certain HIV-1 strains (such as HIVB) resistant to TPV but also predisposes cHIVNL4-3 to more readily develop resistance to TPV. The determination of nucleic acid sequences revealed that at passage 10 cHIVNL4-3I54V/V82T had acquired three new substitutions, E34D, L63P, and A71V. At passage 20, cHIVNL4-3 had acquired two relatively unique amino acid substitutions, K45I and V82L. Thus, we again examined whether these five substitutions caused any changes in the ability of TPV to block protease dimerization. The FRET-based HIV-1 expression assay using newly generated recombinant protease with each of the substitutions showed that only E34D compromised TPV's protease dimerization inhibition activity. These data again confirmed our present interpretation, in that although the loss of TPV's protease dimerization inhibition due to the E34D substitution is involved in the acquisition of TPV resistance of HIV, other amino acid substitutions (such as L63P, A71V, K45I, and V82L) are not related to the protease susceptibility of TPV's dimerization inhibition but are associated with the acquisition of viral resistance to TPV. In this regard, HIVA did not become predominant by passage 5 under TPV selection (Fig. 3A), although HIVA originally had both I54V and V82T before TPV selection (see Fig. S1 in the supplemental material), suggesting that the acquisition of high levels of resistance to TPV requires the acquisition of mutations associated with not only the blockage of replicative activity but also dimerization inhibition activity.
It is of note that the altered susceptibility of protease to TPV's dimerization inhibition activity differs substantially with the genetic background of HIV. The impact of amino acid substitutions in protease on TPV's protease dimerization inhibition activity and antiviral activity is summarized in Table 5.
TABLE 5.
Summary of impacts of amino acid substitutions in protease on TPV's protease dimerization inhibition and anti-HIV activity
| Genetic background | Reduction of TPV's activity to inhibit | Amino acid substitution in PR |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L24I | L24 M | L33I | L33F | E34D | K45I | I54V | L63P | A71V | V82L | V82T | I54V/V82T | ||
| cHIVNL4-3 | Protease dimerization | − | + | + | + | + | − | − | − | − | − | − | − |
| HIV replication | Ea | E | − | − | NDb | ND | − | ND | ND | − | − | − | |
| cHIVB | Protease dimerization | ND | ND | + | ND | ND | ND | − | ND | ND | ND | − | − |
| HIV replication | ND | ND | +c | ND | ND | ND | + | ND | ND | ND | + | + | |
| cHIVC | Protease dimerization | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| HIV replication | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | + | ND | |
E, TPV's activity to inhibit HIV replication was enhanced with certain genetic backgrounds.
ND, not determined.
When the L33I mutation exists with the genetic background of cHIVB carrying I54V or I54V/V82T, L33I reduces the antiviral activity of TPV.
Importantly, TPV's activity to block protease dimerization is compromised mostly with a single amino acid substitution, while the loss of DRV's ability to block protease dimerization requires as many as 4 amino acid substitutions (20). In addition, the activity of TPV to block protease dimerization per se is significantly less potent than that of DRV. These data together should explain why the genetic barrier of TPV against viral resistance is substantially lower than that of DRV. The present data on TPV as well as thus far published data on DRV (20, 21) demonstrate that the inhibition of protease dimerization contributes to the overall anti-HIV-1 activity of TPV and DRV, and it is hoped that agents with further potent activity to block protease dimerization can be developed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by a grant for global education and a research center aiming at the control of AIDS (Global Center of Excellence supported by Monbu-Kagakusho), a grant for the promotion of AIDS research from the Ministry of Health, Welfare, and Labor of Japan, a grant to the Cooperative Research Project on Clinical and Epidemiological Studies of Emerging and Re-Emerging Infectious Diseases (Renkei Jigyo no. 78, Kumamoto University) of Monbu-Kagakusho and a Grant-in-Aid for Young Scientists (B) (grant 21790527) of Monbu-Kagakusho and in part by the Intramural Research Program of Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Published ahead of print 26 September 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Amano M, et al. 2007. A novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI), GRL-98065, is potent against multiple-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 51:2143–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoki M, et al. 2009. Non-cleavage site gag mutations in amprenavir-resistant human immunodeficiency virus type 1 (HIV-1) predispose HIV-1 to rapid acquisition of amprenavir resistance but delay development of resistance to other protease inhibitors. J. Virol. 83:3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Back NK, et al. 2000. In-vitro tipranavir susceptibility of HIV-1 isolates with reduced susceptibility to other protease inhibitors. AIDS 14:101–102 [DOI] [PubMed] [Google Scholar]
- 4. Baxter JD, et al. 2006. Genotypic changes in human immunodeficiency virus type 1 protease associated with reduced susceptibility and virologic response to the protease inhibitor tipranavir. J. Virol. 80:10794–10801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper D, Zajdenverg R, Ruxrungtham K, Scherer J, Chaves R. 2006. Efficacy and safety of two doses of tipranavir/ritonavir versus lopinavir/ritonavir-based therapy in antiretroviral-naïve patients: results of BI 1182.33. Abstr. 8th Int. Cong. Drug Ther. HIV Infect., Glasgow, Scotland [Google Scholar]
- 6. De Meyer S, et al. 2005. TMC114, a novel human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob. Agents Chemother. 49:2314–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Meyer S, et al. 2008. Resistance profile of darunavir: combined 24-week results from the POWER trials. AIDS Res. Hum. Retroviruses 24:379–388 [DOI] [PubMed] [Google Scholar]
- 8. Dierynck I, et al. 2007. Binding kinetics of darunavir to human immunodeficiency virus type 1 protease explain the potent antiviral activity and high genetic barrier. J. Virol. 81:13845–13851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Doyon L, Tremblay S, Bourgon L, Wardrop E, Cordingley MG. 2005. Selection and characterization of HIV-1 showing reduced susceptibility to the non-peptidic protease inhibitor tipranavir. Antiviral Res. 68:27–35 [DOI] [PubMed] [Google Scholar]
- 10. Fang G, Weiser B, Visosky A, Moran T, Burger H. 1999. PCR-mediated recombination: a general method applied to construct chimeric infectious molecular clones of plasma-derived HIV-1 RNA. Nat. Med. 5:239–242 [DOI] [PubMed] [Google Scholar]
- 11. Gatanaga H, et al. 2002. Amino acid substitutions in Gag protein at non-cleavage sites are indispensable for the development of a high multitude of HIV-1 resistance against protease inhibitors. J. Biol. Chem. 277:5952–5961 [DOI] [PubMed] [Google Scholar]
- 12. Ghosh AK, et al. 1998. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg. Med. Chem. Lett. 8:687–690 [DOI] [PubMed] [Google Scholar]
- 13. Ghosh AK, et al. 1998. Structure based design: novel spirocyclic ethers as nonpeptidal P2-ligands for HIV protease inhibitors. Bioorg. Med. Chem. Lett. 8:979–982 [DOI] [PubMed] [Google Scholar]
- 14. Ghosh AK, Leshchenko S, Noetzel M. 2004. Stereoselective photochemical 1,3-dioxolane addition to 5-alkoxymethyl-2(5H)-furanone: synthesis of bis-tetrahydrofuranyl ligand for HIV protease inhibitor UIC-94017 (TMC-114). J. Org. Chem. 69:7822–7829 [DOI] [PubMed] [Google Scholar]
- 15. Harada S, Hazra R, Tamiya S, Zeichner SL, Mitsuya H. 2007. Emergence of human immunodeficiency virus type 1 variants containing the Q151M complex in children receiving long-term antiretroviral chemotherapy. Antiviral Res. 75:159–166 [DOI] [PubMed] [Google Scholar]
- 16. Henderson GJ, et al. 2012. Interplay between single resistance-associated mutations in the HIV-1 protease and viral infectivity, protease activity, and inhibitor sensitivity. Antimicrob. Agents Chemother. 56:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsieh SM, et al. 2011. Emerging HIV-1 resistance to tipranavir and darunavir in patients with virological failure to first-generation protease inhibitors in Taiwan. Int. J. STD AIDS 22:617–620 [DOI] [PubMed] [Google Scholar]
- 18. Ide K, et al. 2011. Novel HIV-1 protease inhibitors (PIs) containing a bicyclic P2 functional moiety, tetrahydropyrano-tetrahydrofuran, that are potent against multi-PI-resistant HIV-1 variants. Antimicrob. Agents Chemother. 55:1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Koh Y, et al. 2010. In vitro selection of highly darunavir-resistant and replication-competent HIV-1 variants by using a mixture of clinical HIV-1 isolates resistant to multiple conventional protease inhibitors. J. Virol. 84:11961–11969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koh Y, et al. 2011. Loss of protease dimerization inhibition activity of darunavir is associated with the acquisition of resistance to darunavir by HIV-1. J. Virol. 85:10079–10089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koh Y, et al. 2007. Potent inhibition of HIV-1 replication by novel non-peptidyl small molecule inhibitors of protease dimerization. J. Biol. Chem. 282:28709–28720 [DOI] [PubMed] [Google Scholar]
- 22. Koh Y, et al. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 47:3123–3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohl NE, et al. 1988. Active human immunodeficiency virus protease is required for viral infectivity. Proc. Natl. Acad. Sci. U. S. A. 85:4686–4690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larder BA, et al. 2000. Tipranavir inhibits broadly protease inhibitor-resistant HIV-1 clinical samples. AIDS 14:1943–1948 [DOI] [PubMed] [Google Scholar]
- 25. Larder BA, Kemp SD, Harrigan PR. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696–699 [DOI] [PubMed] [Google Scholar]
- 26. Maeda Y, Venzon DJ, Mitsuya H. 1998. Altered drug sensitivity, fitness, and evolution of human immunodeficiency virus type 1 with pol gene mutations conferring multi-dideoxynucleoside resistance. J. Infect. Dis. 177:1207–1213 [DOI] [PubMed] [Google Scholar]
- 27. Mitsuya Y, Liu TF, Rhee SY, Fessel WJ, Shafer RW. 2007. Prevalence of darunavir resistance-associated mutations: patterns of occurrence and association with past treatment. J. Infect. Dis. 196:1177–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naeger LK, Struble KA. 2007. Food and Drug Administration analysis of tipranavir clinical resistance in HIV-1-infected treatment-experienced patients. AIDS 21:179–185 [DOI] [PubMed] [Google Scholar]
- 29. Panel on Antiretroviral Guidelines for Adults and Adolescents 2012. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf Accessed 13 August 2012 [Google Scholar]
- 30. Poppe SM, et al. 1997. Antiviral activity of the dihydropyrone PNU-140690, a new nonpeptidic human immunodeficiency virus protease inhibitor. Antimicrob. Agents Chemother. 41:1058–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poveda E, et al. 2010. Drug resistance mutations in HIV-infected patients in the Spanish drug resistance database failing tipranavir and darunavir therapy. Antimicrob. Agents Chemother. 54:3018–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poveda E, et al. 2006. Successful rescue therapy with darunabir (TMC114) in HIV-infected patients who have failed several ritonavir-boosted protease inhibitors. AIDS 20:1558–1560 [DOI] [PubMed] [Google Scholar]
- 33. Rhee SY, et al. 2010. HIV-1 protease mutations and protease inhibitor cross-resistance. Antimicrob. Agents Chemother. 54:4253–4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schapiro JM, et al. 2010. Improving the prediction of virological response to tipranavir: the development and validation of a tipranavir-weighted mutation score. Antivir. Ther. 15:1011–1019 [DOI] [PubMed] [Google Scholar]
- 35. Shirasaka T, et al. 1993. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc. Natl. Acad. Sci. U. S. A. 90:562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamiya S, Mardy S, Kavlick MF, Yoshimura K, Mistuya H. 2004. Amino acid insertions near Gag cleavage sites restore the otherwise compromised replication of human immunodeficiency virus type 1 variants resistant to protease inhibitors. J. Virol. 78:12030–12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Temesgen Z, Feinberg J. 2007. Tipranavir: a new option for the treatment of drug-resistant HIV infection. Clin. Infect. Dis. 45:761–769 [DOI] [PubMed] [Google Scholar]
- 38. Van Marck H, et al. 2009. The impact of individual human immunodeficiency virus type 1 protease mutations on drug susceptibility is highly influenced by complex interactions with the background protease sequence. J. Virol. 83:9512–9520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wlodawer A, et al. 1989. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science 245:616–621 [DOI] [PubMed] [Google Scholar]
- 40. Yoshimura K, et al. 2002. A potent human immunodeficiency virus type 1 protease inhibitor, UIC-94003 (TMC-126), and selection of a novel (A28S) mutation in the protease active site. J. Virol. 76:1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yoshimura K, et al. 1999. JE-2147: a dipeptide protease inhibitor (PI) that potently inhibits multi-PI-resistant HIV-1. Proc. Natl. Acad. Sci. U. S. A. 96:8675–8680 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.