Abstract
The specific cell pathways involved in bovine ephemeral fever virus (BEFV) cell entry have not been determined. In this work, colocalization of the M protein of BEFV with clathrin or dynamin 2 was observed under a fluorescence microscope. To better understand BEFV entry, we carried out internalization studies with a fluorescently labeled BEFV by using a lipophilic dye, 3,30-dilinoleyloxacarbocyanine perchlorate (DiO), further suggesting that BEFV uses a clathrin-mediated endocytosis pathway. Our results suggest that clathrin-mediated and dynamin 2-dependent endocytosis is an important avenue of BEFV entry. Suppression of Rab5 or Rab7a through the use of a Rab5 dominant negative mutant and Rab7a short hairpin RNA (shRNA) demonstrated that BEFV requires both early and late endosomes for endocytosis and subsequent infection in MDBK and Vero cells. Treatment of BEFV-infected cells with nocodazole significantly decreased the M protein synthesis and viral yield, indicating that microtubules play an important role in BEFV productive infection, likely by mediating trafficking of BEFV-containing endosomes. Furthermore, BEFV infection was strongly blocked by different inhibitors of endosomal acidification, suggesting that virus enters host cells by clathrin-mediated and dynamin 2-dependent endocytosis in a pH-dependent manner.
INTRODUCTION
Endocytosis is required for a number of cellular functions that include nutrient uptake, drug delivery, cell adhesion and migration, membrane receptor recycling and downregulation, pathogen entry, neurotransmission, antigen presentation, cell polarity, mitosis, cell growth, and cell differentiation. A number of viruses have evolved to exploit endocytosis to enter host cells after initial attachment of virions to a receptor(s) on the cell membrane. After attachment to the respective cell surface receptor(s), internalization usually follows directly from the plasma membrane or via endocytosis. To date, a number of different routes of endocytosis exploited by viruses have been demonstrated. The well-understood endocytic pathways include clathrin-dependent and lipid raft/caveola-dependent entry pathways. Clathrin-mediated endocytosis is a major endocytic pathway from the plasma membrane to early endosomes. Clathrin is made up of light chain and heavy chain and forms a unique structure called clathrin triskelion (19). In clathrin-mediated endocytosis, clathrin is assembled on the inside face of the plasma membrane to form a characteristic coated pit (CCP) and in response to receptor-mediated internalization signals. Once assembled, CCPs pinch off from the cell membrane and mature into clathrin-coated vesicles (19), which then deliver the cargo into endosomes. Dynamin is required for clathrin- or caveola-mediated endocytosis and phagocytosis, but it is not necessary for macropinocytosis (13, 16). A number of viruses have been proved to enter host cells via the clathrin-dependent endocytosis pathway (12, 33, 36). Many other viruses have also been reported to enter host cells through the caveola-dependent pathway (3, 17).
Bovine ephemeral fever virus (BEFV) is an economically important pathogen of cattle and water buffalo. BEFV is an arthropod-borne rhabdovirus which has been classified in the genus Ephemerovirus. Bovine ephemeral fever (BEF) is characterized clinically by sudden onset of fever, stiffness, depression, serous oral and nasal discharges, joint pain, and lameness (14, 29, 41). BEFV virions are bullet or cone shaped (28) and are composed of a single-stranded, negative-sense RNA genome with a lipid envelope and five structural proteins. The five structural proteins include the large RNA-dependent RNA polymerase (L), the polymerase-associated protein (P), the envelope glycoprotein (G), the nucleoprotein (N), and the matrix protein (M) (8, 39). As is the case for several enveloped RNA viruses, the M protein of rhabdoviruses is critical for virus assembly and budding. In the absence of other viral proteins, M protein alone could bud from the cell surface in the form of lipid-enveloped and virus-like particles (VLPs).
Despite its economic importance, little is known of the pathogenesis of BEFV. To extend our understanding of the pathways required for productive BEFV infection and to facilitate new strategies for combating BEFV-caused diseases, earlier studies from our team have demonstrated that signaling pathways triggered by BEFV promote apoptosis and virus replication (5, 18, 24). However, the mechanism for cellular entry of BEFV remains unclear. The present study was aimed at elucidating the mechanism by which BEFV gains access into host cells. For this work, we have undertaken a detailed investigation of BEFV entry. Using a combination of pharmacological, biochemical, and genetic approaches, we have found that BEFV enters cells by clathrin-mediated and dynamin 2-dependent endocytosis in a pH-dependent manner that requires small GTPases Rab5 and Rab7 as well as microtubules for entry and subsequent infection in host cells.
MATERIALS AND METHODS
Cells and viruses.
Two cell lines, Madin-Darby bovine kidney (MDBK) cells (Sigma-Aldrich Co., St. Louis, MO) and African green monkey kidney (Vero) cells (Sigma-Aldrich Co.), used in this study were maintained in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 10 mM HEPES (pH 7.2). Cells (1 × 106) were seeded in 6-cm cell culture dishes 1 day before each experiment in a 37°C incubator with 5% CO2. All experimental procedures were started in serum-free medium for 2 h, and then the medium was refreshed with medium containing 5% FBS overnight once cell confluence reached about 75%. The 2004/TW/TN1 strain of BEFV, isolated in Taiwan, was propagated in MDBK cells. At about 70 to 80% cytopathic effect (CPE), the supernatants of BEFV-infected cells were harvested and stored at −70°C for further studies.
Antibodies.
A monoclonal antibody against the M protein of BEFV was kindly provided by M. H. Liao, National Pingtung University of Science and Technology, Taiwan. Rabbit anti-dynamin 2 polyclonal antibody was from Abcam Co. (Cambridge, MA). Rabbit anti-clathrin heavy chain (CHC) polyclonal antibody was purchased from Cell Signaling Technology (Danvers, MA). Mouse anti-β-actin antibody was from Millipore (Billerica, MA).
Chemical inhibitors and reagents.
The mechanism of BEFV entry was investigated by using different inhibitors of endocytosis. Chlorpromazine (CPZ), methyl-β-cyclodextrin (MβCD), nystatin, Dynasore, ammonium chloride (NH4Cl), and nocodazole were purchased from Sigma-Aldrich Co. Bafilomycin A1 was purchased from Calbiochem Co. (San Diego, CA). Cytochalasin D was from MG Scientific Inc. (San Diego, CA). Except for MβCD, all inhibitors or reagents were present during the whole infection period. The cell lysates and supernatants of BEFV-infected cells were collected at 24 h postinfection (hpi) for Western blotting and viral titration, respectively. Rhodamine phalloidin for β-actin staining was purchased from Molecular Probes (Eugene, OR).
Transferrin uptake.
To confirm that we had a functional block in clathrin-mediated endocytosis, we first investigated the cellular uptake of transferrin, which is generally used as a marker for clathrin-mediated endocytosis. MDBK cells were starved for 2 h in serum-free medium and then were treated with CPZ (5 μM), sucrose (100 mM), and dimethyl sulfoxide (DMSO), respectively. Alexa 568-labeled transferrin (10 μg/ml) was added for 20 min at 4°C for binding and then transferred to 37°C for 15 min to allow internalization. Treated cells were acid washed (0.1 M glycine and 0.1 M NaCl, pH 3.0) to remove uninternalized ligands. Images were collected with a Zeiss LSM 510 Meta confocal microscope with the pinhole set to achieve 1 airy unit. Transferrin uptake control assays were carried out using Alexa 568-labeled human transferrin (Molecular Probes).
Colocalization of BEFV with clathrin and dynamin 2 by immunostaining.
MDBK cells in 24-well culture plates were infected with BEFV at a multiplicity of infection (MOI) of 15 for 15 min. BEFV-infected cells were then fixed by methanol and stained with BEFV M, clathrin, and dynamin 2 antibodies, respectively. Colocalization of BEFV M protein with clathrin or dynamin 2 was observed under a fluorescence microscope.
Preparation of BEFV labeled with a fluorescence dye, DiO.
A lipophilic dye, 3,30-dilinoleyloxacarbocyanine perchlorate (DiO; Molecular Probes), was used to label virus particles as described previously (37). All procedures for labeling of the virus were performed without light. The virus (106 focus-forming units [FFU]/ml) was incubated at room temperature for 10 min in serum-free medium (Invitrogen, Carlsbad, CA) containing 6.4 μM Fast DiO. The labeling solution contained 8% polyethylene glycol (PEG) 6000 and 2.2% NaCl at the final concentration. The solution was then kept at 4°C overnight. The labeled virus was sedimented at 4°C for 1 h and resuspended in phosphate-buffered saline (PBS). The labeled virus was stored at −80°C before use. To study whether CPZ affects virus internalization, MDBK cells were starved for 2 h in serum-free medium and then were treated with CPZ (5 μM) for 1 h. MDBK cells were then infected with DiO-labeled BEFV at an MOI of 2 for 15 min. Viral particles were observed with a fluorescence microscope.
Clathrin, dynamin 2, and Rab7a shRNA constructs.
In order to define the role of clathrin, dynamin 2, and Rab7a in the virus entry, MDBK or Vero cells at 75% confluence were transfected with clathrin heavy chain (CHC) gene-specific short hairpin RNA (shRNA) expression pGFP-V-RS vector (CLTC gene identifier [ID] 281080), dynamin 2 gene-specific shRNA expression pGFP-V-RS vector (dynamin 2 gene ID 511691), Rab7a gene-specific shRNA expression pGFP-V-RS vector (Rab7a gene ID 7879), and control shRNAs, respectively. All shRNAs and the scrambled negative shRNA (TR30013) from OriGene Co. (Rockville, MD) were constructed in the vector pGFP-V-RS (TR30007) plasmid. Each set of clathrin, dynamin 2, and Rab7a shRNAs containing four different shRNA sequences was tested in this study. Initial experiments revealed that the CHC shRNA construct GI355357 (GCTGCTTATCTCTTCAAAGGCAACAATCG), dynamin 2 shRNA construct GI353620 (CAGAAGACGCTGAATCAGCAACTGACCAA), and Rab7a shRNA construct GI339928 (GACTTTCTGACCAAGGAGGTGATGGTGGA) resulted in the most significant downregulation of clathrin, dynamin 2, and Rab7a expression, respectively. Hence, these shRNA constructs were chosen for our further studies. To transfect cells from each well on a 6-cm cell culture dish, 6 μl of TurboFect was mixed with 4 μg of plasmid in 400 μl serum-free MEM for 20 min at room temperature. After 20 min of incubation, the TurboFect-plasmid mixture was added to each well. Cells were infected with BEFV at an MOI of 2 at 24 h posttransfection. The cell lysates and supernatants of BEFV-infected cells were harvested after 24 h postinfection and processed for Western blotting and viral titration, respectively. The effect of clathrin, dynamin 2, and Rab7a gene silencing on BEFV entry was assessed by examination of the expression level of M protein and progeny virus titer of BEFV.
Effect of inhibitors and shRNAs on entry as a measure of viral transcription.
To ensure that inhibitors of viral entry or knockdown of clathrin or dynamin 2 will lead to blockage of transcription in addition to lowering the protein expression and virus progeny production, we used semiquantitative reverse transcription-PCR (RT-PCR) to compare the levels of the M-encoding mRNAs of BEFV in the presence or absence of CPZ, sucrose, Dynasore, or shRNA treatments. Briefly, MDBK cells were infected as described above and at 24 hpi were either mock treated or treated with CPZ, sucrose, Dynasore, and shRNAs. All cultures were harvested and lysed at 24 hpi, and total RNA was extracted and subjected to semiquantitative RT-PCR. ReverTra Ace reverse transcriptase (Toyobo Co., Japan) was used for reverse transcription. The primer sequences for semiquantitative RT-PCR were chosen based on the full-length M-encoding gene sequences of BEFV (GenBank accession number AF234533). Primers were as follows: forward primer, 5′-ATGGTTACCCTTTTCAAGAAAGG-3′ (positions 1 to 23), and antisense primer, 5′-TCATGACTTAACTAAGTTAGTGAAACCATG-3′ (positions 672 to 643). The expected size of the PCR product is 672 bp. The primer sequences for amplifying an internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene were as follows: forward primer, 5′-CAAGGTCATCCATGACAACTTTG-3′ (positions 477 to 499), and antisense primer, 5′-GTCCACCACCCTGTTGCTGTAG-3′ (positions 972 to 951). The expected size of the PCR product is 496 bp. The primers designed were based on published sequences (GenBank accession number NM-001034034). The amount of specific PCR product semiquantitatively reflected the level of mRNA. The PCR products were analyzed by agarose gel electrophoresis.
Virus titration.
The virus titer in the BEFV-infected cell supernatant collected at 24 hpi was determined by an agar overlay plaque assay performed in triplicate. Cells in 6-cm cell culture dishes were incubated for 1 h with diluted virus in 100 μl serum-free MEM. The cells were then washed twice with MEM to remove unabsorbed viruses and overlaid with 2 ml of 1% agarose in MEM containing 2% FBS and antibiotics. Plaques were examined after an incubation period of 2 to 3 days at 37°C by staining with neutral red for 3 h.
Overcoming bafilomycin A1-mediated entry block by low-pH pulse.
MDBK and Vero cells were pretreated with 25 nM bafilomycin A1 for 1 h and then incubated with BEFV at 4°C for 1 h. Following three rounds of washes with cold MEM, the virus-cell complexes either were directly exposed to a low-pH solution (MEM-10 mM HEPES adjusted with HCl at pHs ranging from 4.5 to 7.2) for 15 min or were preincubated at 37°C for 1 h in the presence of 25 nM bafilomycin A1 and then incubated in a low-pH solution for 15 min. The cells were washed two times with MEM, and the medium was replaced with fresh medium containing 25 nM bafilomycin A1. Viral infectivity was determined by immunoblotting at 24 h after the initiation of infection.
Vector encoding Rab5 DN mutant.
To elucidate the exact endosomal compartments traversed by endocytosed BEFV, we used the dominant negative (DN) mutants of Rab5 and Rab7a shRNA to assay the requirement of transport to early or late endosomes in BEFV entry. Transfections were performed as described above. The Rab5 DN mutants (S34N and Q79L) were kindly provided by Chi-Hung Lin, National Yang-Ming University, Taiwan. The expression level of M protein was analyzed by Western blotting. The progeny virus titer of BEFV was determined by plaque assay.
Electrophoresis and Western blot assay.
All the tested cells in a 6-cm dish were washed twice with phosphate-buffered saline (PBS) and lysed in 60 μl of 2.5× Laemmli loading buffer and then harvested by scraping and boiling for 10 min. Equal amounts of samples were separated by 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes. Expression levels of individual proteins were examined using the respective antibodies, followed by the respective secondary antibodies (goat anti-mouse or goat anti-rabbit immunoglobulin G conjugated with horseradish peroxidase [HRP]). After incubation with enhanced chemiluminescence (ECL Plus; Amersham Biosciences), the membranes were exposed to X-ray films (Kodak). The intensity of each protein was calculated using Photocapt (Vilber Lourmat).
Statistical analysis.
All data were analyzed using an independent sample t test and are expressed as averages of three independent experiments. P values of less than 0.05 were considered significant.
RESULTS
BEFV enters cells via clathrin-mediated endocytosis.
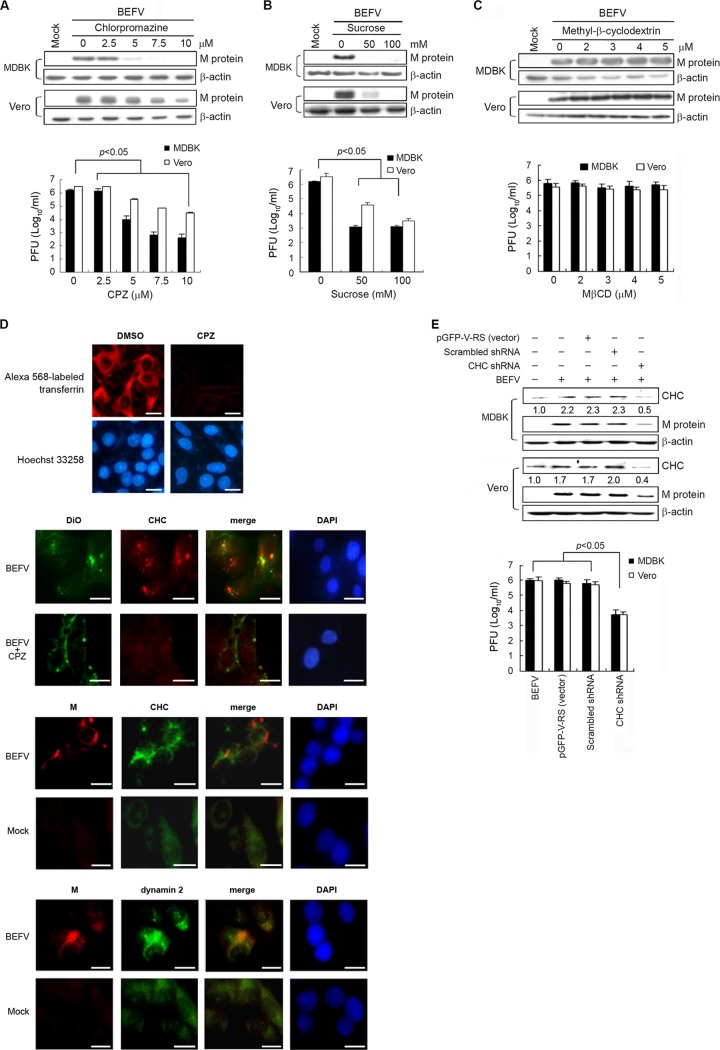
It has been well documented that cell entry of viruses via endocytosis can occur either depending on clathrin or independently of clathrin. CPZ was used to disrupt clathrin-mediated endocytosis (34). To determine whether the endocytic pathway used by BEFV involves clathrin, MDBK and Vero cells were pretreated with various concentrations of CPZ and sucrose for 1 h at 37°C and then infected with BEFV at an MOI of 2 for 24 h. In this study, CPZ greatly inhibited M protein synthesis in a dose-dependent manner compared to mock-treated controls (Fig. 1A, upper panel). Accordingly, the progeny virus titer of BEFV was also obviously reduced (3-log10 reduction) in MDBK cells by CPZ treatment compared to negative controls (Fig. 1A, lower panel). The inhibitory effect of CPZ on BEFV infection was more evident in MDBK cells. In a parallel experiment, we also employed a physiological method to interfere with the clathrin pathway (15). The same trend was seen in sucrose treatment (Fig. 1B).
Fig 1.
BEFV infection impaired by inhibition of clathrin-coated pit formation. (A and B) MDBK and Vero cells were pretreated with various concentrations of CPZ (A) and hypertonic sucrose (B) for 1 h, followed by infection with BEFV at an MOI of 2. The level of M protein and the progeny virus titer of BEFV were examined by Western blotting and by plaque assay, respectively. (C) MDBK and Vero cells were pretreated with various concentrations of MβCD for 1 h, followed by infection with BEFV at an MOI of 2. The cell lysates and supernatants of BEFV-infected cells were collected at 24 hpi for Western blotting and viral titration, respectively. The level of M protein was detected by Western blotting. The progeny virus titer of BEFV was determined by plaque assay. The results are from three triplicate experiments; error bars indicate the means ± standard deviations. The β-actin was used as an internal control for normalization. (D) Fluorescence of Alexa 568-labeled transferrin (Tfn) is shown, along with a Hoechst 33258 counterstain for cell nuclei. To study whether CPZ affects virus internalization, MDBK cells were starved for 2 h in serum-free medium and then were pretreated with CPZ (5 μM) for 1 h. MDBK cells were then infected with DiO-labeled BEFV. DiO-labeled viral particles were observed with a fluorescence microscope. Colocalization of the M protein of BEFV with clathrin or dynamin 2 was also observed by fluorescence microscopy. Immunostaining of clathrin, dynamin 2, and BEFV M was performed using respective antibodies. Mock-infected cells were used as negative controls. Cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Bars, 25 μm. (E) BEFV infection is inhibited in cells transfected with shRNAs specific to clathrin heavy chain. MDBK and Vero cells were transfected with CHC shRNA and mock control vectors (scrambled pGFP-V-RS and pGFP-V-RS), respectively. MDBK and Vero cells were infected with BEFV at an MOI of 2. The cell lysates and supernatants of BEFV-infected cells were collected at 24 hpi for Western blotting and viral titration, respectively. The β-actin was included as an internal control for normalization. Numbers below each lane are percentages of the control level of a specific protein in mock-treated cells. The results are from three triplicate experiments; error bars indicate the means ± standard deviations.
To further investigate whether BEFV uses the lipid raft/caveolin-dependent endocytic pathway, both MβCD and nystatin were used to block cholesterol-dependent, raft/caveola-mediated endocytosis (6, 27). MβCD disrupts a cholesterol-rich microdomain, resulting in the inhibition of both the caveolin-dependent endocytosis and the caveolin-independent lipid raft-dependent endocytosis. Cells were pretreated with various concentrations of MβCD for 1 h at 37°C and thereafter infected with BEFV at an MOI of 2 for 24 h in the absence of MβCD to avoid any potential effect of MβCD on the viral envelopes. The results showed that there was no significant difference between virus-infected cells with MβCD treatment and those without MβCD treatment (Fig. 1C). The results revealed that a caveolin-/lipid raft-dependent pathway does not represent the main entry route for BEFV. In addition, blockade of transferrin internalization by CPZ (5 μM) was observed (Fig. 1D). Transferrin internalization was also significantly blocked by the presence of hypertonic sucrose medium. These data demonstrated that clathrin-mediated endocytosis was indeed inhibited by these treatments under our experimental conditions. In the present study, colocalization of BEFV with clathrin or dynamin 2 in BEFV-infected MDBK cells was also observed during the very early stage of the life cycle (Fig. 1D). Furthermore, by using DiO-labeled BEFV to track particle entry, we found that DiO-labeled BEFVs colocalize with clathrin (Fig. 1D). Our results also reveal that CPZ inhibits DiO-labeled BEFV entry (Fig. 1D).
To further confirm our findings, we used shRNA to specifically knock down the expression of clathrin heavy chain (CHC). At 24 h posttransfection, we found that transfection of CHC shRNA indeed reduced clathrin expression in BEFV-infected cells compared to negative controls (Fig. 1E, upper panel). A marked reduction of the expression level of M protein and progeny virus titer of BEFV in the presence of CHC shRNA was seen compared to negative controls (Fig. 1E, lower panel). Taken together, these results reinforced the critical role of clathrin during BEFV entry, suggesting that clathrin-mediated endocytosis is the main entry route for BEFV.
BEFV entry into cells is dynamin 2 dependent.
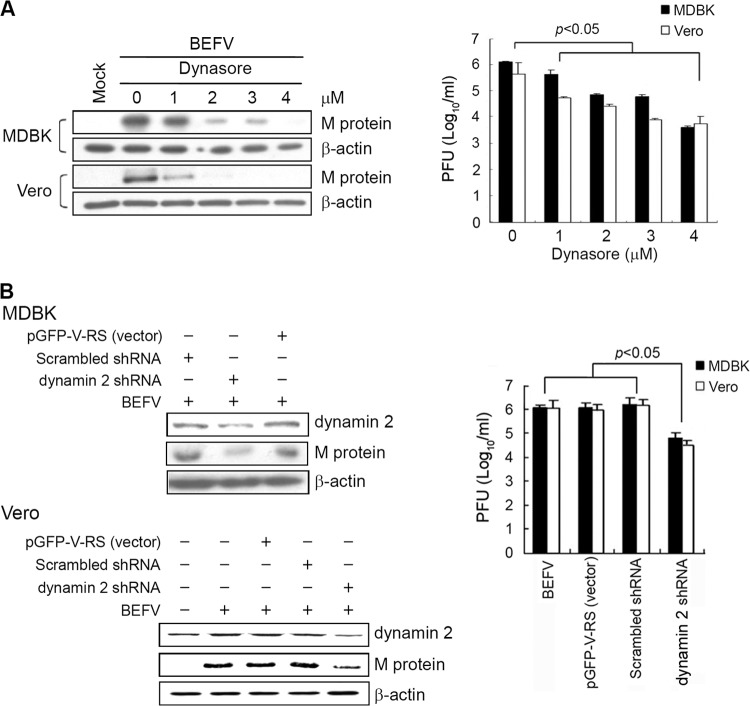
To examine whether dynamin 2 expression is necessary for virus uptake, MDBK and Vero cells were pretreated with Dynasore, a small-molecule inhibitor of the dynamin GTPase activity (26), for 1 h and infected with BEFV for 24 h. As shown in Fig. 2A (left panel), Dynasore significantly impaired viral M protein synthesis in a dose-dependent manner in both cell lines. In the presence of Dynasore, the progeny virus titer of BEFV was also abrogated in a dose-dependent manner (Fig. 2A, right panel). The results revealed that dynamin 2 is important for BEFV entry. To further validate the idea that dynamin 2 is an important factor in virus entry, an shRNA-mediated gene silencing against dynamin 2 was used. Compared to negative-control shRNAs, dynamin 2 levels were reduced by shRNA-mediated knockdown. A significant decrease in the expression level of M protein and the progeny virus titer of BEFV was shown in the dynamin 2 shRNA-transfected cells compared to negative controls (Fig. 2B, left and right panels).
Fig 2.
BEFV infection impaired by inhibition of dynamin 2 activity. (A) MDBK and Vero cells were pretreated with different amounts of Dynasore for 1 h and infected with BEFV at an MOI of 2. The cell lysates and supernatants of BEFV-infected cells were collected at 24 hpi for Western blotting and viral titration, respectively. Cell lysates were analyzed by Western blotting using anti-BEFV M antibody. The results are from three triplicate experiments; error bars indicate the means ± standard deviations. (B) BEFV infection is inhibited in cells transfected with shRNAs specific to dynamin 2. MDBK and Vero cells were transfected with dynamin 2 shRNA and mock control vectors (scrambled pGFP-V-RS and pGFP-V-RS), respectively, for 24 h and then infected with BEFV at an MOI of 2. The cell lysates and supernatants of BEFV-infected cells were collected at 24 hpi for Western blotting and viral titration, respectively. The cell lysates were analyzed by Western blotting using anti-dynamin 2, anti-BEFV M, and anti-β-actin antibodies. The β-actin was included as an internal control for normalization.
Additionally, in the semiquantitative RT-PCR, the transcription of the M-encoding gene of BEFV was also inhibited (up to 70% to 90% inhibition) in the presence of inhibitors or shRNAs (Fig. 3), suggesting that these inhibitors and shRNAs affect viral transcription.
Fig 3.
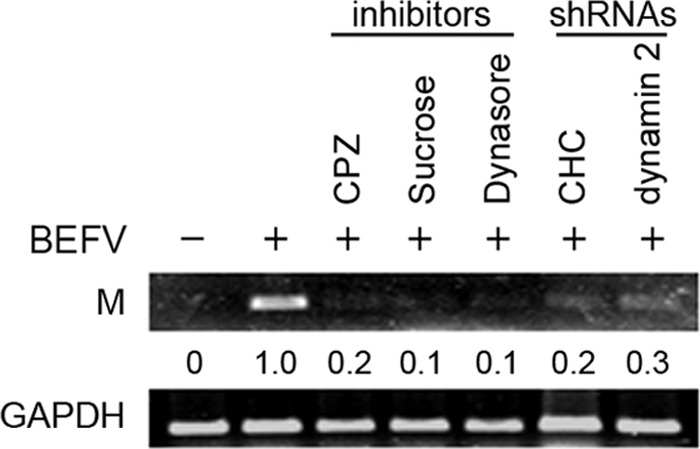
The effect of clathrin and dynamin 2 inhibitors or shRNAs on viral transcription. MDBK cells were pretreated with different inhibitors, respectively, for 1 h or transfected with specific clathrin or dynamin 2 shRNAs for 24 h and then infected with BEFV at an MOI of 2. The BEFV-infected cells were collected at 24 hpi, and total RNAs were extracted for semiquantitative RT-PCR. The inhibitors and shRNAs used are indicated on the top of the gel. The GAPDH gene was used as an internal control for normalization. The levels of M and GAPDH mRNA were compared. Numbers below each lane are percentages of the control level of a specific protein in mock-treated cells. Similar results were obtained from three independent experiments.
Microtubules are required for BEFV productive infection.
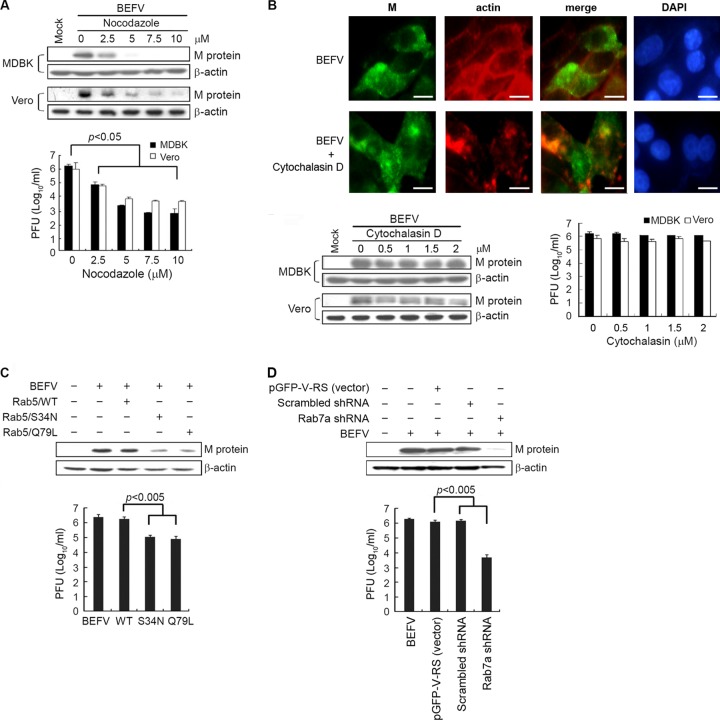
Microtubules contribute to regulation of cargo trafficking within endosomal compartments and serve as scaffolding structures for a variety of cellular proteins, including Rab5 (30). Consistent with this, microtubules have been implicated in cell entry of several viruses (31). To examine the potential role of microtubules in BEFV productive infection, we assessed the effect of cytochalasin D and nocodazole on BEFV infection. These inhibitors were used to disrupt actin filament and microtubules, respectively (38). In this work, MDBK and Vero cells were treated with nocodazole at different concentrations prior to infection with BEFV. Treatment of cells with nocodazole significantly decreased the expression level of M protein and the progeny virus titer of BEFV (Fig. 4A). In contrast, cells treated with cytochalasin D did not affect M protein synthesis and progeny virus production (Fig. 4B, lower panel), indicating that actin fibers are not required for the BEFV entry. To demonstrate that cytochalasin D was active at the concentration used, we used rhodamine phalloidin to stain cytochalasin D-treated cells. As shown in Fig. 4B (upper panel), the actin network has been disassembled in these cells. Taken together, we demonstrated that microtubules, but not actin fibers, play roles in BEFV productive infection, likely by mediating trafficking of BEFV-containing endosomes to the subcellular location where viral and endosomal membranes fuse. This finding is in contrast to vesicular stomatitis virus (VSV), which utilizes an actin-dependent pathway (7, 36).
Fig 4.
Effect of inhibition of BEFV infection by the disrupted microtubule assemble and functional repression of Rab5 or Rab7a. (A and B) MDBK and Vero cells were pretreated with different concentrations of nocodazole (A) or cytochalasin D (B) or with DMSO as a mock control for 1 h and then infected with BEFV at an MOI of 2 for 24 h. The cells were harvested at 24 hpi. The level of M protein of BEFV and β-actin was examined by Western blotting. The progeny virus titer of BEFV was examined by plaque formation assay. To demonstrate that cytochalasin D was active at the concentration used, rhodamine phalloidin was used to stain cytochalasin D-treated cells or BEFV-infected and cytochalasin D-treated cells (B, upper panel). MDBK cells were pretreated with cytochalasin D (1 μM) for 1 h before infection with BEFV at an MOI of 2. (C and D) MDBK cells transfected with Rab5 DN mutants (C) or Rab7a shRNAs (D) for 24 h were infected with BEFV at an MOI of 2 for 24 h. The level of BEFV M protein and supernatants of BEFV-infected cells were analyzed by Western blotting and viral titration, respectively. The results are from triplicate experiments; error bars indicate the means ± standard deviations.
Expression of Rab5 dominant negative mutant Rab5 and Rab7a shRNA prevents BEFV infection.
To further identify the exact endosomal compartment(s) traversed by endocytosed BEFV, we used a Rab5 DN mutant and Rab7a shRNAs to investigate the requirement of transport to early or late endosomes in the cell entry of BEFV. The results shows that Rab5 DN mutant overexpression (Fig. 4C) or depletion of Rab7a reduced the expression level of the M protein and virus production up to 1 to 2 log compared to mock-infected controls (Fig. 4C and D). Our results suggest that both Rab5 and Rab7a are required for endocytosis of BEFV and subsequent productive infection.
BEFV infection requires a low-pH compartment.
Having demonstrated that BEFV enters the host cells via clathrin-mediated and dynamin 2-dependent endocytic pathways, we next wanted to explore whether BEFV infection needs the acidic environment of the endosomal compartment. To this end, we incubated cells with substances preventing endosomal acidification. MDBK or Vero cells were pretreated with different concentrations of NH4Cl and bafilomycin A1 before BEFV absorption or treated with NH4Cl (20 nM) and bafilomycin A1 (25 nM) at different time points before, during, and after BEFV absorption, followed by infection with BEFV at an MOI of 2 for 24 h. Preincubation of MDBK and Vero cells with bafilomycin A1 showed a marked inhibitory effect on viral M protein synthesis and progeny virus production (Fig. 5A, upper panels). Incubation of cells with NH4Cl also resulted in a strong inhibitory effect on viral M protein synthesis and progeny virus titer of BEFV (Fig. 5B). MDBK cells treated with bafilomycin A1 or NH4Cl for 1 h before, during, and after infection with BEFV at an MOI of 2 significantly decreased the level of M protein (Fig. 5A, lower panel). The expression level of M protein was not detectable until 20 min postinfection in bafilomycin A1- or NH4Cl-treated cells. These results suggested that endosomal acidification is important for BEFV internalization. It is interesting that there is a postentry effect of bafilomycin A1 or NH4Cl on BEFV replication. To elucidate the mechanism of the effect of both bafilomycin A1and NH4Cl on BEFV replication, further study is ongoing.
Fig 5.
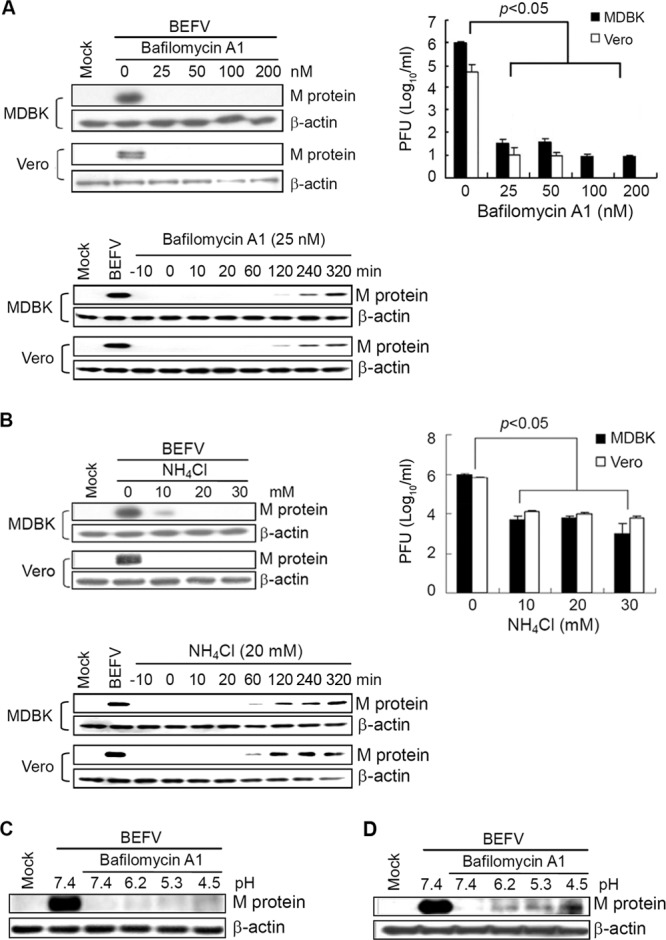
Endosomal acidification is required for BEFV entry at a low pH. (A) MDBK and Vero cells were pretreated with different concentrations of bafilomycin A1 for 1 h or at different time points (before, during, and after virus absorption), followed by infection with BEFV at an MOI of 2. Each value represents the mean of three independent experiments ± standard deviation. (B) MDBK and Vero cells were pretreated with different concentrations (upper panel) of NH4Cl for 1 h or at different time points (lower panel), followed by infection with BEFV at an MOI of 2. The cell lysates and supernatants of BEFV-infected cells were collected at 24 hpi. The synthesis of M protein was detected by Western blotting. The progeny virus titer of BEFV was determined by plaque assay. (C and D) Unbound virus was removed by washing cells with cold MEM, and virus-bound cells either were directly treated with low-pH solutions at different pHs for 10 min (C) or were preincubated at 37°C for 1 h in the presence of bafilomycin A1 and then treated with low-pH solutions at different pHs for 10 min (D). Cell lysates were collected at 24 hpi, and the level of M protein was detected by Western blotting. The β-actin was used as an internal control. The results are from triplicate experiments.
The bafilomycin A1 block is overcome at low pH.
To confirm the importance of acidic pH for the initiation of BEFV productive infection, we carried out “low-pH bypass” experiments. These experiments examine whether cells treated with inhibitors of virus entry can be affected by triggering fusion of viruses with the plasma membrane at low pH. For this purpose, we pretreated MDBK cells with 25 nM bafilomycin A1 for 1 h and incubated the cells with BEFV at 4°C for 1 h. Following extensive washes with cold medium, the virus-cell complexes were incubated directly with a low-pH solution for 10 min (Fig. 5C) or were preincubated at 37°C for 1 h to permit endocytosis and subsequently incubated with a low-pH solution for 10 min (Fig. 5D). The low-pH solution did not rescue the BEFV infectivity when the cells were directly treated after the virus binding (Fig. 5C), indicating that the low-pH environment alone was not enough for triggering fusion. Notably, the preincubation of the virus-cell complex for 1 h at 37°C followed by treatment with a low-pH solution substantially reduced the bafilomycin A1-mediated block of BEFV entry (Fig. 5D). This result demonstrated that acidic pH overcomes the bafilomycin A1 block of BEFV entry, implying that other important factors had contributed to viral disassembly at endosome or lysosome.
DISCUSSION
The mechanisms involved in the cell entry process of BEFV are poorly understood. Understanding the strategies used by viruses for cellular uptake can provide detailed information that might be used to prevent infection. To date, the exact cellular mechanism of BEFV entry remains unknown. In this study, the use of a panel of chemical inhibitors, DiO-labeled BEFV, DN mutants, and shRNA to inhibit or modify cellular processes has provided important insight into the early events of viral infections, suggesting that BEFV entry is through a clathrin-mediated and dynamin 2-dependent endocytic pathway. The strong inhibition by CPZ or sucrose points to clathrin-mediated endocytosis as an important avenue of BEFV entry. In order to examine more precisely the role of clathrin-mediated endocytosis in BEFV entry, we used clathrin or dynamin 2 shRNAs to disrupt the endocytic pathway. By the silencing of clathrin, BEFV infection was specifically reduced. Both the use of dynamin 2 shRNA and the use of Dynasore to disrupt dynamin 2-dependent endocytosis, even though the assays are different, led to similar conclusions, indicating that BEFV entry is dynamin 2 dependent. The sensitivity of infection to lysosomotropic agents is consistent with such an entry pathway, since clathrin-coated vesicles deliver their content to endosomes with an acidic pH environment. BEFV enters host cells after receptor-mediated endocytosis and depends on acidic cellular compartments for productive infection. Gaining insights into the initial virus-cell interactions is valuable in the search for an effective treatment or prevention of BEF.
Sensitivity to lysosomotropic agents has often been considered good evidence for endocytosis. This is the case for BEFV infection, which was influenced by these drugs. In this study, the ability of BEFV to infect cells was restricted by using a lysosomotropic agent and a specific inhibitor of the vacuolar proton-ATPase activity. Notably, bafilomycin A1 may interfere with the endocytic route in two different ways. Bafilomycin A1 is well known to block endosomal acidification by suppressing vacuolar proton ATPases (9), but it also inhibits the transport from early to late endosomes (19). Direct evidence for the relevance of endocytic uptake for BEFV entry was provided by the inhibition of BEFV infection with CPZ together with clathrin or dynamin 2 shRNAs. It has been shown that CPZ abolishes the formation of clathrin-coated vesicles by interfering with the interaction of the adapter protein AP2 with the clathrin-coated pit lattice (40) and thus inhibiting clathrin-mediated endocytosis (2, 36). BEFV infection was strongly inhibited by various inhibitors of endosomal acidification, indicating that virus enters host cells by clathrin-mediated and dynamin 2-dependent endocytosis in a pH-dependent manner. These data further reinforce the fact that the pH dependence of BEFV entry and acidification of virus-containing endosomes are required for BEFV to replicate within the host cells. The study provided the first evidence suggesting that BEFV infection depends on endosomal acidification and also on dynamin GTPase activity, which are hallmarks of clathrin-mediated endocytosis.
Interaction with the microtubule network and movement upon it are necessary for maintaining the normal intracellular distribution of endocytic organelles as well as for vesicular trafficking steps between the structures (10). The use of nocodazole to disrupt microtubules emphasizes the microtubule-mediated trafficking of the bulk virion population to the late endosome. Different Rab GTPases are associated with sorting of cargo to further destinations (34). Previous studies suggested that the small GTPase Rab5 regulates early endosome (EE) interactions with the microtubule network, membrane docking, and fusion in the early endocytic pathway (30). Cargo internalized by clathrin-mediated endocytosis is typically delivered to EE 2 to 5 min after internalization (21). Modulation of cytoskeleton organization is important for efficient replication of a number of viruses (4, 32). In the case of Junin virus (JV), cytoskeleton is required for the initiation of the assembly and budding processes at the plasma membrane. Inhibition of assembly of cytoskeletons by chemicals can dramatically reduce virion production or viral protein synthesis. In this study, microtubule disruption impairs BEFV infectivity, and because early endosomal transport requires microtubules and Rab5, suggests that BEFV requires transport to early endosome for infection. However, microtubule disruption may also influence multiple cellular functions. Hence, the specificity of the requirement of early endosomal compartments for the completion of the life cycle of BEFV was examined. Results obtained in this study provided evidence demonstrating that microtubules play a critical role during the early stage of the viral life cycle. Furthermore, we found that blockade of Rab5 or Rab7a has a strong impact on viral protein expression and viral yield, indicating that BEFV requires trafficking through early and late endosomes. In contrast to BEFV, VSV was inhibited only by the expression of dominant negative Rab5 and not by dominant negative Rab7, indicating an independence of late endosome function for infection by VSV (34). The requirement of both Rab5 and Rab7 for endocytosis has also been seen in influenza virus and some enveloped viruses (34). In contrast, several viruses appear to require trafficking only through early endosome (20, 34). The dependence of BEFV on early and late endosomes suggests that virus must be able to reach bona fide late endosomes for fusion and release from the endocytic pathway. Rab5 and Rab7 have also been demonstrated to regulate autophagy and maturation of late autophagic vacuoles, respectively (23, 35). Previously, several studies demonstrated that autophagosomes or autophagic vacuoles fuse with early and late endosomes (1, 25) as well as lysosome (11, 22). Hence, the relationship between BEFV-containing early and late endosomes and autophagy needs to be addressed in the future. Taken together, we provide initial evidence suggesting that the pH dependence of BEFV entry via the clathrin-mediated and dynamin 2-dependent endocytic pathway requires delivery to early and late endosomes.
The present study provides the first model of the events leading to BEFV internalization. It is possible that multiple factors regulate different steps of internalization. After endocytosis, BEFV requires transport to late endosomes before an acidic pH-dependent step presumably leads to the release of viral genome into the cytoplasm, resulting in successful infection. The molecular mechanisms elucidated in this study broaden our understanding of the pathways required for BEFV entry and facilitate new strategies for combating BEFV-caused diseases.
ACKNOWLEDGMENTS
This work was supported by the grants from the National Science Council (NSC 99-2321-B-005-015-MY3) and the Ministry of Education, Taiwan, Republic of China, under the ATU plan.
Footnotes
Published ahead of print 10 October 2012
REFERENCES
- 1. Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. 1998. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J. Biol. Chem. 273:21883–21892 [DOI] [PubMed] [Google Scholar]
- 2. Blanchard E, et al. 2006. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J. Virol. 80:6964–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bousarghin L, Touze A, Sizaret PY, Coursaget P. 2003. Human papillomavirus types 16, 31, and 58 use different endocytosis pathways to enter cells. J. Virol. 77:3846–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Campbell EM, Nunez R, Hope TJ. 2004. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 78:5745–5755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang CJ, Shih WL, Yu FL, Liao MH, Liu HJ. 2004. Apoptosis induced by bovine ephemeral fever virus. J. Virol. Methods 122:165–170 [DOI] [PubMed] [Google Scholar]
- 6. Chazal N, Gerlier D. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cureton DK, Massol RH, Saffarian S, Kirchhausen TL, Whelan SP. 2009. Vesicular stomatitis virus enters cells through vesicles incompletely coated with clathrin that depend upon actin for internalization. PLoS Pathog. 5(4):e1000394 doi:10.1371/journal.ppat.1000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Della-Porta AJ, Brown F. 1979. The physico-chemical characterization of bovine ephemeral fever virus as a member of the family Rhabdoviridae. J. Gen. Virol. 44:99–112 [DOI] [PubMed] [Google Scholar]
- 9. Drose S, Altendorf K. 1997. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 200:1–8 [DOI] [PubMed] [Google Scholar]
- 10. Goodson HV, Valetti C, Kreis TE. 1997. 1997. Motors and membrane traffic. Curr. Opin. Cell Biol. 9:18–28 [DOI] [PubMed] [Google Scholar]
- 11. Gordon PB, Hoyvik H, Seglen PO. 1992. Prelysosomal and lysosomal connections between autophagy and endocytosis. Biochem. J. 283:361–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helenius A, Kartenbeck J, Simons K, Fries E. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henley JR, Krueger EW, Oswald BJ, McNiven MA. 1998. Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hertig C, et al. 1996. Vaccinia virus-expressed bovine ephemeral fever virus G but not G(NS) glycoprotein induces neutralizing antibodies and protects against experimental infection. J. Gen. Virol. 77:631–640 [DOI] [PubMed] [Google Scholar]
- 15. Heuser JE, Anderson RG. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinshaw JE. 2000. Dynamin and its role in membrane fission. Annu. Rev. Cell Dev. Biol. 16:483–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang WR, et al. 2011. Cell entry of avian reovirus follows a caveolin-1-mediated and dynamin 2-dependent endocytic pathway that requires activation of p38 MAPK and Src signaling pathways as well as microtubules and small GTPase Rab5 protein. J. Biol. Chem. 286:30780–30794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ji WT, et al. 2011. Inhibitors of PI3K and mTOR but not Akt enhance replication of bovine ephemeral fever virus. Vet. J. 187:119–123 [DOI] [PubMed] [Google Scholar]
- 19. Keen JH. 1990. Clathrin and associated assembly and disassembly proteins. Annu. Rev. Biochem. 59:415–438 [DOI] [PubMed] [Google Scholar]
- 20. Krishnan MN, et al. 2007. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J. Virol. 81:4881–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakadamyali M, Rust MJ, Zhuang X. 2006. Ligands for clathrin-mediated endocytosis are differentially sorted into distinct populations of early endosomes. Cell 124:997–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawrence BP, Brown WJ. 1992. Autophagic vacuoles rapidly fuse with pre-existing lysosomes in cultured hepatocytes. J. Cell Sci. 102:515–526 [DOI] [PubMed] [Google Scholar]
- 23. Li L, et al. 2010. Regulation of mTORC1 by the Rab and Arf GTPases. J. Biol. Chem. 285:19705–19709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin CH, et al. 2009. Bovine ephemeral fever virus-induced apoptosis requires virus gene expression and activation of Fas and mitochondrial signaling pathway. Apoptosis 14:864–877 [DOI] [PubMed] [Google Scholar]
- 25. Liou W, Geuze HJ, Geelen MJH, Slot JW. 1997. The autophagic and endocytic pathways converge at the nascent autophagic vacuole. J. Cell Biol. 136:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macia E, et al. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- 27. Manes S, del Real G, Martinez AC. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557–568 [DOI] [PubMed] [Google Scholar]
- 28. Murphy FA, Taylor WP, Mims CA, Whitfield SG. 1972. Bovine ephemeral fever virus in cell culture and mice. Arch. Gesamte Virusforsch. 38:234–249 [DOI] [PubMed] [Google Scholar]
- 29. Nandi S, Negi BS. 1999. Bovine ephemeral fever: a review. Comp. Immunol. Microbiol. Infect. Dis. 22:81–91 [DOI] [PubMed] [Google Scholar]
- 30. Nielsen E, Severin F, Backer JM, Hyman AA, Zerial M. 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1:376–382 [DOI] [PubMed] [Google Scholar]
- 31. Roohvand F, et al. 2009. Initiation of hepatitis C virus infection requires the dynamic microtubule network: role of the viral nucleocapsid protein. J. Biol. Chem. 284:13778–13791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruthel G, et al. 2005. Association of Ebola virus matrix protein VP40 with microtubules. J. Virol. 79:4709–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sieczkarski SB, Whittaker GR. 2005. Characterization of the host cell entry of filamentous influenza virus. Arch. Virol. 150:1783–1796 [DOI] [PubMed] [Google Scholar]
- 34. Sieczkarski SB, Whittaker GR. 2003. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic 4:333–343 [DOI] [PubMed] [Google Scholar]
- 35. Su WC, et al. 2011. Rab5 and class III PI3K Vps34 are involved in hepatitis C virus NS4B-induced autophagy. J. Virol. 85:10561–10571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun X, Yau VK, Briggs BJ, Whittaker GR. 2005. Role of clathrin-mediated endocytosis during vesicular stomatitis virus entry into host cells. Virology 338:53–60 [DOI] [PubMed] [Google Scholar]
- 37. van der Schaar HM, et al. 2007. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J. Virol. 81:12019–12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. 1997. Nanomolar concentrations of nocodazole alter microtubule dynamic instability in vivo and in vitro. Mol. Biol. Cell 8:973–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker PJ, Byrne KA, Cybinski DH, Doolan DL, Wang YH. 1991. Proteins of bovine ephemeral fever virus. J. Gen. Virol. 72:67–74 [DOI] [PubMed] [Google Scholar]
- 40. Wang LH, Rothberg KG, Anderson RG. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang FI, Hsu AM, Huang KJ. 2001. Bovine ephemeral fever in Taiwan. J. Vet. Diagn. Invest. 13:462–467 [DOI] [PubMed] [Google Scholar]