Abstract
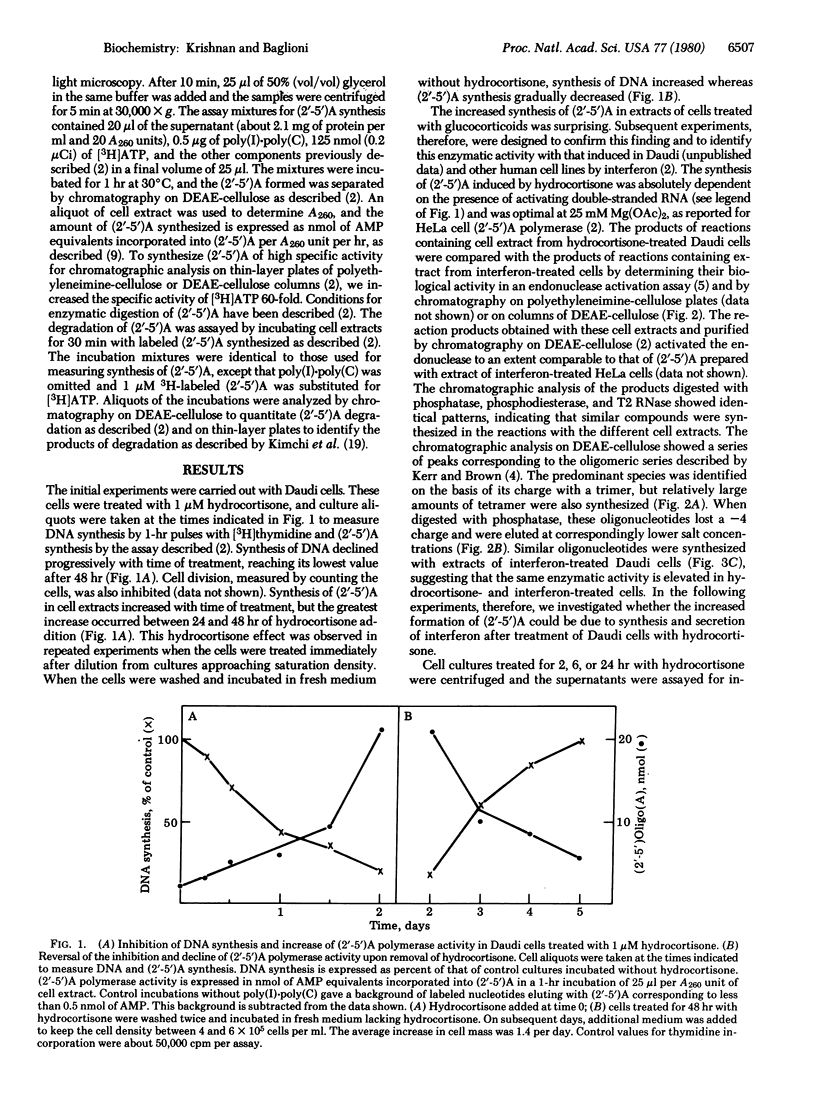
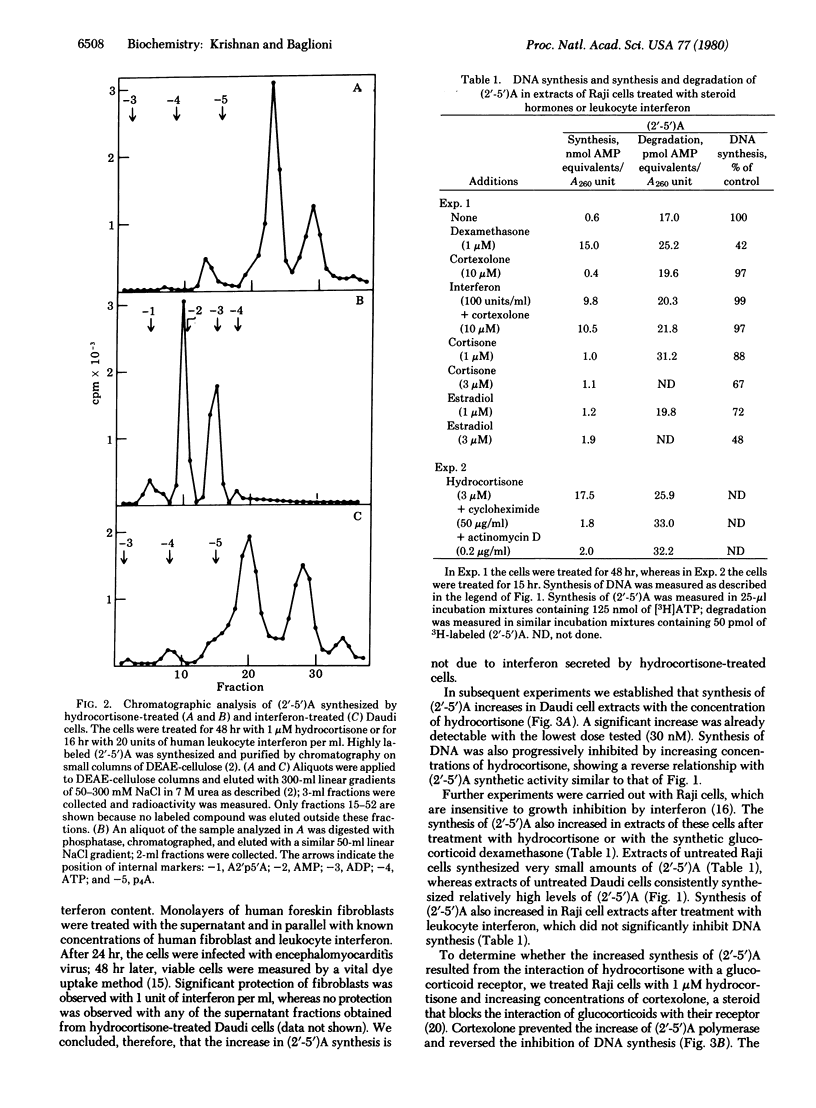
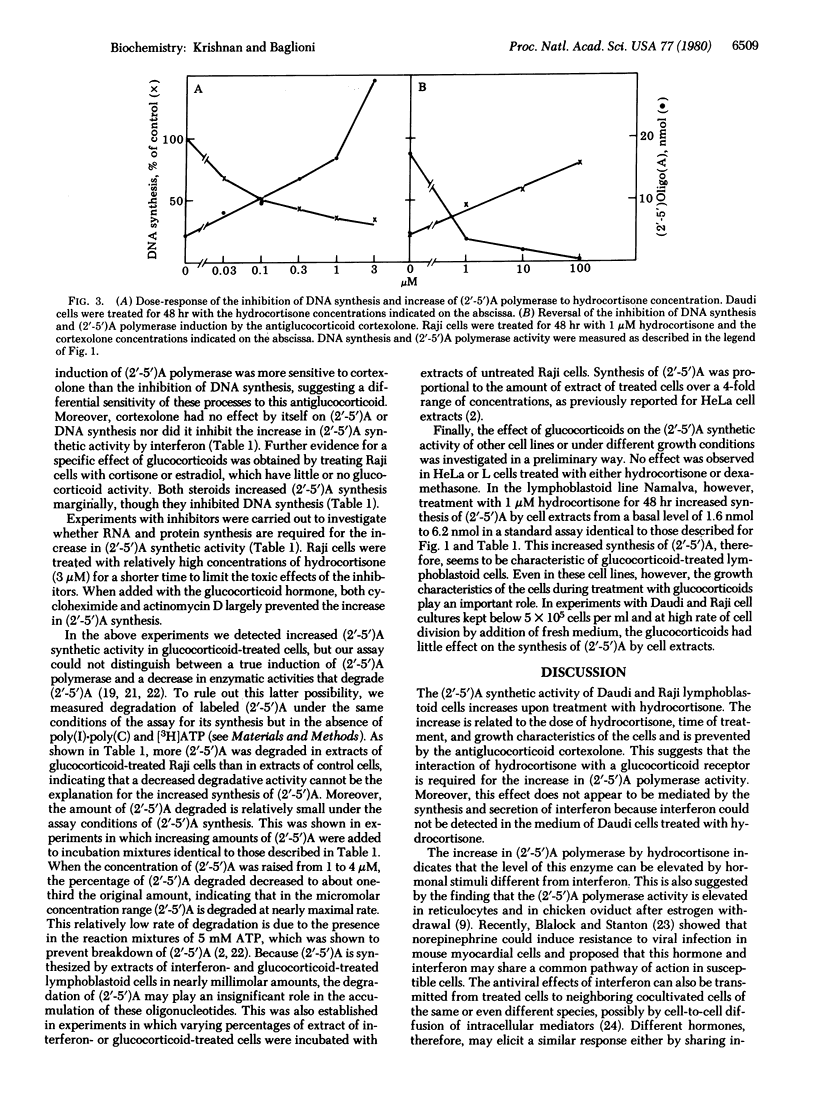
An enzymatic activity that synthesizes (2'-5')-oligo(A) from ATP is induced in animal cells treated with interferon. This activity, designated (2'-5')A polymerase, is also elevated in human lymphoblastoid Daudi and Raji cells treated with hydrocortisone. The polymerase activity increases significantly after 24 hr of treatment and declines when hydrocortisone is removed from the culture medium. The product of the enzyme prepared from hydrocortisone-treated cells is indistinguishable from (2'-5')oligo(A) synthesized with polymerase of interferon-treated cells either by an endonuclease activation assay or by chromatographic analysis. The increase in (2'-5')A polymerase is not mediated by secretion of interferon by hydrocortisone-treated cells; less than 1 unit of interferon per ml is present in the culture medium during treatment with this glucocorticoid hormone. Moreover, this increase is related to the concentration of hydrocortisone in the culture medium and is inhibited by the addition of cortexolone. This steroid interferes with the interaction between glucocorticoid hormones and their receptor. Cortexolone has no effect, however, on the induction of (2'-5')A polymerase by interferon. The synthetic glucocorticoid dexamethasone also increases the polymerase activity. Experiments with inhibitors show that such an increase requires RNA and protein synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lidin B., Strander H., Cantell K. Spontaneous interferon production and Epstein-Barr virus antigen expression in human lymphoid cell lines. J Gen Virol. 1975 Aug;28(2):219–223. doi: 10.1099/0022-1317-28-2-219. [DOI] [PubMed] [Google Scholar]
- Adams A., Strander H., Cantell K. Sensitivity of the Epstein-Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975 Aug;28(2):207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- Adolf G. R., Swetly P. Glucocorticoid hormones inhibit DNA synthesis and enhance interferon production in human lymphoid cell line. Nature. 1979 Dec 13;282(5740):736–738. doi: 10.1038/282736a0. [DOI] [PubMed] [Google Scholar]
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Baron S. Interferon-induced transfer of viral resistance between animal cells. Nature. 1977 Sep 29;269(5627):422–425. doi: 10.1038/269422a0. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Stanton J. D. Common pathways of interferon and hormonal action. Nature. 1980 Jan 24;283(5745):406–408. doi: 10.1038/283406a0. [DOI] [PubMed] [Google Scholar]
- Claman H. N. Corticosteroids and lymphoid cells. N Engl J Med. 1972 Aug 24;287(8):388–397. doi: 10.1056/NEJM197208242870806. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Blackwell G. J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979 Mar 29;278(5703):456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Horoszewicz J. S., Leong S. S., Carter W. A. Noncycling tumor cells are sensitive targets for the antiproliferative activity of human interferon. Science. 1979 Nov 30;206(4422):1091–1093. doi: 10.1126/science.493995. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Joncas J., Boucher J., Boudreault A., Granger-Julien M. Effect of hydrocortisone on cell viability, Epstein-Barr virus genome expression, and interferon synthesis in human lymphoblastoid cell lines. Cancer Res. 1973 Sep;33(9):2142–2148. [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Regulation of lymphocyte mitogenesis by (2'--5') oligo-isoadenylate. Nature. 1979 Dec 20;282(5741):849–851. doi: 10.1038/282849a0. [DOI] [PubMed] [Google Scholar]
- Lindahl-Magnusson P., Leary P., Gresser I. Interferon inhibits DNA synthesis induced in mouse lymphocyte suspensions by phytohaemagglutinin or by allogeneic cells. Nat New Biol. 1972 May 24;237(73):120–121. doi: 10.1038/newbio237120a0. [DOI] [PubMed] [Google Scholar]
- Magrath I. T., Pizzo P. A., Novikovs L., Levine A. S. Enhancement of Epstein-Barr virus replication in producer cell lines by a combination of low temperature and corticosteroids. Virology. 1979 Sep;97(2):477–481. doi: 10.1016/0042-6822(79)90360-x. [DOI] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Metabolic stability of 2' 5'oligo (A) and activity of 2' 5'oligo (A)-dependent endonuclease in extracts of interferon-treated and control HeLa cells. Nucleic Acids Res. 1979 Feb;6(2):767–780. doi: 10.1093/nar/6.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Synthesis of 2'5'-oligo(A) in extracts of interferon-treated HeLa cells. J Biol Chem. 1979 Jun 25;254(12):5058–5064. [PubMed] [Google Scholar]
- Minks M. A., West D. K., Benvin S., Baglioni C. Structural requirements of double-stranded RNA for the activation of 2',5'-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells. J Biol Chem. 1979 Oct 25;254(20):10180–10183. [PubMed] [Google Scholar]
- Minks M. A., West D. K., Benvin S., Greene J. J., Ts'o P. O., Baglioni C. Activation of 2',5'-oligo(A) polymerase and protein kinase of interferon-treated HeLa cells by 2'-O-methylated poly (inosinic acid) . poly(cytidylic acid), Correlations with interferon-inducing activity. J Biol Chem. 1980 Jul 10;255(13):6403–6407. [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Dower W. J., Schimke R. T., Brown R. E., Kerr I. M. 2-5A synthetase: assay, distribution and variation with growth or hormone status. Nature. 1979 Mar 29;278(5703):471–473. doi: 10.1038/278471a0. [DOI] [PubMed] [Google Scholar]
- Vengris V. E., Stollar B. D., Pitha P. M. Interferon externalization by producing cell before induction of antiviral state. Virology. 1975 Jun;65(2):410–417. doi: 10.1016/0042-6822(75)90046-x. [DOI] [PubMed] [Google Scholar]
- West D. K., Baglioni C. Induction of interferon in HeLa cells of a protein kinase activated by double-stranded RNA. Eur J Biochem. 1979 Nov;101(2):461–468. doi: 10.1111/j.1432-1033.1979.tb19740.x. [DOI] [PubMed] [Google Scholar]
- Williams B. R., Kerr I. M., Gilbert C. S., White C. N., Ball L. A. Synthesis and breakdown of pppA2'p5'A2'p5'A and transient inhibiton of protein synthesis in extracts from interferon-treated and control cells. Eur J Biochem. 1978 Dec;92(2):455–462. doi: 10.1111/j.1432-1033.1978.tb12767.x. [DOI] [PubMed] [Google Scholar]
- Zajac B. A., Henle W., Henle G. Autogenous and virus-induced interferons from lines of lymphoblastoid cells. Cancer Res. 1969 Aug;29(8):1467–1475. [PubMed] [Google Scholar]


