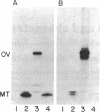
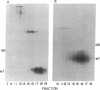
Abstract
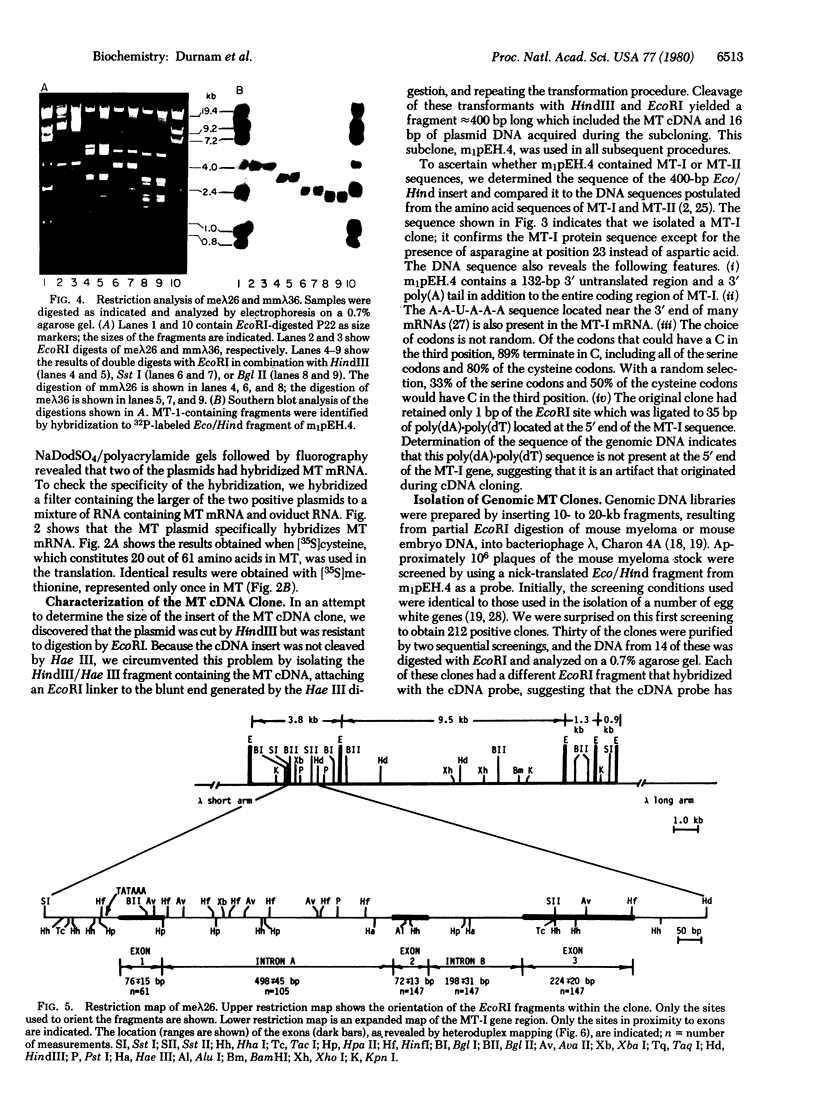
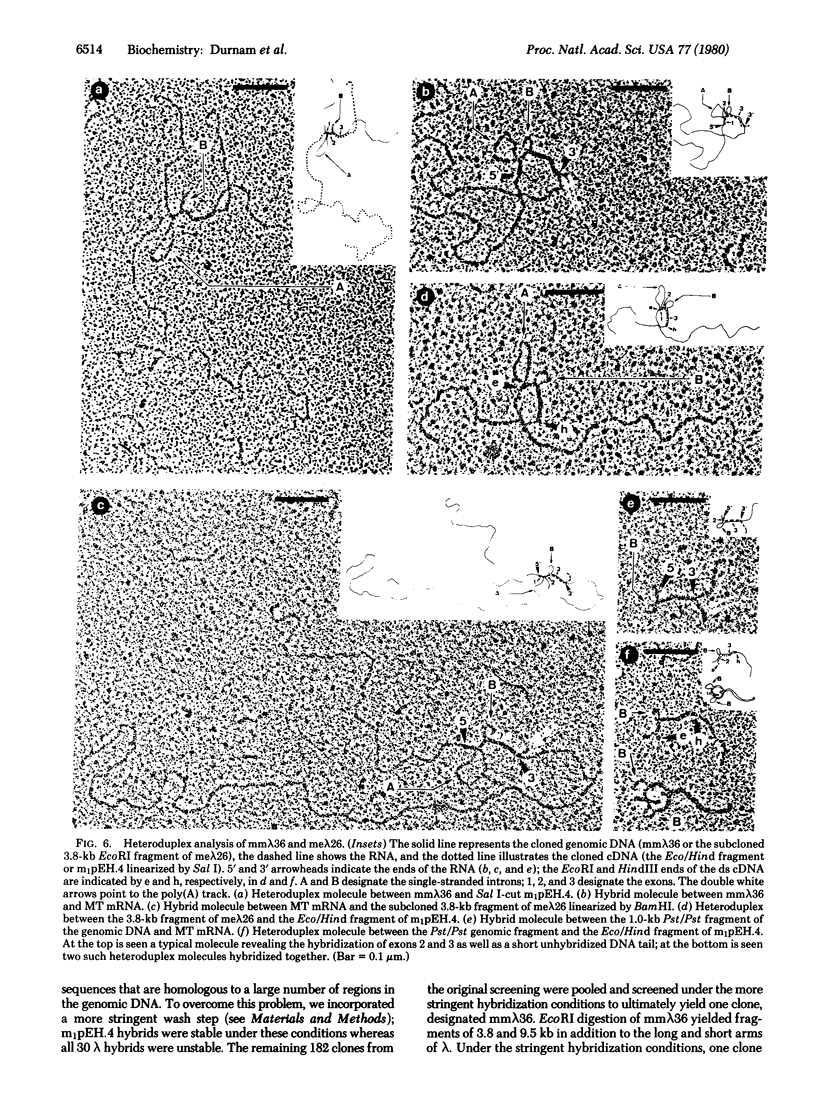
Double-stranded cDNA was synthesized from a mouse liver mRNA fraction enriched for metallothionein mRNA activity, ligated to restriction site linkers, inserted into pBR322, and used to transform Escherichia coli chi 1776. The sequence of the largest plasmid containing DNA that hybridized to metallothionein mRNA was determined and shown to contain a 380-base-pair insert that includes the entire coding region and 3' untranslated region of metallothionein-I. The metallothionein-I insert was nick-translated and used to screen both a mouse myeloma and a mouse embryo DNA library in bacteriophage lambda. A metallothionein-I genomic clone containing 13-15 kilobase pairs of mouse DNA was isolated from each library. Both contain a 3.8-kilobase-pair EcoRI fragment that hybridizes to the metallothionein-I probe. The location, size, and orientation of the metallothionein-I gene within the 3.8-kilobase-pair fragment were determined by heteroduplex and restriction mapping. The gene spans 1.1 kilobase pairs and contains at least two introns.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Weser U. Partial purification, characterization and translation in vitro of rat liver metallothionein messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):841–852. doi: 10.1042/bj1750841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian M. G. Isolation and purification of cadmium binding proteins from rat liver. Biochem Biophys Res Commun. 1974 Dec 11;61(3):920–926. doi: 10.1016/0006-291x(74)90243-5. [DOI] [PubMed] [Google Scholar]
- Cochet M., Gannon F., Hen R., Maroteaux L., Perrin F., Chambon P. Organization and sequence studies of the 17-piece chicken conalbumin gene. Nature. 1979 Dec 6;282(5739):567–574. doi: 10.1038/282567a0. [DOI] [PubMed] [Google Scholar]
- Cochet M., Perrin F., Gannon F., Krust A., Chambon P., McKnight G. S., Lee D. C., Mayo K. E., Palmiter R. Cloning of an almost full-length chicken conalbumin double-stranded cDNA. Nucleic Acids Res. 1979 Jun 11;6(7):2435–2452. doi: 10.1093/nar/6.7.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzel K. R., Shapiro S. G., Cousins R. J. Regulation of liver metallothionein and plasma zinc by the glucocorticoid dexamethasone. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1120–1126. doi: 10.1016/0006-291x(79)92124-7. [DOI] [PubMed] [Google Scholar]
- Garapin A. C., Lepennec J. P., Roskam W., Perrin F., Cami B., Krust A., Breathnach R., Chambon P., Kourilsky P. Isolation by molecular cloning of a fragment in the split ovalbumin gene. Nature. 1978 Jun 1;273(5661):349–354. doi: 10.1038/273349a0. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., MacDonald R. J. Cloning of hormone genes from a mixture of cDNA molecules. Methods Enzymol. 1979;68:75–90. doi: 10.1016/0076-6879(79)68007-2. [DOI] [PubMed] [Google Scholar]
- Hildebrand C. E., Tobey R. A., Campbell E. W., Enger M. D. A cadmium-resistant variant of the Chinese hamster (CHO) cell with increased metallothionein induction capacity. Exp Cell Res. 1979 Dec;124(2):237–246. doi: 10.1016/0014-4827(79)90199-x. [DOI] [PubMed] [Google Scholar]
- Huang I. Y., Yoshida A. Mouse liver metallothioneins. Complete amino acid sequence of metallothionein-I. J Biol Chem. 1977 Nov 25;252(22):8217–8221. [PubMed] [Google Scholar]
- Karin M., Herschman H. R. Dexamethasone stimulation of metallothionein synthesis in HeLa cell cultures. Science. 1979 Apr 13;204(4389):176–177. doi: 10.1126/science.432639. [DOI] [PubMed] [Google Scholar]
- Maki R., Traunecker A., Sakano H., Roeder W., Tonegawa S. Exon shuffling generates an immunoglobulin heavy chain gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2138–2142. doi: 10.1073/pnas.77.4.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Keem K., Monahan J. J. Factors affecting the transformation of Escherichia coli strain chi1776 by pBR322 plasmid DNA. Gene. 1978 Jul;3(4):279–292. doi: 10.1016/0378-1119(78)90038-0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Ericsson L. H., Walsh K. A. Precursor of egg white lysozyme. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1977 Sep 25;252(18):6386–6393. [PubMed] [Google Scholar]
- Probst G. S., Bousquet W. F., Miya T. S. Kinetics of cadmium-induced hepatic and renal metallothionein synthesis in the mouse. Toxicol Appl Pharmacol. 1977 Jan;39(1):51–60. doi: 10.1016/0041-008x(77)90176-4. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Moen R. C., Davidson J. M., Byers P. H., Bornstein P., Palmiter R. D. Correlation of procollagen mRNA levels in normal and transformed chick embryo fibroblasts with different rates of procollagen synthesis. Biochemistry. 1978 May 2;17(9):1581–1590. doi: 10.1021/bi00602a001. [DOI] [PubMed] [Google Scholar]
- Rudd C. J., Herschman H. R. Metallothionein accumulation in response to cadmium in a clonal rat liver cell line. Toxicol Appl Pharmacol. 1978 Jun;44(3):511–521. doi: 10.1016/0041-008x(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Rugstad H. E., Norseth T. Cadmium resistance and content of cadmium-binding protein in two enzyme-deficient mutants of mouse fibroblasts (L-cells). Biochem Pharmacol. 1978 Mar 1;27(5):647–650. doi: 10.1016/0006-2952(78)90499-9. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B., Brown D. D., Reeder R. H. The molecular basis for length heterogeneity in ribosomal DNA from Xenopus laevis. J Mol Biol. 1976 Aug 25;105(4):461–486. doi: 10.1016/0022-2836(76)90229-1. [DOI] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]