Immune response in the arthropod vector to virus infection is a critical determinant of transmission for arboviruses such as West Nile virus (WNV). The immune response modulates outcomes of infection such as viral load, incubation period required for transmission, and viral pathogenesis in the vector (1, 2). As insects appear to lack an adaptive immune response characteristic of vertebrates, this controlling response occurs through activation of apparently simple, linear innate immunity pathways. However, in PNAS, Paradkar et al. (3) describe a mechanism connecting two of these innate immunity pathways (the RNAi pathway and the Jak-STAT pathway) in mosquitoes through the action of a secreted signaling molecule, Vago, leading to an antiviral state in uninfected, responsive cells. This work provides evidence of an integrated, versatile insect immune system capable of communication between pathways and cells to give rise to an effective response.
Innate immunity pathways have been well characterized in Drosophila, and orthologous pathways have been identified in a number of mosquito species (4, 5). These pathways are sufficient to mount protective responses to a variety of pathogens, including bacteria, fungi, and viruses. When considering the antiviral response, the RNAi, Toll, Imd, and Jak-STAT pathways have been shown to contribute to innate immunity (reviewed in ref. 4). The RNAi pathway recognizes viral dsRNA that is produced as a result of viral RNA genome replication (6, 7). Through the RNAi pathway, viral dsRNA is cleaved by Dicer-2 and incorporated into the RNA-induced silencing complex, where it is used to bind viral RNA genomes, targeting them for degradation. The RNAi response is essential for controlling virus replication and limiting virus-induced pathology in insects (2, 6), and inherently provides response specificity. Signaling pathways such as the Toll, Imd, and Jak-STAT pathways have also been implicated in the insect antiviral response (8–11). These signaling pathways lead to the activation of transcription factors and the subsequent expression of antimicrobial peptides. Although these innate immune responses are typically described as linear pathways, it has long been assumed that the pathways must communicate to form an integrated and pathogen-specific immune response; however, the mechanisms and molecules that connect the innate immune pathways were to this point unknown.
The Jak-STAT pathway has been well studied in mammals and is a complex system that uses multiple ligands, receptors, kinases, and transcription factors. There are four mammalian Jaks that associate with the cytoplasmic domains of a variety of membrane-bound receptors, including IFN and IL receptors (reviewed in ref. 12). The ligands (interferons and cytokines) bind to these receptors, inducing dimerization and bringing the Jaks into close proximity, allowing for transphosphorylation of the kinases as well as the cytoplasmic tails of the receptors. Phosphorylation recruits the STAT proteins; seven STATs are found in mammals (12). The STATs are phosphorylated by Jak and dimerize as homo- or heterodimers. Following dimerization, the STATs translocate to the nucleus and activate transcription of antimicrobial peptide genes, among others (12, 13). Combinations of various ligands, receptors, Jaks, and STATs increase the versatility and specificity of the immune response in mammals.
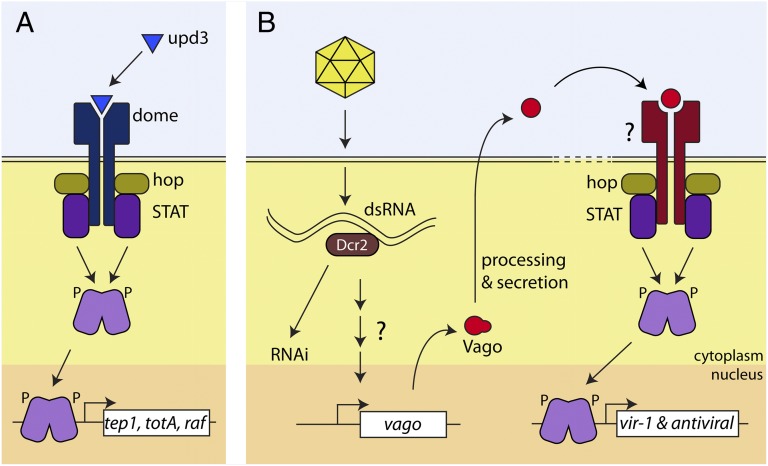
In contrast, the insect Jak-STAT pathway is relatively simple (Fig. 1A), possessing three ligands (Upd, Upd2, Upd3), one receptor (Domeless), one Jak (Hopscotch), and one STAT (STAT92E), as characterized in Drosophila (4). The ligands differentially activate the pathway; Upd and Upd2 are involved in developmental processes, whereas Upd3 responds to septic injury and bacterial challenge (14–16). Several genes have been reported to be STAT-responsive following pathogen-induced activation of the pathway. Some of these target genes are associated with phagocytosis (tep1), whereas others have been implicated in the general stress response and hemocyte proliferation (totA and raf, respectively) (14). When specifically considering viral infection, the Jak-STAT pathway has been shown to be activated by and produce an antiviral response to Drosophila C virus, Sindbis virus, WNV, and Dengue virus, among others (10, 17, 18). Virus infection induces a specific STAT-responsive transcription profile, characterized by increased transcription of previously unidentified genes and negligible change in standard pathway targets identified following bacterial infection in Drosophila (10). Specifically, the transcription of the gene vir-1 is highly up-regulated during Drosophila C virus infection of Drosophila, but not following bacterial infection (10). Likewise, Dengue virus infection of mosquitoes induces the expression of Dengue virus restriction factors 1 and 2 through activation of the Jak-STAT pathway (18).
Fig. 1.
(A) Characterized pathway for activation of STAT. The Upd3 ligand binds Domeless, leading to recruitment of hopscotch (Jak) and STAT. Phosphorylation of STAT allows nuclear translocation, promoter binding, and activation of transcription. (B) Dicer-2–dependent activation of STAT transcription via Vago signaling. Dicer-2 binds viral dsRNA and, through an unknown mechanism, activates expression of Vago. Processed and secreted Vago binds to an unknown cellular receptor, resulting in Jak-STAT activation and expression of vir-1 and yet-uncharacterized antiviral genes.
Recently, Deddouche et al. observed increased expression of vago (a gene encoding a protein with a single von Willebrand factor type C motif) in response to virus infection of Drosophila, but unchanged expression in response to bacterial challenge (17). Further investigation determined that vago expression was dependent on Dicer-2 activity in the presence of viral RNA replication, but did not require the other proteins required for the RNAi response (17). This led to the conclusion that Dicer-2 has signaling capabilities in response to dsRNA detection that are independent of the RNAi pathway.
Paradkar et al. report the observation of increased expression of a Vago orthologue in Culex mosquito cells infected with WNV (3). By using knockdown of Dicer-2, the authors demonstrate that the expression of Culex Vago was dependent on Dicer-2, but not the downstream components of the RNAi pathway. Interestingly, this response is specific for WNV dsRNA, implying that Dicer-2 is capable of differentiating between dsRNA sources. Strikingly, the authors demonstrate that Vago is secreted from cells following infection and induces an antiviral state in uninfected cells. This type of protective paracrine signaling response is reminiscent of IFN signaling in mammals that functions through the Jak-STAT pathway, and indeed Vago treatment of cells with reduced levels of STAT fails to induce the antiviral state observed in cells with WT levels of STAT. Finally, and perhaps most intriguingly, the authors show that activation of STAT-dependent transcription by Vago does not occur through the previously characterized cellular receptor, Domeless,
Paradkar et al. report the observation of increased expression of a Vago orthologue in Culex mosquito cells infected with WNV.
implying the involvement of a yet-unidentified Vago receptor linked to STAT activation (putative pathway is shown in Fig. 1B).
The recognition of the cytokine function of Vago offers significant insight into the integration of innate immune pathways, identifying a link between dsRNA recognition and Jak-STAT activation resulting in the establishment of an antiviral state in responsive cells. However, numerous questions arise from these findings. One question involves the detection of dsRNA by Dicer-2; how is the recognition of dsRNA transduced through Dicer-2 to bring about the expression of Vago? Another line of questions concerns the downstream gene targets of STAT. Vago brings about an antiviral state indirectly by activating the Jak-STAT pathway, resulting in the transcription of downstream effector genes. It will be important to determine how these gene products inhibit viral replication, and also investigate whether Vago-STAT–dependent expression of particular proteins leads to the activation of other antimicrobial pathways, such as Imd and Toll, establishing a network of connections between pathways. Finally, the ability of Vago to activate the Jak-STAT pathway in a manner independent of Domeless raises the question of Vago receptor identity. Identification of the receptor will allow investigation into the relationship between Vago and its receptor and Upd-Domeless in Jak-STAT signaling; do the two inputs cooperate or compete during an immune response? It is also possible that Vago-induced Jak-STAT signaling may play a role in processes outside of the immune response such as cell fate and tissue differentiation during insect development. Open questions in this field may be resolved by recognition of an alternative input to the Jak-STAT pathway.
With the work in PNAS, Paradkar et al. (3) open up new areas of investigation in insect immunity. The demonstration of communication between two pathways resulting in an infected cell stimulating an antiviral state in an uninfected cell suggests that other infectious agents may also stimulate intercellular crosstalk between antimicrobial pathways giving rise to a complex, systemic, and specific response to pathogen challenge.
Acknowledgments
The authors thank Alan Moore for assistance with illustrations.
Footnotes
The authors declare no conflict of interest.
See companion article on page 18915.
References
- 1.Cirimotich CM, Scott JC, Phillips AT, Geiss BJ, Olson KE. Suppression of RNA interference increases alphavirus replication and virus-associated mortality in Aedes aegypti mosquitoes. BMC Microbiol. 2009;9:49. doi: 10.1186/1471-2180-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Vargas I, et al. Dengue virus type 2 infections of Aedes aegypti are modulated by the mosquito’s RNA interference pathway. PLoS Pathog. 2009;5(2):e1000299. doi: 10.1371/journal.ppat.1000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paradkar PN, et al. Secreted Vago restricts West Nile virus infection in Culex mosquito cells by activating the Jak-STAT pathway. Proc Natl Acad Sci USA. 2012;109:18915–18920. doi: 10.1073/pnas.1205231109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 5.Waterhouse RM, et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science. 2007;316(5832):1738–1743. doi: 10.1126/science.1139862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffmann JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in drosophila. Nat Immunol. 2006;7(6):590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 7.Wang XH, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312(5772):452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avadhanula V, Weasner BP, Hardy GG, Kumar JP, Hardy RW. A novel system for the launch of alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009;5(9):e1000582. doi: 10.1371/journal.ppat.1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arjona A, Wang P, Montgomery RR, Fikrig E. Innate immune control of West Nile virus infection. Cell Microbiol. 2011;13(11):1648–1658. doi: 10.1111/j.1462-5822.2011.01649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dostert C, et al. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat Immunol. 2005;6(9):946–953. doi: 10.1038/ni1237. [DOI] [PubMed] [Google Scholar]
- 11.Fragkoudis R, Attarzadeh-Yazdi G, Nash AA, Fazakerley JK, Kohl A. Advances in dissecting mosquito innate immune responses to arbovirus infection. J Gen Virol. 2009;90(Pt 9):2061–2072. doi: 10.1099/vir.0.013201-0. [DOI] [PubMed] [Google Scholar]
- 12.Schindler C, Levy DE, Decker T. JAK-STAT signaling: From interferons to cytokines. J Biol Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 13.Li WX. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008;18(11):545–551. doi: 10.1016/j.tcb.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agaisse H, Perrimon N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol Rev. 2004;198:72–82. doi: 10.1111/j.0105-2896.2004.0133.x. [DOI] [PubMed] [Google Scholar]
- 15.Hombría JC, Brown S, Häder S, Zeidler MP. Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev Biol. 2005;288(2):420–433. doi: 10.1016/j.ydbio.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Wright VM, Vogt KL, Smythe E, Zeidler MP. Differential activities of the Drosophila JAK/STAT pathway ligands Upd, Upd2 and Upd3. Cell Signal. 2011;23(5):920–927. doi: 10.1016/j.cellsig.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Deddouche S, et al. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat Immunol. 2008;9(12):1425–1432. doi: 10.1038/ni.1664. [DOI] [PubMed] [Google Scholar]
- 18.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106(42):17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]