Abstract
Tomato (Solanum lycopersicum) fruit accumulate the red carotenoid pigment lycopene. The recessive mutation yellow-flesh (locus r) in tomato eliminates fruit carotenoids by disrupting the activity of the fruit-specific phytoene synthase (PSY1), the first committed step in the carotenoid biosynthesis pathway. Fruits of the recessive mutation tangerine (t) appear orange due to accumulation of 7,9,7′,9′-tetra-cis-lycopene (prolycopene) as a result of a mutation in the carotenoid cis–trans isomerase. It was established 60 y ago that tangerine is epistatic to yellow-flesh. This uncharacteristic epistasis interaction defies a paradigm in biochemical genetics arguing that mutations that disrupt enzymes acting early in a biosynthetic pathway are epistatic to other mutations that block downstream steps in the same pathway. To explain this conundrum, we have investigated the interaction between tangerine and yellow-flesh at the molecular level. Results presented here indicate that allele r2997 of yellow-flesh eliminates transcription of PSY1 in fruits. In a genetic background of tangerine, transcription of PSY1 is partially restored to a level sufficient for producing phytoene and downstream carotenoids. Our results revealed the molecular mechanism underlying the epistasis of t over r and suggest the involvement of cis-carotenoid metabolites in a feedback regulation of PSY1 gene expression.
Keywords: fruit development, tomato breeding
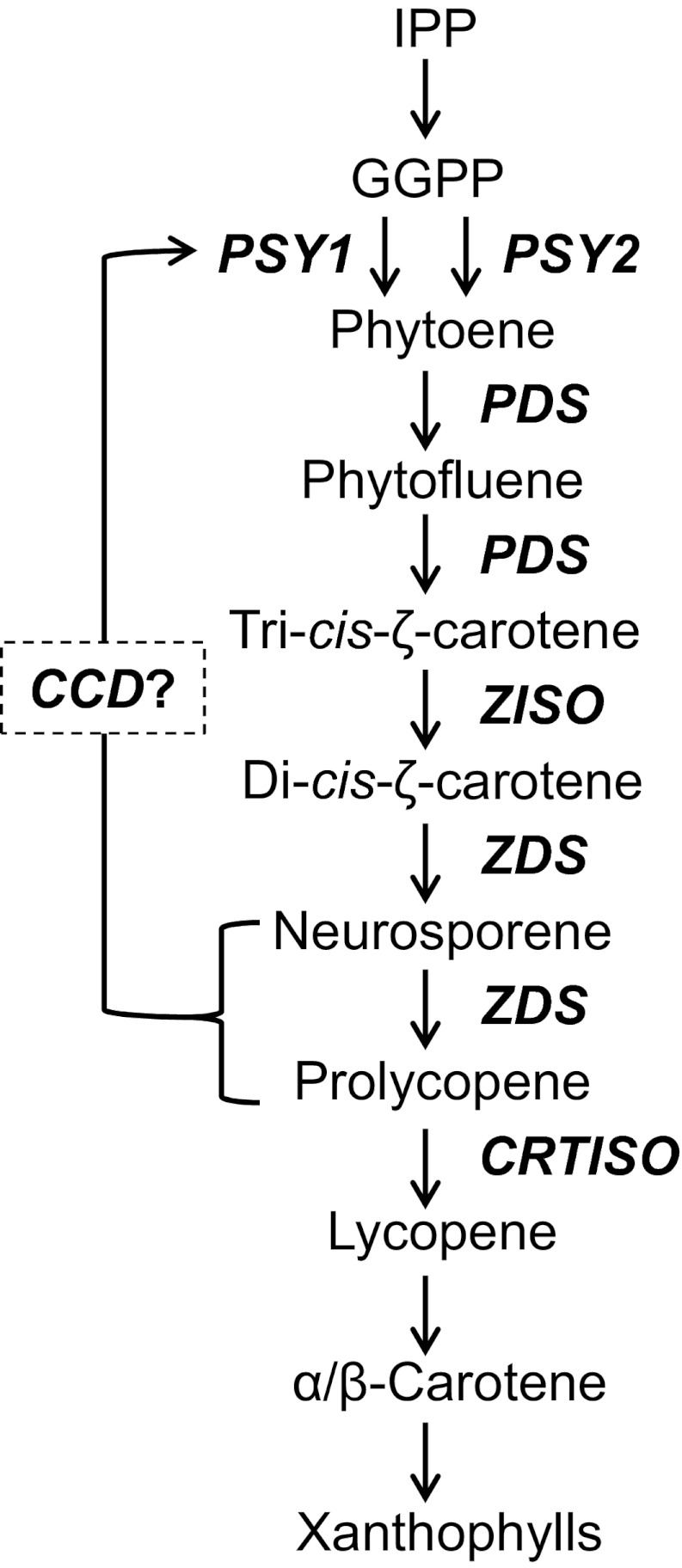
Epistasis is a genetic phenomenon where an allele of one gene masks the phenotype of an allele of another gene. Originally, it was postulated to explain deviations from the expected Mendelian segregation ratios of certain mutations and was extended later to include deviations from additivity in quantitative traits. Investigation of epistasis in metabolic pathways has established a paradigm in biochemical genetics that postulates that a mutation disrupting an enzyme that acts early in a biosynthetic pathway is epistatic to other mutations that block downstream steps in the same pathway (1). This principle has been extensively used in biological research to determine in double mutants the order of steps and functional relationship between genes in biosynthetic and developmental pathways as well as the contribution of alleles to quantitative traits (reviewed in refs. 2 and 3). Here we report on a peculiar epistatic interaction between mutations in two genes that govern the accumulation of the carotenoid pigment lycopene in tomato fruit wherein a mutation in a downstream enzyme for lycopene synthesis is epistatic to a mutation in an earlier step. Carotenoids are 40-carbon isoprenoid pigments that serve indispensable functions in photosynthesis (4). In plants, they furnish flowers and fruits with unique colors and provide precursors for the phytohormones abscisic acid and strigolactones. Carotenoid biosynthesis in plants has been described at the molecular level (reviewed in refs. 5–11). The first committed step in the pathway is the formation of phytoene from two molecules of geranylgeranyl diphosphate by the enzyme phytoene synthase (PSY). Four double bonds are then introduced by two enzymes, phytoene desaturase (PDS) and ζ-carotene desaturase (ZDS), and each catalyzes two symmetric desaturation steps to yield ζ-carotene and lycopene, respectively (Fig. 1). Intermediates in this part of the pathway are cis-configured (12, 13). A specific enzyme, ζ-carotene isomerase (ZISO), is required for cis-to-trans conversion of the 15—15′ cis double bond in tri-cis-ζ-carotene (14), and another isomerase, carotenoid cis–trans isomerase (CRTISO), produces all-trans-lycopene from tetra-cis-lycopene (prolycopene) (15–17). Further cyclizations and oxygenations give rise to α- and β-carotene and various xanthophylls.
Fig. 1.
The carotenoid biosynthesis pathway in plants. Mutations in tomato that impair specific enzymes are indicated. GGPP, geranylgeranyl diphosphate; OPP, pyrophosphate.
The red color of tomato (Solanum lycopersicum) fruit is provided by lycopene. The elevated carotenoid biosynthesis in tomato fruit during the “breaker” stage of fruit ripening is mainly regulated at the transcriptional level of carotenoid biosynthesis enzymes (10, 18). Expression of genes for the enzymes upstream of lycopene is up-regulated, whereas the transcription of enzymes that metabolize lycopene is turned off (19, 20). These transcriptional changes are developmentally coordinated. However, the molecular mechanism underlying the regulation of carotenoid biosynthesis genes is unknown.
Characterization of tomato mutants has greatly contributed to our knowledge of carotenoid biosynthesis in plants. The recessive mutation yellow-flesh, recognized by pale yellow fruit, was described over 100 y ago (21, 22). It was linked to a single locus, r, which was mapped on chromosome 3 in chromosomal bin IL3-2 (23) (http://solgenomics.net/locus/1599/view). Locus r encodes a fruit-specific phytoene synthase (PSY1) (24), which catalyzes a rate-limiting step in the carotenoid pathway (25). There are two additional PSY genes in tomato—PSY2, which functions solely in chloroplast-containing tissues (26, 27), and PSY3, whose existence has been discovered in the tomato genome. PSY3 is predicted to function in roots under stress conditions similarly to cereals (28, 29). Fruits of tomato with the recessive mutation tangerine (locus t) appear orange due to the accumulation of 7,9,9′,7′-tetra-cis-lycopene (prolycopene) (30, 31). Several studies in the 1950s reported that the mutation tangerine is epistatic to yellow-flesh because the phenotype of the double mutant ttrr was that of tangerine, namely, orange fruits (32–34). It was therefore surprising to discover that tangerine encodes CRTISO (15), an enzyme responsible for reconfiguration of two adjacent cis double bonds in neurosporene and cis-lycopene (13). Because these reactions are downstream of phytoene in the carotenoid biosynthesis pathway, blocking phytoene synthesis by yellow-flesh should be epistatic to tangerine. To understand this paradox, we analyzed the interaction between tangerine and yellow-flesh at the molecular level. Here we present results indicating that allele r2997 of yellow-flesh eliminates transcription of PSY1 in fruit. In the genetic background of tangerine, transcription of PSY1 is partially restored to a level sufficient for producing phytoene and downstream carotenoids at a significant level. Our results revealed the molecular mechanism underlying the epistasis of t over r and suggest the involvement of carotenoid metabolites in the regulation of gene expression.
Results
Epistasis of tangerine to yellow-flesh.
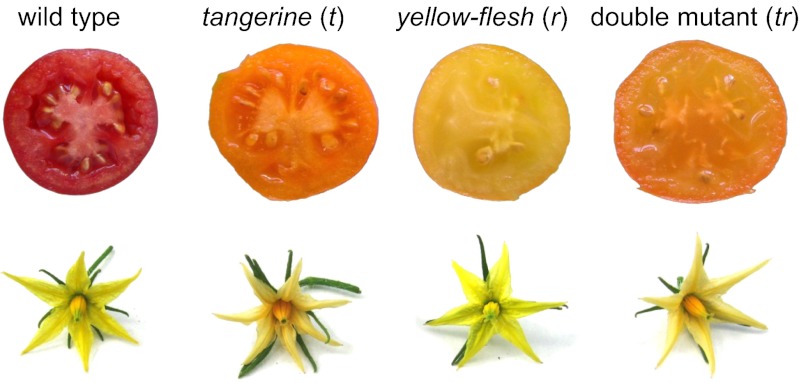
To confirm the original reports from the 1950s on the epistasis of tangerine over yellow-flesh, we repeated the experiments of Jenkins and Mackinney (33) and Tomes et al. (32). To this end, the original mutation lines tangerine3002 (t3002) and yellow-flesh2997 (r2997) were crossed and the phenotypes of F2 generation plants were analyzed. The results substantiated a phenotype distribution of a 9:4:3 ratio of red:tangerine:yellow-flesh. However, about a quarter of the tangerine fruit appeared paler (Fig. 2). The genotypes of the F2 plants were confirmed with DNA markers and genetically in F3 plants. Carotenoid analysis showed that wild-type fruit contained mainly all-trans-lycopene, whereas t3002 accumulated prolycopene and upstream intermediates (Table 1). The concentration of total carotenoids in r2997 fruits was only 3.6% of that in the wild type, and their makeup of lutein and β-carotene resembled the composition in green leaves. As previously reported (24, 35), this phenotype is characteristic of a block in the carotenoid pathway at the phytoene synthase step. Although fruit of the double mutant r2997/t3002 appeared similar to tangerine, they were paler because they contained less total carotenoids but retained a typical tangerine composition (Table 1). These results confirmed previous reports on epistasis of t to r (32, 33) and suggested that phytoene synthesis was restored to a certain degree in the double mutant r2997/t3002. Carotenoid composition in mature leaves of the mutants was similar to that of the wild type (Rutgers) (Table S1).
Fig. 2.
Typical fruits and flowers of wild type (Rutgers), tangerine (t3002), yellow-flesh (r2997), and the double mutant.
Table 1.
Carotenoids in ripe fruits of tangerine3002, yellow-flesh2997, the double mutant, and the corresponding isogenic wild type, Rutgers (µg/g fresh weight) (n = 5, ±SEM)
| Carotenoid concentration (μg/g fresh weight) |
||||
| Rutgers | t3002 | r2997 | t3002/r2997 | |
| Phytoene | 10.3 ± 1.7 | 31.0 ± 7.8 | 0 | 0.9 ± 0.1 |
| Phytofluene | 1.7 ± 1.3 | 9.5 ± 0.5 | 0 | 0 |
| ζ-Carotene | 0 | 10.1 ± 0.4 | 0 | 0 |
| Neurosporene | 0 | 18.4 ± 5.5 | 0 | 0 |
| Prolycopene | 0 | 64.1 ± 8.2 | 0 | 7.7 ± 3.1 |
| Lycopene | 72.0 ± 27.1 | 0 | 1.0 ± 0.6 | 1.6 ± 0.6 |
| β-Carotene | 25.6 ± 10.4 | 0 | 1.0 ± 0.1 | 1.9 ± 0.6 |
| Lutein | 5.6 ± 3.5 | 2.9 ± 0.4 | 2.3 ± 0.3 | 1.7 ± 0.5 |
| Others | 4.1 ± 1.0 | 0.2 ± 0.3 | 0 | 0.2 ± 0.3 |
| Total | 118.4 ± 50.7 | 136.2 ± 12.6 | 4.3 ± 0.7 | 14.0 ± 5.6 |
Mutations Responsible for tangerine3002 and yellow-flesh2997.
It has been previously established in two alleles, t3183 and tmic, that tangerine is mutated in the gene CRTISO, which encodes a carotene cis–trans isomerase (15). We have determined the mutation in t3002 (LA3002) along with three other tangerine alleles (Figs. S1 and S2) that were identified in the tomato variety M82 following ethyl methanesulfonate (EMS) mutagenesis. A frame-shift mutation due to a single-nucleotide insertion in codon 226 of CRTISO, which leads to an early stop codon, was found in t3002 (Fig. S1). The other alleles in the M82 variety carry missense mutations: L241K in t4838, G520R in t3406, and G546E in t9776 (Fig. S1).
Two lines of evidence demonstrate that yellow-flesh allele r2997 is associated with the gene for the fruit-specific phytoene synthase (PSY1). The locus r on chromosome 3 cosegregated with PSY1 (23). A mutant line, e3756, with a typical yellow-flesh phenotype, was isolated by mutagenesis in the tomato variety M82 (36). A transition mutation, changing tryptophan 151 to an early stop codon, was found in the PSY1 gene of e3756 (r3756) (Fig. S3). Fruit in all F1 plants from a cross between r2997 and r3756 were yellow because of a lack of carotenoids (Table S2). Absence of complementation in F1 demonstrates that r3756 and r2997 are alleles of the same genetic locus and confirms that r2997 is impaired in PSY1.
The full-length cDNA of PSY1 (24, 27, 37) was sequenced in r2997 and its presumed parental line, Rutgers. The two sequences were found to be identical to Unigene sequence no. SGN-U580527 (http://solgenomics.net/search/unigene.pl?unigene_id=SGN-U580527) from the variety Heinz 1706. The genomic DNA of PSY1 and its neighboring chromosomal regions was sequenced in r2997 and in varieties Rutgers and M82. These sequences were compared with the published genomic sequence of Heinz 1706 (38). The PSY1 gene (Solyc03g031860) is ca. 5 kb long. Based on alignment with the full-length cDNA of SGN-U580527, PSY1 contains nine exons (Fig. S4). A potential transcription start site was identified with TSSP software (SoftBerry; www.softberry.com) 10 bp upstream of the 5′ end of the sequence of Unigene SGN-U580527 (Fig. S4). Five single-nucleotide polymorphic (SNP) sites and one single-nucleotide deletion were found between r2997 and Rutgers in a DNA sequence of ca. 19,600 bp encompassing the PSY1 gene (Fig. 3). Only one of these SNPs, a transition from C to T at nucleotide SL2.40ch03:8604745, is close to the PSY1 gene, 472 bp upstream of the predicted transcription start site. However, all of the sequence disparities in r2997 compared with Rutgers also exist in cultivar M82. Because PSY1 in M82 is active during fruit ripening, these SNPs are probably unrelated to the yellow-flesh mutation.
Fig. 3.
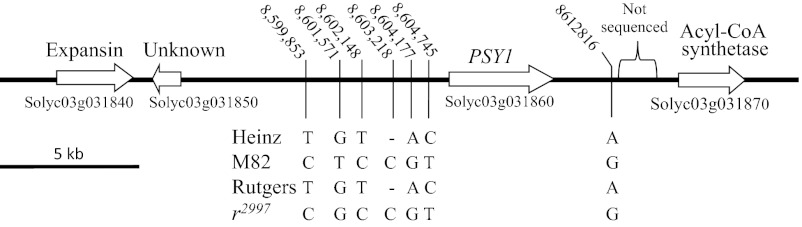
The genomic region around the PSY1 gene (Solyc03g031860) with all SNPs identified among tomato varieties Heinz 1706, M82, Rutgers, and mutant yellow-flesh2997. The nucleotide numbers correspond to Heinz 1706 chromosome 3, scaffold SL2.40ch03 (Sol Genomics Network genome browser: http://solgenomics.net/gbrowse/bin/gbrowse/ITAG2.3_genomic). The region marked “not sequenced” contains repetitive sequences and is also absent in the published sequence of Heinz 1706.
Effect of tangerine on the Expression of PSY1.
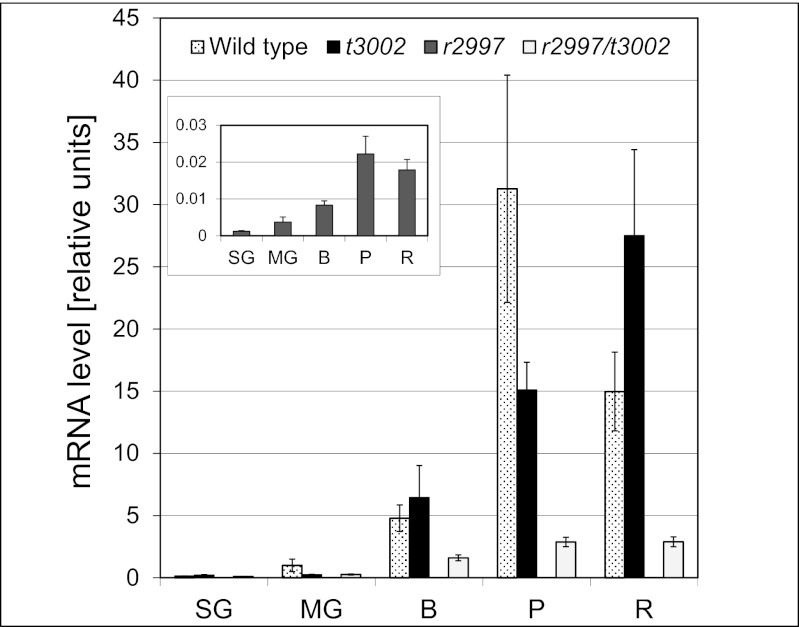
To explain the phenotype of r2997, the mRNA level of PSY1 was measured in fruit of r2997, t3002, the double mutant r2997/t3002, and Rutgers (Fig. 4). The results indicated the absence of PSY1 mRNA in r2997 fruits. This result provides a molecular explanation for the phenotype in yellow-flesh, r2997. Because PSY1 is exclusively responsible for phytoene synthesis in fruit (26), lack of expression of this gene eliminates carotenoid biosynthesis altogether. In tangerine3002, the level of PSY1 transcripts was higher than in the wild type, but it peaked in the “red” stage rather than in breaker. Surprisingly, transcription of PSY1 was partially restored in the double mutant r2997/t3002. Although the level of PSY1 mRNA was lower in the double mutant relative to the wild type, it was nearly 200-fold higher than in r2997, sufficient to produce phytoene synthase to sustain carotenoid synthesis. Careful measurements of PSY1 mRNA in r2997 fruits indicated that the pattern of its expression during fruit ripening was similar to that of the wild type, although at >1,000-fold lower concentration, making PSY1 in r2997 nonfunctional for any practical consideration. The transcript levels of PSY2 and CRTISO during fruit ripening in r2997 were generally similar to the wild type (Fig. S5). However, higher mRNA of PSY2 was measured in ripe fruit of t3002, although this change did not affect carotenoid composition. The reduced transcript level of CRTISO in t3002 is attributed to the phenomenon of nonsense-mediated mRNA decay (39).
Fig. 4.
Expression of PSY1 during fruit development of wild type (parental variety Rutgers), t3002, r2997, and the double mutant. RNA was extracted from fruit samples at five developmental stages: small green (SG), mature green (MG), breaker (B), pink (P), and red (R). Relative amounts of mRNA were determined by quantitative RT-PCR and were normalized with HIS3 transcript. (Inset) The relative amount of PSY1 in r2997 is shown (n = 5, ±SEM).
To test whether epistasis of tangerine to yellow-flesh is a general or allele-specific phenomenon, we created double mutants of tangerine with the loss-of-function allele of yellow-flesh, r3756. Fruit of the double mutant r3756/t3406 exhibited a characteristic yellow-flesh phenotype and lacked carotenoids (Table 2), indicating that in contrast to r2997, allele r3756 is epistatic to tangerine. These results imply that the phenomenon of epistasis of tangerine to yellow-flesh is unique to the r2997 allele.
Table 2.
Carotenoids in ripe fruits of tangerine3406, yellow-flesh3756, and the double mutant, compared with their corresponding isogenic wild type, M82 (µg/g fresh weight) (n = 5, ±SEM)
| Carotenoid concentration (μg/g fresh weight) |
|||||||
| M82 | t3406 | r3756 | t3406/r3756 | z2803 | r2997 | z2803/r2997 | |
| Phytoene | 10.9 ± 1.2 | 29.3 | 0 | 0 | 39.4 ± 3.0 | <1.0 | 0 |
| Phytofluene | 5.9 ± 0.7 | 11.4 | 0 | 0 | 19.7 ± 2.0 | 0 | 0 |
| ζ-Carotene | 1.6 ± 0.1 | 12.1 | 0 | 0 | 15.8 ± 0.1 | 0 | <1.0 |
| cis-neurosporene | 0 | 23.4 | 0 | 0 | <1.0 | 0 | <1.0 |
| Prolycopene | 4.5 ± 0.8 | 61.0 | 0 | 0 | <1.0 | 0 | 0 |
| trans-lycopene | 72.4 ± 2.2 | 0 | 0.9 | <1.0 | 1.5 ± 0.4 | <1.0 | <1.0 |
| β-Carotene | 3.4 ± 1.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lutein | 1.0 ± 0.7 | <1.0 | 1.0 | <1.0 | 3.7 ± 0.1 | 1.5 ± 0.4 | 1.1 ± 0.01 |
| Others | <1.0 | — | — | <1.0 | <1.0 | <1.0 | <1.0 |
| Total | 114.5 ± 6.9 | 137.2 | 1.9 | 1.4 | 81.3 ± 4.7 | 2.5 ± 1.3 | 2.5 ± 0.5 |
To confirm that restoration of PSY1 expression in r2997/t3002 is a consequence of the tangerine phenotype and not due to other interactions at the genetic level between the mutated CRTISO gene in t3002 and PSY1 in r2997, we examined the phenotype of r2997 in double mutants with two different tangerine alleles, t3406 and tmic, which not only carry different mutations in CRTISO but also exist in different genetic backgrounds, M82 and MicroTom, respectively. Epistasis of tangerine to yellow-flesh was observed in both r2997/t3406 and r2997/tmic double mutants (Fig. S6). Therefore, the recovery of PSY1 activity in r2997 by tangerine can be attributed to the altered carotenoid phenotype of tangerine.
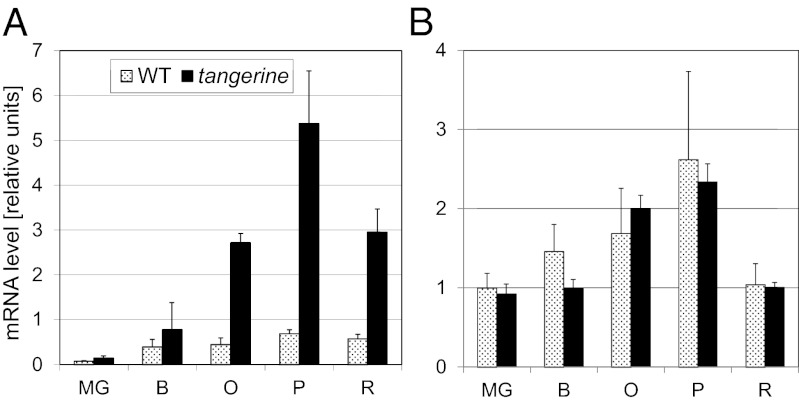
Two main changes occur in tangerine fruit carotenoids: elimination of trans-lycopene, β-carotene, and downstream xanthophylls and accumulation of prolycopene and carotenoid intermediates upstream of it. To test which one of them plays a role in activating PSY1 expression, the mutant t2997 was crossed with the recessive mutant zeta (z2803), which blocks carotenoid biosynthesis in fruit at ζ-carotene due to a mutation in the gene ZISO (Table 2). Fruits of the double mutant r2997/z2803 did not contain carotenoids, demonstrating that yellow-flesh r2997 is epistatic over zeta (Table 2). This result proves that restoration of PSY1 transcription in t2997 fruit by tangerine is not caused by the elimination of carotenoids downstream of lycopene, because they also are missing in zeta. For the same reason, phytoene, phytofluene, and ζ-carotene can be ruled out as well. The only unique carotenoids present at high concentration in tangerine are tri-cis-neurosporene and prolycopene. Correlation of high concentrations of tri-cis-neurosporene and prolycopene with increased PSY1expression also emerges from the expression data of wild-type PSY1 (allele R) in tangerine3002 (Fig. 4) and in MicroTom tangerine (tmic), where expression of PSY1 is markedly higher than in the wild type whereas expression of PDS does not change (Fig. 5).
Fig. 5.
Expression of PSY1 (A) and PDS (B) during fruit development of tangerine (tmic), and the corresponding isogenic wild type MicroTom. O, orange (n = 3, ±SEM).
Discussion
Epistasis Interactions Between tangerine and yellow-flesh.
The mutations yellow-flesh and tangerine affect carotenoid biosynthesis enzymes that act in a linear enzymatic pathway. The phenotype of the double mutants r2997/t3002, r2997/tmic, and r2997/t3406 described in this work confirmed previous reports that tangerine is epistatic to yellow-flesh (32–34, 40). This result contradicts the characteristic epistasis pattern of genes for enzymes acting in a biosynthetic pathway and thus challenges a classical paradigm in genetics. Our results demonstrate that the mutation in allele r2997 of yellow-flesh eliminates the transcription of the fruit-specific phytoene synthase gene PSY1. Under the genetic background of tangerine, transcription of PSY1 in r2997 is partially recovered, leading to an increase of PSY activity. This makes the double mutant capable of synthesizing phytoene and enables the carotenoid biosynthesis pathway to proceed until it is blocked at the cis–trans isomerization of prolycopene due to the tangerine mutation. The involvement of regulation of gene expression in the case of the double mutant r2997/t changes a simple interaction between mutations in an enzymatic pathway into an interaction often found in regulatory pathways where mutations in downstream-acting genes can be epistatic to mutations in earlier-acting genes (41).
The nature of the mutation in r2997 that abolishes transcription of PSY1 remains elusive. Genetic evidence unequivocally linked the r2997 locus to PSY1. However, none of the SNPs in the genomic DNA that spans the whole sequence of the PSY1 gene and its flanking regions is unique to r2997. One likely explanation is that r2997 is an epimutation caused by DNA methylation, similar to tomato Cnr (42), or by chromatin modifications. Alternatively, the mutation could be in an enhancer-type sequence that, in accordance with the recessive nature of the mutation, is a cis-acting element that exists farther than PSY1’s neighboring genes. The results suggest that the mutation affects a transcriptional activation mechanism necessary for high expression of PSY1 in fruit but does not alter the fruit-specific regulation of expression, as evident from the characteristic pattern of the low PSY1 expression during fruit ripening, which is still active in r2997 (Fig. 4).
Irrespective of the mechanism by which the mutation in r2997 affects the transcription of PSY1, it is apparent that its response to tangerine is mediated by the aberrant carotenoid composition, because increased PSY1 transcription occurred in all tangerine alleles and in different genetic backgrounds. There are two possibilities for signals originating from carotenoids in tangerine that can be transduced to affect other biochemical processes. One is a signal related to the absence of carotenoids downstream of prolycopene, such as trans-lycopene, β-carotene, and xanthophylls, all of which are missing in tangerine. Alternatively, an increase in the concentration of prolycopene, cis-neurosporene, and upstream intermediates may produce signals that are transduced to regulate the transcription of PSY1. Because removal of trans-lycopene and β-carotene accompanied by accumulation of cis-ζ-carotene and upstream carotenes also occurs in the mutant zeta (Table 2), the absence of epistasis by zeta in the case of the double mutant r2997/z2803 suggests that the effect of tangerine on PSY1 transcription in r2997 is originated from prolycopene and/or tri-cis-neurosporene. In fruit of tangerine, these cis-configured carotenes are present at significant concentrations of 5–10% of total carotenoids already at green stages of development, and in ripe fruit their concentration is >1,000-fold higher than in wild type. The identity of the signaling molecules could be the cis-carotenes or cis-configured apocarotenoid molecules derived from them following cleavage by dioxygenases (Fig. 6).
Fig. 6.
A proposed model of feedback regulation of carotenoid biosynthesis in tomato fruit. A signal generated from prolycopene and/or neurosporene is transduced to up-regulate the expression of PSY1, the rate-limiting step of the pathway. IPP, isopentenyl diphosphate; CCD, carotenoid cleavage dioxygenase.
The exact origin of the yellow-flesh allele r2997 is unknown. Yellow tomato varieties have been documented since the 16th century, when the tomato was introduced to Europe (22). Allele r2997, described as a “spontaneous” mutation (43), is one of the oldest known yellow-flesh alleles. It was introduced into the Rutgers variety to create LA2997 from the original yellow-flesh mutation, MacArthur’s “tester stock 90” (44) used in previous epistasis studies (32, 33, 40). We speculate that r2997 was originally derived by genetic introgression from a green-fruited wild tomato species, because all of them were shown to carry the recessive r allele as demonstrated, for example, in the Solanum pennellii chromosomal segment in introgression line IL3-2 (23). A functional PSY1 gene in these species may be related to its possible role in the flowers.
The premise that prolycopene and tri-cis-neurosporene produce regulatory signaling for gene expression offers a possible explanation for why plants use four enzymes to convert 15-cis-phytoene to all-trans-lycopene whereas in bacteria this pathway is catalyzed by a single enzyme, phytoene desaturase (CRTI). CRTI forms four double bonds at C-11, C-7, C-7′, and C-11′, and carries out cis–trans isomerizations to produce all-trans-lycopene (reviewed in ref. 45). Moreover, this enzyme is active in transgenic plants (46–48). Considering a simple one-enzyme alternative, the requirement in plants for two desaturases, PDS and ZDS, and two isomerases, ZISO and CRTISO, is not trivial. Although both pathways convert 15-cis-phytoene to trans-lycopene, they are not biochemically identical, because the plant pathway proceeds through cis-configured intermediates that exist in detectable concentrations (13). Both neurosporene and prolycopene contain 7,9-di-cis double bonds which, following enzymatic cleavage, would give cis-configured apocarotenoids that by themselves or through other intermediates may serve as signaling molecules. Concentration of these molecules is likely to vary by any disturbance of carotenoid biosynthesis, for example due to stress. Support of this hypothesis comes from the recent discovery of an uncharacteristic regulation of expression of the gene CRTISO in Arabidopsis by a Set Domain Group 8 (SDG8) chromatin-modifying histone methyltransferase (49). As proposed by Cazzonelli et al. (50), SDG8 is required to maintain permissive expression of CRTISO in specific tissues. Epigenetic regulation of an enzyme in the middle of the carotenoid biosynthesis pathway that is not rate-limiting is unusual. This regulation denotes that the importance of CRTISO activity transcends synthesis of trans-lycopene perhaps to control the level of cis-neurosporene and prolycopene as precursors for yet-unknown regulating molecules. The possibility that tetra-cis-lycopene might play a regulatory role was raised to explain the yellow phenotype of the endosperm of “golden rice,” which expresses the bacterial phytoene desaturase CRTI (51). However, because no change in expression was observed in the rice intrinsic carotenoid biosynthesis genes, it was speculated that the effect of lack of cis-carotenes is at the enzymatic level.
Phytoene synthesis is the first committed enzymatic reaction and a rate-limiting step in carotenoid biosynthesis. Expression in roots of PSY3 is subject to developmental and environmental regulations (28, 29, 52–56). Feedback regulation by disruption of carotenoid biosynthesis is attributed to reduction in abscisic acid, which is derived from xanthophylls (reviewed in ref. 11). Another example of feedback regulation is the case of the gene encoding 1-deoxy-d-xylulose 5-phosphate synthase, which is regulated in Arabidopsis by the activity of PSY (25, 57), but the nature of the signaling is unknown. The implication of cis-neurosporene and prolycopene in the regulation of PSY1 expression is a case of feedback regulation in the carotenoid pathway where specific carotenoid species are identified.
Materials and Methods
Plant Material and Growth Conditions.
Tomato (S. lycopersicum) varieties Rutgers and M82 were used as the wild-type references for the relevant mutants. The tangerine mutant LA3002 (t3002) and yellow-flesh mutant LA2997 (r2997) were obtained from the Tomato Genetics Resource Center (University of California, Davis, CA). Mutants e3406 (t3406), e4838 (t4838), e9776 (t9776), e3756 (r3756), and e2803 (z2803) were isolated from the variety M82 following mutagenesis and screening (36) (http://zamir.sgn.cornell.edu/mutants). Plants were grown in the greenhouse for crossing and in both the greenhouse and field for fruit analysis. For comparison of carotenoids and mRNA concentrations between different varieties, plants in the same experiment were grown simultaneously under the same conditions and sampled at the same time.
Measurement of mRNA with Quantitative Real-Time RT-PCR.
RNA extraction from fruits and leaves was performed with TRI Reagent RNA isolation reagent (Sigma-Aldrich) according to the manufacturer’s protocol from 1 g of frozen tissue. About 200 mg of powdered tissue was used for RNA extraction. Reverse transcription was performed with the ImProm-II Reverse Transcription System (Promega) following the manufacturer’s protocol, using 2 μg of RNA in a total volume of 40 μL and a degenerated 5′-T16NN-3′ primer. The RT product was diluted to a final volume of 800 μL. The cDNA was amplified with the Absolute PCR System (ABgene; Thermo Scientific) using 7 μL of the cDNA sample solution in a total reaction volume of 15 μL. RT-PCR was done in a Rotor-Gene 2000 thermocycler (Corbett Research). Following heating of the sample to 95 °C for 15 min, the cycling conditions were 95 °C for 15 s, 60 °C for 10 s, 72 °C for 15 s, and fluorescence acquisition at 79 °C. The relative mRNA level was determined in five biological replicates for each gene and developmental stage. The genes ACTIN and HIS3 served as controls for normalization. Primers used for PCR amplifications are described in Table S3.
Carotenoid Analysis.
Fruit pigments were extracted from ∼500 mg of fruit sample using a 1:1 mixture of acetone and diethyl ether. Leaf pigments were extracted with acetone from 100 mg of young leaves. The extracts were filtered, dried under a stream of nitrogen, and dissolved in 100% acetone. Analysis by HPLC using a Waters 996 photodiode array detector has been previously described (20). Carotenoids were identified by their characteristic absorption spectra, distinctive retention times, and, in some cases, comparison with standards. Quantitation was performed by integrating the peak areas of the HPLC results using Millennium chromatography software (Waters).
Genotyping of F2 Population of Double Mutants.
F2 progeny of a cross between t3002 and r2997 were screened visually, and seedlings showing virescent shoots were selected. These plants were further confirmed by the tawny flower color typical of tangerine. DNA was extracted from 150 mg of leaf tissue by the DNAzolES reagent according to the manufacturer’s recommended protocol (Molecular Research Center). The genotype of the double mutant was confirmed by sequence analysis with an ABI Prism 377 DNA sequencer (PerkinElmer) of PCR-amplified DNA of CRTISO using the primers PDH14 (forward) 5′-CTGCAGTGCTTCCACGTGAT-3′ and PDH10 (reverse) 5′-CGAAACACAACACGGCCTAA-3′ as a marker for t3002 and SNP SL2.40ch03:8604745 in PSY1 (Fig. 3) for r2997. F2 progeny of a cross between t3406 and r3756 mutants were analyzed similarly, using the mutation in r3756 as a marker. Sequence analysis of PCR products of PSY1 and CRTISO confirmed homozygosity for both t3406 and r3756, respectively. Seedlings of F2 progeny from a cross between zeta2083 and r2997 were visually screened for virescent shoots typical of zeta. The z2083 mutation was verified by sequencing of PCR-amplified DNA fragments using the primers 5′-GTGTTTCGGGACAAGGCACA-3′ (forward) and 5′-TGCCATTGGCAGTCAGCACA-3′ (reverse). Two plants with characteristic yellow-flesh fruit due to lack of carotenoids were validated as being homozygous z2083. This phenotype has not segregated in F3 plants.
Supplementary Material
Acknowledgments
We thank Prof. Dani Zamir for kindly providing tomato lines e3756 and e2803. This research was supported by Grant 1685/09 from the Israel Science Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214808109/-/DCSupplemental.
References
- 1.Beadle GW. Genetics and metabolism in Neurospora. Physiol Rev. 1945;25:643–663. doi: 10.1152/physrev.1945.25.4.643. [DOI] [PubMed] [Google Scholar]
- 2.Huang LS, Sternberg PW. 2006. in WormBook, ed. The C. elegans Research Community (WormBook Research Community, Pasadena, CA), pp 1–19. Available at http://www.ncbi.nlm.nih.gov/books/NBK19777/
- 3.Phillips PC. Epistasis—The essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet. 2008;9(11):855–867. doi: 10.1038/nrg2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cazzonelli CI. Carotenoids in nature: Insights from plants and beyond. Funct Plant Biol. 2011;38:833–847. doi: 10.1071/FP11192. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham FX, Jr, Gantt E. Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:557–583. doi: 10.1146/annurev.arplant.49.1.557. [DOI] [PubMed] [Google Scholar]
- 6.Hirschberg J. Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol. 2001;4(3):210–218. doi: 10.1016/s1369-5266(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 7.Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43(3):228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 8.DellaPenna D, Pogson BJ. Vitamin synthesis in plants: Tocopherols and carotenoids. Annu Rev Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- 9.Walter MH, Strack D. Carotenoids and their cleavage products: Biosynthesis and functions. Nat Prod Rep. 2011;28(4):663–692. doi: 10.1039/c0np00036a. [DOI] [PubMed] [Google Scholar]
- 10.Cazzonelli CI, Pogson BJ. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010;15(5):266–274. doi: 10.1016/j.tplants.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Sola MA, Rodriguez-Concepcion M. Carotenoid biosynthesis in Arabidopsis: A colorful pathway. The Arabidopsis Book. 2012;Vol 10 doi: 10.1199/tab.0158. Article e0158. www.bioone.org/doi/full/10.1199/tab.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartley GE, Scolnik PA, Beyer P. Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and ζ-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem. 1999;259(1-2):396–403. doi: 10.1046/j.1432-1327.1999.00051.x. [DOI] [PubMed] [Google Scholar]
- 13.Isaacson T, Ohad I, Beyer P, Hirschberg J. Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol. 2004;136(4):4246–4255. doi: 10.1104/pp.104.052092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Li F, Wurtzel ET. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010;153(1):66–79. doi: 10.1104/pp.110.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isaacson T, Ronen G, Zamir D, Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell. 2002;14(2):333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell. 2002;14(2):321–332. doi: 10.1105/tpc.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu Q, Ghisla S, Hirschberg J, Mann V, Beyer P. Plant carotene cis-trans isomerase CRTISO: A new member of the FAD(RED)-dependent flavoproteins catalyzing non-redox reactions. J Biol Chem. 2011;286(10):8666–8676. doi: 10.1074/jbc.M110.208017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bramley PM. Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot. 2002;53(377):2107–2113. doi: 10.1093/jxb/erf059. [DOI] [PubMed] [Google Scholar]
- 19.Pecker I, Gabbay R, Cunningham FX, Jr, Hirschberg J. Cloning and characterization of the cDNA for lycopene β-cyclase from tomato reveals decrease in its expression during fruit ripening. Plant Mol Biol. 1996;30(4):807–819. doi: 10.1007/BF00019013. [DOI] [PubMed] [Google Scholar]
- 20.Ronen G, Cohen M, Zamir D, Hirschberg J. Regulation of carotenoid biosynthesis during tomato fruit development: Expression of the gene for lycopene ε-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J. 1999;17(4):341–351. doi: 10.1046/j.1365-313x.1999.00381.x. [DOI] [PubMed] [Google Scholar]
- 21.Fleming HK, Myers CE. Tomato inheritance with special reference to skin and flesh color in the orange variety. Proc Am Soc Hortic Sci. 1937;35:609–623. [Google Scholar]
- 22.Jenkins JA. The origin of the cultivated tomato. Econ Bot. 1948;2:379–392. [Google Scholar]
- 23.Liu YS, et al. There is more to tomato fruit colour than candidate carotenoid genes. Plant Biotechnol J. 2003;1(3):195–207. doi: 10.1046/j.1467-7652.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 24.Fray RG, Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol. 1993;22(4):589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Villalón A, Gas E, Rodríguez-Concepción M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. Plant J. 2009;60(3):424–435. doi: 10.1111/j.1365-313X.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 26.Fraser PD, Kiano JW, Truesdale MR, Schuch W, Bramley PM. Phytoene synthase-2 enzyme activity in tomato does not contribute to carotenoid synthesis in ripening fruit. Plant Mol Biol. 1999;40(4):687–698. doi: 10.1023/a:1006256302570. [DOI] [PubMed] [Google Scholar]
- 27.Giorio G, Stigliani AL, D’Ambrosio C. Phytoene synthase genes in tomato (Solanumlycopersicum L.)—New data on the structures, the deduced amino acid sequences and the expression patterns. FEBS J. 2008;275(3):527–535. doi: 10.1111/j.1742-4658.2007.06219.x. [DOI] [PubMed] [Google Scholar]
- 28.Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008;146(3):1333–1345. doi: 10.1104/pp.107.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsch R, Wüst F, Bär C, Al-Babili S, Beyer P. A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 2008;147(1):367–380. doi: 10.1104/pp.108.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zechmeister L, Lerosen AL, Went FW, Pauling L. Prolycopene, a naturally occurring stereoisomer of lycopene. Proc Natl Acad Sci USA. 1941;27(10):468–474. doi: 10.1073/pnas.27.10.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clough JM, Pattenden G. Naturally occurring poly-cis carotenoids. Stereochemistry of poly-cis lycopene and in congeners in ‘Tangerine’ tomato fruits. J Chem Soc Chem Commun. 1979;(14):616–619. [Google Scholar]
- 32.Tomes ML, Quackenbush FW, Nelson OE, North B. The inheritance of carotenoid pigment system in the tomato. Genetics. 1953;38(2):117–127. doi: 10.1093/genetics/38.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins JA, Mackinney G. Inheritance of carotenoid differences in the tomato hybrid yellow × tangerine. Genetics. 1953;38(2):107–116. doi: 10.1093/genetics/38.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins JA, Mackinney G. Carotenoids of the apricot tomato and its hybrids with yellow and tangerine. Genetics. 1955;40(5):715–720. doi: 10.1093/genetics/40.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan D, Chen J, Shen H, Yang W. Genetics of flesh color and nucleotide sequence analysis of phytoene synthase gene 1 in a yellow-fruited tomato accession PI114490. Sci Hortic (Amsterdam) 2008;118(1):20–24. [Google Scholar]
- 36.Menda N, Semel Y, Peled D, Eshed Y, Zamir D. In silico screening of a saturated mutation library of tomato. Plant J. 2004;38(5):861–872. doi: 10.1111/j.1365-313X.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 37.Ray J, et al. Cloning and characterization of a gene involved in phytoene synthesis from tomato. Plant Mol Biol. 1992;19(3):401–404. doi: 10.1007/BF00023387. [DOI] [PubMed] [Google Scholar]
- 38.Tomato Genome Consortium The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012;485(7400):635–641. doi: 10.1038/nature11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker KE, Parker R. Nonsense-mediated mRNA decay: Terminating erroneous gene expression. Curr Opin Cell Biol. 2004;16(3):293–299. doi: 10.1016/j.ceb.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 40.MacArthur JW. Linkage groups in the tomato. J Genet. 1934;9:123–133. [Google Scholar]
- 41.Avery L, Wasserman S. Ordering gene function: The interpretation of epistasis in regulatory hierarchies. Trends Genet. 1992;8(9):312–316. doi: 10.1016/0168-9525(92)90263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning K, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet. 2006;38(8):948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- 43.Chetelat RT. Revised list of monogenic stocks. Rep Tomato Genet Coop. 2002;52:41–62. [Google Scholar]
- 44.Mackinney G, Jenkins JA. Inheritance of carotenoid differences in Lycopersicon esculentum strains. Proc Natl Acad Sci USA. 1949;35(6):284–291. doi: 10.1073/pnas.35.6.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandmann G. Evolution of carotene desaturation: The complication of a simple pathway. Arch Biochem Biophys. 2009;483(2):169–174. doi: 10.1016/j.abb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 46.Misawa N, et al. Functional expression of the Erwinia uredovora carotenoid biosynthesis gene crtl in transgenic plants showing an increase of β-carotene biosynthesis activity and resistance to the bleaching herbicide norflurazon. Plant J. 1993;4(5):833–840. doi: 10.1046/j.1365-313x.1993.04050833.x. [DOI] [PubMed] [Google Scholar]
- 47.Römer S, et al. Elevation of the provitamin A content of transgenic tomato plants. Nat Biotechnol. 2000;18(6):666–669. doi: 10.1038/76523. [DOI] [PubMed] [Google Scholar]
- 48.Ye X, et al. Engineering the provitamin A (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science. 2000;287(5451):303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 49.Cazzonelli CI, et al. Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell. 2009;21(1):39–53. doi: 10.1105/tpc.108.063131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cazzonelli CI, Roberts AC, Carmody ME, Pogson BJ. Transcriptional control of SET DOMAIN GROUP 8 and CAROTENOID ISOMERASE during Arabidopsis development. Mol Plant. 2010;3(1):174–191. doi: 10.1093/mp/ssp092. [DOI] [PubMed] [Google Scholar]
- 51.Schaub P, Al-Babili S, Drake R, Beyer P. Why is golden rice golden (yellow) instead of red? Plant Physiol. 2005;138(1):441–450. doi: 10.1104/pp.104.057927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welsch R, Medina J, Giuliano G, Beyer P, Von Lintig J. Structural and functional characterization of the phytoene synthase promoter from Arabidopsis thaliana. Planta. 2003;216(3):523–534. doi: 10.1007/s00425-002-0885-3. [DOI] [PubMed] [Google Scholar]
- 53.Meier S, Tzfadia O, Vallabhaneni R, Gehring C, Wurtzel ET. A transcriptional analysis of carotenoid, chlorophyll and plastidial isoprenoid biosynthesis genes during development and osmotic stress responses in Arabidopsis thaliana. BMC Syst Biol. 2011;5:77. doi: 10.1186/1752-0509-5-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arango J, Wüst F, Beyer P, Welsch R. Characterization of phytoene synthases from cassava and their involvement in abiotic stress-mediated responses. Planta. 2010;232(5):1251–1262. doi: 10.1007/s00425-010-1250-6. [DOI] [PubMed] [Google Scholar]
- 55.Toledo-Ortiz G, Huq E, Rodríguez-Concepción M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc Natl Acad Sci USA. 2010;107(25):11626–11631. doi: 10.1073/pnas.0914428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Tsfadia O, Wurtzel ET. The phytoene synthase gene family in the Grasses: Subfunctionalization provides tissue-specific control of carotenogenesis. Plant Signal Behav. 2009;4(3):208–211. doi: 10.4161/psb.4.3.7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodríguez-Villalón A, Gas E, Rodríguez-Concepción M. Colors in the dark: A model for the regulation of carotenoid biosynthesis in etioplasts. Plant Signal Behav. 2009;4(10):965–967. doi: 10.4161/psb.4.10.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.