Abstract
The cellular response to DNA damage is mediated through multiple pathways that regulate and coordinate DNA repair, cell cycle arrest, and cell death. We show that the DNA damage response (DDR) induced by ionizing radiation (IR) is coordinated in breast cancer cells by selective mRNA translation mediated by high levels of translation initiation factor eIF4G1 (eukaryotic initiation factor 4γ1). Increased expression of eIF4G1, common in breast cancers, was found to selectively increase translation of mRNAs involved in cell survival and the DDR, preventing autophagy and apoptosis [Survivin, hypoxia inducible factor 1α (HIF1α), X-linked inhibitor of apoptosis (XIAP)], promoting cell cycle arrest [growth arrest and DNA damage protein 45a (GADD45a), protein 53 (p53), ATR-interacting protein (ATRIP), Check point kinase 1 (Chk1)] and DNA repair [p53 binding protein 1 (53BP1), breast cancer associated proteins 1, 2 (BRCA1/2), Poly-ADP ribose polymerase (PARP), replication factor c2–5 (Rfc2-5), ataxia telangiectasia mutated gene 1 (ATM), meiotic recombination protein 11 (MRE-11), and others]. Reduced expression of eIF4G1, but not its homolog eIF4G2, greatly sensitizes cells to DNA damage by IR, induces cell death by both apoptosis and autophagy, and significantly delays resolution of DNA damage foci with little reduction of overall protein synthesis. Although some mRNAs selectively translated by higher levels of eIF4G1 were found to use internal ribosome entry site (IRES)-mediated alternate translation, most do not. The latter group shows significantly reduced dependence on eIF4E for translation, facilitated by an enhanced requirement for eIF4G1. Increased expression of eIF4G1 therefore promotes specialized translation of survival, growth arrest, and DDR mRNAs that are important in cell survival and DNA repair following genotoxic DNA damage.
Keywords: translational control, eIF
Cells have developed complex, coordinated DNA repair responses to protect and repair their genomes from genotoxic damage, prevent mitotic entry, and promote survival (1). Paradoxically, although DNA damage induces the transcription of a number of genes involved in the DNA damage response (DDR), cells typically respond to DNA damage by downregulating protein synthesis (2, 3). Translational regulation is a poorly explored but fundamental component of the cellular response to DNA damage (2). Down-regulation of protein synthesis after ionizing radiation (IR) involves inhibition of the cap-dependent mRNA translation machinery, controlled by the protein kinase mammalian target of rapamycin (mTOR), a downstream effector of the PI3 kinase–Akt signaling pathway (2, 4).
Translation in eukaryotes is mediated by multiple factors primarily at the step of initiation on mRNA, through assembly of the eukaryotic initiation factor (eIF)4F. eIF4F is composed of eIF4E, which binds the m7GTP cap, eIF4G (eukaryotic, initiation factor 4γ), which is a scaffold protein upon which ribosomes and eIFs assemble, and eIF4A, an ATP-dependent RNA helicase. eIF4E availability is regulated by eF4E binding protein 1 (4E-BP1), a competitive eIF4E inhibitor, which is inactivated through hyperphosphorylation by mTOR (5). Translation of most mRNAs requires eIF4E interaction with the mRNA cap, controlled by eIF4E/4E-BP regulation in response to growth and metabolic demands. A small number of mRNAs that maintain translation during cell stress responses contain in the 5′ untranslated region (5′ UTR) both a cap and an internal ribosome entry site (IRES), which permits eIF4E-independent translation (6). Cellular IRES models of translation initiation generally involve the binding of eIF4G to secondary structures in the IRES (7, 8). In mammalian cells increased levels of eIF4G facilitate translation of strictly cap (eIF4E)-dependent mRNAs, mRNAs containing multiple upstream (u)ORFs or IRES elements, and those of low abundance (7, 9, 10). Furthermore, different mRNAs display a wide range of dependence on eIF4E levels and yet lack an IRES (11). In yeast, higher expression levels of eIF4G promote translation of mRNAs with longer polyA tails (12) or promote direct ribosome–mRNA interactions (13), increasing translation of mRNAs with intrinsically higher translation efficiencies. Thus, in both yeast and mammalian cells, reduced levels of eIF4G do not strongly impair translation of many mRNAs (9, 12, 13), suggesting that certain mRNAs are selectively increased in their translation with higher levels of eIF4G.
eIF4G1 is the most abundant member of the eIF4G family. Preferential translation by the two eIF4G isoforms in flies, nematodes, and mammals has been reported, where a preferential requirement for eIF4G2-like homologs was found in germline cell developmental mRNA translation (14, 15). A poorly studied related eIF4G family member, death associated protein 5 (DAP5)/protein (p)97, lacks the NH2-terminal eIF4E and poly(A) binding protein (PABP) interaction sites (16), and either stimulates (17) or inhibits (16) translation of certain IRES-containing mRNAs.
eIF4G1 expression is strongly increased in breast cancers (10, 18, 19) and squamous cell lung cancers (20), associated with metastatic progression and reduced survival. The mechanism of overexpression has not been well-studied. Given that eIF4G1, like eIF4E, is also phosphorylated by, and is an effector of, mTOR activity (9), that mTOR inhibition is associated with increased sensitivity to IR-mediated DNA damage (21), and that eIF4G1 depletion does not strongly down-regulate overall protein synthesis (9), we investigated whether the elevated expression of eIF4G1 associated with breast cancer progression is responsible for resistance to genotoxic DNA damage responses.
Results
eIF4G1 Expression Promotes Resistance to Genotoxic DNA Damage with Little Effect on Overall Protein Synthesis.
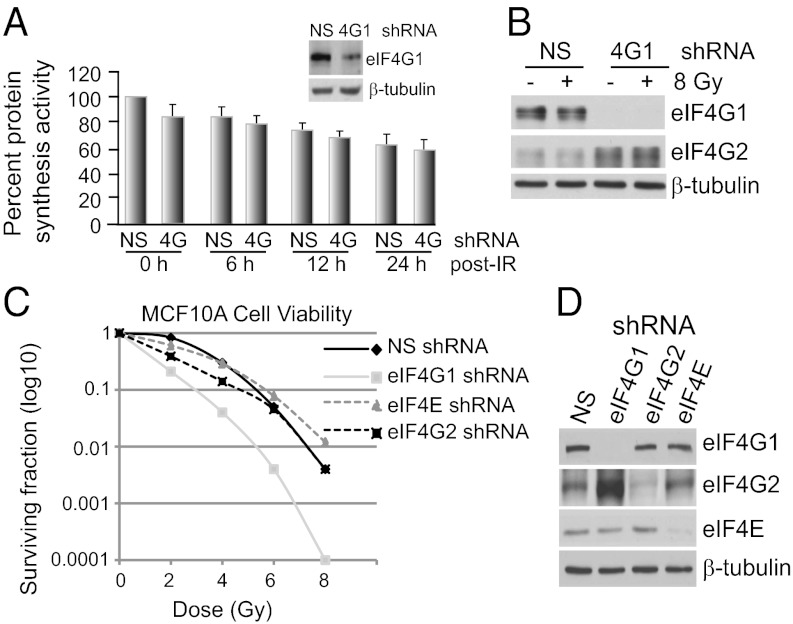
Reduced eIF4G1 expression only moderately down-regulates overall protein synthesis rates in mammalian and yeast cells (9, 12, 13). Accordingly, stable reduced expression of eIF4G1 by inducible shRNA silencing in MCF10A immortalized/partially transformed breast epithelial cells (Fig. 1A) or in MDA-MB-231, MCF-7, or HTB20 breast cancer cells (Fig. S1A), reduced protein synthesis rates by only 15–20% irrespective of exposure to a high dose (8 Gy) of IR and is, therefore, a common feature. Silencing of eIF4G1 resulted in a compensatory increased expression of eIF4G2 regardless of IR treatment (Fig. 1B). Cosilencing of eIF4G2 and eIF4G1 was shown previously not to further reduce protein synthesis (9). As described previously (2), exposure to IR does not result in eIF2α Ser51 phosphorylation and eIF2 inhibition (Fig. S2), unlike UVB irradiation (3).
Fig. 1.
Effect of eIF4G1, eIF4G2 and eIF4E silencing on overall protein synthesis and cell survival in IR-treated cells. (A) NS (non-silencing) and eIF4G1-silenced MCF10A cells were treated with 8 Gy IR or mock-treated. Protein synthesis rates determined by [35S]methionine incorporation, normalized to NS-shRNA nonirradiated control (0 h). SEMs are shown. Immunoblot is eIF4G1 in silenced and control cells. (B) Equal amounts of protein at 24 h post-IR were resolved by SDS/PAGE and immunoblotted with the indicated antibodies. (C) Clonogenic survival assays performed on MCF10A cells following IR, with stable shRNA to eIF4E, eIF4G1, eIF4G2, or an NS random sequence. Cells were plated overnight and irradiated, and colonies were scored after 2 wk (n = 3). (D) Immunoblot of equal protein amounts of lysates from C.
Cells reduced in eIF4G1 expression by stable shRNA silencing demonstrated up to a 50-fold decline in viability with increasing IR dose compared with nonsilenced (NS) control cells (Fig. 1 C and D). Surprisingly, silencing of eIF4E or eIF4G2 only partially increased sensitivity to DNA damage by IR compared with eIF4G1. Silencing of overexpressed eIF4G1 in other breast cancer cell lines also conferred significant sensitivity to IR-mediated DNA damage (Fig. S1C). We, therefore, pursued the mechanism for eIF4G1 promotion of cell survival.
Increased Expression of eIF4G1 Prevents Autophagy, Apoptosis, and Senescence Mediated by IR DNA Damage.
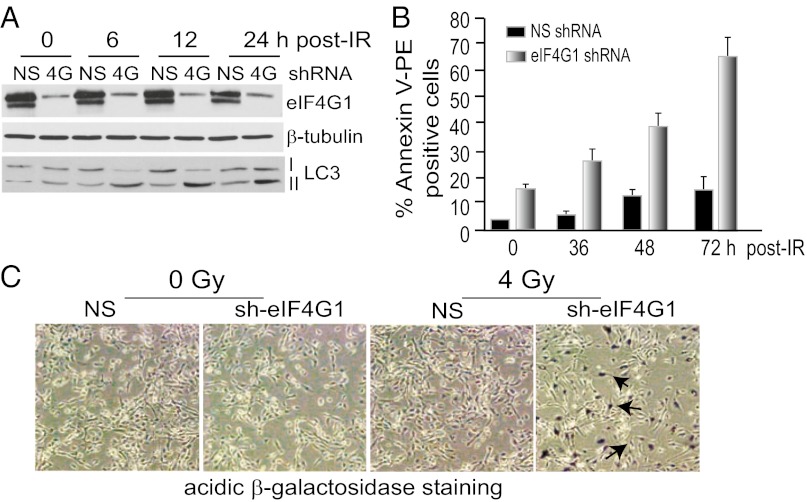
Genotoxic DNA damage can promote cell death by both autophagy and apoptosis (22). Lysates of irradiated cells were first examined for formation of LC3-II, a marker of autophagy that decorates autophagosomes. As early as 6 h post-IR, eIF4G1-silenced cells undergo autophagy, as evidenced by increased LC3-II (Fig. 2A), whereas NS cells showed only slight autophagy at 24 h. LC3 fused to GFP also decorated autophagosomes with strong punctate staining only in IR-treated cells, consistent with induction of autophagy (Fig. S3A), which in eIF4G1-silenced cells, was strongly and persistently increased even at 24 h.
Fig. 2.
Depletion of eIF4G1 sensitizes cells to IR-mediated autophagy, apoptosis, and senescence. (A) Equal amounts of protein lysates from MCF10A cells stably silenced for eIF4G1 or NS, treated with 8 Gy IR, resolved by SDS/PAGE, and immunoblotted with antibodies as shown. LC3-II is a marker for induction of autophagy. (B) FACS analysis conducted on 8 Gy–irradiated NS or eIF4G1-silenced MCF10A cells stained with PE-Annexin V, a marker of apoptosis. (C) Cells stably transfected with NS or eIF4G1 shRNA lentiviruses were subjected to 0 or 4 Gy irradiation, and β-gal assays for cellular senescence were performed. Representative phase-contrast photomicrographs of cells of triplicate studies shown at 100× magnification. Arrows mark β-gal staining. Assays were n = 3.
Silencing of eIF4G1 increased apoptosis even in the absence of IR, shown by a 20% increase in baseline Annexin V staining by FACS analysis, a marker of apoptosis (Fig. 2B). Post-IR, annexin staining increased by 40% at 48 h and ∼75% at 72 h. Cleaved caspase 3, another marker of apoptosis, was not detected in NS cells regardless of irradiation, whereas the two major cleavage forms were increased by eIF4G1 silencing, and further increased by IR (Fig. S3B). Increased expression of eIF4G1 therefore facilitates resistance to both apoptosis and autophagy following IR-mediated genotoxic DNA damage.
Prolonged DNA-damage is associated with terminal withdrawal from the cell cycle causing a senescence-like phenotype (SLP) (23) and cell death by both apoptosis and SLP [SLP demonstrated by acidic β-galactosidase (β-gal) (23)]. Both were clearly evident in irradiated eIF4G1-silenced cells and not in controls (Fig. 2C). eIF4G1 silencing alone increased β-gal activity to include 30% of cells, which was strongly increased in intensity and extent when combined with IR (>90% of cells). Increased p14ARF expression, another marker of SLP (23), was also strongly enhanced (Fig. S3C). We, therefore, investigated the association of the DDR with eIF4G1 levels, as eIF4G1 links autophagy and apoptosis.
Reduced Expression of eIF4G1 Delays and Prolongs Assembly of DDR Complex Proteins at DNA Double-Strand Breaks and Impairs G2/M Cell Cycle Arrest.
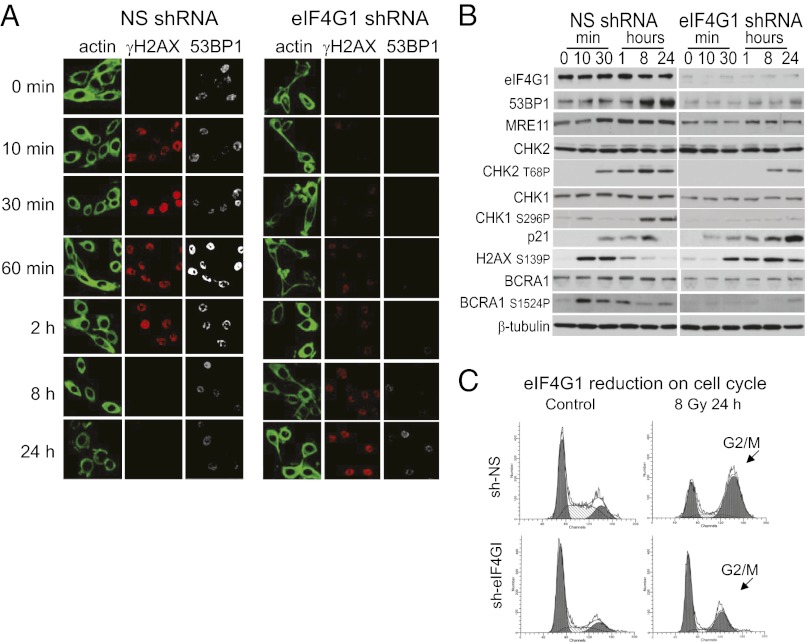
The abrogated expression of components of the DDR is reflected in overall delayed and mitigated signaling throughout the DDR response. Formation of IR-induced foci (IRIF) in eIF4G1-silenced cells at DNA double-strand break (DSB) sites [marked by DNA repair proteins p53 binding protein 1 (53BP1) and phosphorylated histone 2 AX protein (γH2AX)] was aberrantly delayed and prolonged compared with NS controls (Fig. 3A), with little expression of 53BP1 and reduced expression and delayed assembly of γH2AX. This is consistent with a failure to properly assemble and/or resolve DSB repair complexes (24). Phosphorylation of H2AX (γH2AX) generally occurs immediately following DNA damage (25). In eIF4G1-silenced and irradiated cells, H2AX phosphorylation was delayed and sustained without resolution, even at 24 h (Fig. 3 A and B), indicative of an inability to resolve IRIFs. An IR-induced increase in 53BP1 levels was also impaired in eIF4G1-silenced cells. With eIF4GI silencing, and consistent with deficient DSB repair, there was a sustained increase in p21 protein levels, reduced signaling (activating phosphorylation) of Check point kinase 1 (Chk1), Chk2, and breast cancer associated protein 1 (BRCA1) [mediated by ataxia telangiectasia mutated gene 1 (ATM), ataxia telangiectasia Rad related kinase (ATR), and Chk2], and reduced BRCA1 and Chk1 protein levels (Fig. 3B). There was also impaired G2/M cell cycle arrest (Fig. 3C). Collectively, these data indicate that there are multiple disruptions within the DDR pathway linked to reduced expression of eIF4G1 and enhanced radiosensitivity.
Fig. 3.
DNA-damage response signaling and G2/M arrest are impaired in eIF4G1-silenced cells. (A) Kinetics of IRIF in MCF10A cells stably expressing NS or eIF4G1 shRNAs assessed by confocal microscopy. Actin: direct immunofluorescence with FITC-phalloidin; γH2AX (H2AX-S139P): primary monoclonal antibody and TRITC anti-mouse secondary antibody; 53BP1: rabbit primary antibody coupled to Alexa Fluor 488 anti-rabbit secondary antibody (n = 3). (B) Equal protein amounts of total cell lysates analyzed by SDS/PAGE and immunoblot with antibodies for protein and phospho-specific proteins as shown. (C) Cell cycle analysis performed by propidium iodide staining of RNase A treated MCF10A NS and eIF4G1-silenced cells following IR. DNA content (x axis) was determined by FACS analysis.
eIF4G1 Promotes Selectively Increased Translation of DDR and Survival mRNAs in Response to IR-Mediated DNA Damage.
Studies previously linked IR resistance to maintenance of protein synthesis (2). We, therefore, determined whether the greatly increased radiosensitivity and depletion of the DDR response in eIF4G1-silenced cells is attributable to selectively reduced mRNA translation. Translation signature studies were carried out in NS and eIF4G1-silenced cells, with or without IR treatment, using gene expression array analysis of mRNAs unassociated with ribosomes (nontranslated), associated with light polyribosomes (one to three ribosomes, poorly translated), or well-translated and associated with “heavy” polyribosomes (four or more ribosomes) (Table 1). Reduction of eIF4G1 levels by RNA silencing produced a ∼20% reduction in the total level of polyribosomes (Fig. 4A), consistent with the reduction in overall protein synthesis.
Table 1.
mRNAs displaying strong dependence on elevated levels of eIF4G1 for increased abundance in heavy (≥four) polyribosomes in response to IR
| GO group and gene | Fold-increased heavy polyribosome abundance* |
| DNA damage and repair | |
| Mre11 | 3× |
| Rad50 | 3.5× |
| Rad51 | 3× |
| Rad52 | 0.5× |
| Rad17 | 2× |
| Rad54 | 4× |
| Rfc2 | 3× |
| Rfc3 | 1.3× |
| Rfc4 | 3× |
| Rfc5 | 3× |
| ATRIP | 2× |
| BRCA1 | 2.5× |
| BRCA2 | 2.5× |
| BRCA1 internal protein | 3.5× |
| PARP | 3× |
| 53BP1 | 3.5× |
| NBS1 | 1.5× |
| γH2AX | 2× |
| HUS1 | 1.5× |
| Effector kinases | |
| Chk1 | 2× |
| ATM | 5× |
| Growth arrest factors | |
| GADD45a | 6× |
| p53 | 3× |
| Survival factors | |
| XIAP† | 1.5× |
| Survivin | 8× |
| HIF1α† | 3× |
*Results show the average increase in mRNA abundance in heavy polyribosomes (≥four ribosomes) in nonsilencing vector-transformed cells at 12 h after 8 Gy IR compared with similarly treated cells silenced for eIF4G1.
†Not detectable on chip; determined by qRT-PCR. NBS1, Nibrin; HUS1, hydroxy urea sensitive protein 1.
Fig. 4.
eIF4E requirement of selectively translated DDR and survival mRNAs with eIF4G1 overexpression. (A) Cells silenced with NS or eIF4G1 shRNAs subjected to 8 Gy IR and fractionated by sucrose density gradient sedimentation into unbound mRNA, light (1–3) and heavy (≥4) ribosomes. (B) Immunoblot of NS and eIF4G1-silenced MCF10A cells at 12 h following 8 Gy IR from equal protein amounts of total cell lysates. (C) Relative rate of protein synthesis assessed by immunoprecipitation (IP) of de novo proteins shown following 2 h metabolic labeling with [35S]methionine with actinomycin D (20 µM) and MG132 (10 µM) at 2 h after 8 Gy IR. (D) Immunoblot of NS, eIF4E silenced or eIF4G1-silenced MCF10A cells following 8 Gy IR at 24 h, from equal protein amounts of total cell lysates. (E) Quantification of immunoblot studies shown in B. EMCV results derived from Renilla luciferase reporter studies (n = 3).
In response to IR, eIF4G1-dependent mRNA translation was found to be reprogrammed. As shown by shRNA silencing of eIF4G1, heavy polysomes were selectively depleted largely of mRNAs comprising gene ontology (GO) categories for DNA damage response and repair (36% of mRNAs), cell cycle inhibition/growth arrest and survival functions (51% of mRNAs), and bioenergetics (5% of mRNAs) (Table 1 and Table S1), when normalized to wild-type control mRNA levels with a ≥twofold enrichment requirement. Thus, ∼87% of mRNAs strongly dependent on increased expression of eIF4G1 in irradiated cells correspond to the central functions of survival, DNA damage and repair, and cell cycle control. Interestingly, there was a small group of mRNAs up-regulated by reduction of eIF4G1 levels with IR, corresponding primarily to functions involved in increased cell adhesion, cell–matrix interactions and cell–cell interactions (70% of mRNAs), as well as immune responsiveness (11%). Of the survival factor mRNAs, those strongly decreased in heavy polysomes with eIF4G1 silencing (Table 1), included Survivin (eightfold), a key antiapoptotic protein, and hypoxia inducible factor (HIF)1α(threefold), which activates the hypoxia response and promotes cell survival from IR-mediated DNA damage (26). Of growth arrest factors, two were prominent: p53 (threefold) and growth arrest and DNA damage protein 45a (GADD45a) (sixfold). GADD45a functions in cell cycle arrest and inhibition of DNA synthesis associated with DNA repair via regulatory interactions with cell division control protein 2 (cdc2), p21, and proliferating cell nuclear antigen (PCNA) (27) and is transcriptionally increased by p53 activation following DNA damage. A compelling list of DNA repair and response factor mRNAs were also increased in heavy polysomes in an IR and eIF4G1-dependent manner, particularly the kinases ATM (fivefold) and Chk1 (twofold), and other response factors such as meiotic recombination protein 11 (MRE-11), radiation induced protein (Rad)50/51/54, Rfc (replication factor c) transcription elongation proteins 2/4/5, PARP, 53BP1, and BRCA1/2. Consistent with the multifactor nature of DDR and survival pathways, enforced overexpression of survivin or HIF1α in eIF4G1-silenced cells by translation of transfected cDNA from the hepatitis C virus (HCV) IRES conferred only a slight survival advantage to cells at lower doses of IR and none at higher levels (Fig. S4A).
To verify these results, we performed quantitative (q)RT-PCR analysis of mRNAs in polyribosomes, coupled with immunoblot studies. Certain mRNAs such as 53BP1, HIF1α, BRCA-1, and GADD45a are strongly transcriptionally induced by DNA damage, whereas Survivin and X-linked inhibitor of apoptosis (XIAP) are increased by cellular transformation regardless of DNA damage (Fig. S4B). All mRNAs were strongly reduced in heavy polyribosomes with eIF4G1 silencing (normalized to total mRNA levels), consistent with their reduced protein levels, as shown for PARP, p53, XIAP, GADD45a, 53BP1, and ATR interacting protein (ATRIP) (Fig. 4B). As shown for 53BP1, XIAP and GADD45a by metabolic labeling with [35S]methionine, the reduced abundance of protein is a result of diminished de novo protein synthesis associated with decreased eIF4G1 expression (Fig. 4C).
eIF4G1 and eIF4E Dependence in Translation of Survival and DDR Pathway mRNAs.
Reduction of cap-dependent mRNA translation by 4E-BP1 sequestration of eIF4E, as facilitated by cellular stress (19), can promote IRES-mediated translation (6, 10, 18). Several DDR and survival mRNAs have suspected IRES elements, including GADD45a and HIF1α, and their translation may be instrumental in cell survival following stress (2, 6, 28). We, therefore, used bicistronic luciferase reporter mRNAs to test IRES activity. Silencing eIF4G1 blocked IRES activity 24 h following 8 Gy IR for the encephalomyocarditis virus (EMCV) (29), HIF1α, and GADD45a 5′ UTRs (Fig. S5). However, none of the other mRNAs selectively translated by high levels of eIF4G1 following IR DNA damage possessed IRES activity, as shown for XIAP, Survivin, and 53BP1 mRNA 5′ UTRs (Fig. S5). Thus, only a small number of mRNAs that are selectively translated by pathologically high levels of eIF4G1 contain IRES elements.
An IRES is only one means for conferring selective mRNA translation under conditions of limiting eIF4E availability, as found during stress conditions. Some mRNAs have been shown to require only low levels of eIF4E for their translation (30) and yet do not use an IRES or internal initiation (11). Mechanistically, these mRNAs share a requirement for higher levels of eIF4G1 but with little requirement for eIF4E (11, 31, 32). We, therefore, reduced the levels of eIF4E or eIF4G1 by shRNA silencing in MDA-MB-231 breast cancer cells in which eIF4G1 is strongly expressed and compared the expression of survival and DDR proteins. Silencing of eIF4G1, as shown earlier, strongly reduced the expression of 53BP1, GADD45a, survivin and XIAP proteins, regardless of the presence of IRES elements (Fig. 4 D and E), which remained impaired despite IR treatment and mRNA induction (Fig. S6). IRES-dependent mRNAs EMCV and GADD45a were fully independent of eIF4E but strongly (fivefold) reduced by lowering eIF4G1 levels (Fig. 4 D and E). β-Actin mRNA has a strong dependence on eIF4E-cap interaction for its translation (11) and was strongly (>20-fold) reduced with eIF4E silencing. In contrast, 53BP1 and XIAP showed only a slight (∼2-fold) reduction with eIF4E silencing but ∼10 fold reduction with eIF4G1 silencing. Survivin showed a greater dependence on eIF4E but still less than for eIF4G1, consistent with its reported stimulation by elevated levels of eIF4E (33). These results are consistent with a common strong requirement for high levels of eIF4G1 but a reduced requirement for eIF4E in the IR-induced selective translation of survival and DDR pathway mRNAs.
Examination of the non-IRES mRNAs for common computationally predicted features was not instructive. The average mammalian mRNA 5′ UTR is 100–200 nt long, ∼60% GC, with a composite stability of <−40 kcal/mol, with fewer than 10% of mRNAs containing a uORF (34). Although about half of the eIF4G1-dependent mRNAs had extremely long 5′ UTRs with extensive predicted stability, such as Chk1, RAD50, ATM, and BRCA1, the others were within the mean range for all predicted features (Table S2). Most of the mRNAs were predicted to contain one or more hairpin structures of somewhat higher than normal GC content (70 to >90%) that can impair translation when located near the cap (35), which constituted a trend but not a statistically significant correlation (P < 0.2) compared with randomly selected mRNAs that did not show increased dependence on high levels of eIF4G1.
Discussion
The increased expression of eIF4G1, which occurs in breast and some other human cancers, is a critical link between the response to DNA damage and expression of DNA repair and cell survival genes, coordinated at the level of translational control. The potential role of eIF4G1 phosphorylation and regulation by MAP kinase interacting kinase 2 (Mnk2) (36) remains to be studied.
Although autophagy is primarily a cytoprotective process and an inhibitor of apoptosis (37), in times of cellular stress such as IR exposure, it can contribute to cell death (22). Dual autophagy and apoptosis likely involve reduced expression/activation of p53 (38). Our studies are consistent with reduced p53 levels in induction of both autophagy and apoptosis, in that p53 translation requires higher levels of eIF4G1 following IR DNA damage. Moreover, reduced activity of mTOR by IR, a master regulator of autophagy, can also induce autophagy (9, 39).
It was reasonable to assume that IRES-mediated translation would serve as the major form of specialized translation initiation during DNA damage mediated by IR. Few of the DDR and survival mRNAs induced by IR-mediated DNA damage appear to use an IRES, including XIAP, reported previously to contain an IRES element (40). However, XIAP shares a common feature with all of the DDR/survival mRNAs that are selectively increased in translation following IR-mediated DNA damage: it has a relaxed requirement for eIF4E.
eIF4G1 mediates a transcriptional–translational feed-forward loop for HIF1α and survivin expression that promotes cell survival, in that Survivin transcription is stimulated by HIF1α, the mRNA of which shows strong eIF4GI-dependent IRES activity. Higher levels of eIF4G1 also establish a feed-forward pathway for increased cell survival through cell cycle arrest following DNA damage. GADD45a translation is strongly eIF4G1-dependent. Activation of GADD45a promotes JNK-mediated activating phosphorylation of p53 at S15, stimulating the DDR pathway, and simultaneously promoting strong G2/M cell cycle arrest (41); 53BP1 also promotes phosphorylation and activation of p53, in addition to driving non-homologous end joining (NHEJ) and inhibiting homologous DNA recombination and repair (42).
DNA damage mediated by both UVB (3) and high-energy (X-ray) IR (this study) both reprogram the protein synthesis machinery to promote selective mRNA translation but of largely nonoverlapping mRNAs. UVB radiation is of lower energy and introduces primarily pyrimidine dimers, stimulating nucleotide excision repair (43). UVB selects for mRNAs with 5′ UTRs containing uORFs, consistent with partial inactivation of eIF2 and transiently delayed initiation further downstream (3). In contrast, high-energy X irradiation is ionizing, producing free radicals and reactive oxygen species that lead to single strand and double-strand DNA breaks (44). IR primarily inhibits mTOR and down-regulates eIF4E availability (2).
The mechanisms for both stringent and relaxed requirements for eIF4E or eIF4G1 in mRNA translation initiation are still poorly understood, as are mechanisms that promote initiation by cellular IRES elements. eIF4G1 clearly has specific functions that do not overlap that of eIF4G2, which remain to be understood mechanistically. With that said, eIF4G1 and eIF4G2 clearly translate many, if not most, of the same mRNAs, but this does not extend to the efficient translation of survival and DDR mRNAs following IR-mediated DNA damage, consistent with results obtained in yeast with eIF4G1 silencing (12, 45). Our findings suggest an alternate means of translation initiation that might involve mRNA-specific recruitment of eIF4G1 that is associated with eIF4E but possibly involves recycling of eIF4G1 on the mRNA to deliver eIF3, 40S ribosome subunits, and associated factors and, therefore, is a requirement for the overexpression of eIF4G1 (Fig. S7).
Materials and Methods
Irradiation.
Cells were irradiated with a Varian Linac 2300 linear accelerator at room temperature for doses as shown. Mock treated cells were handled identically except for irradiation.
Retroviral and Lentivirus Expression Studies.
Cloning of expression vectors into the pBABE and pLKO.1 vectors, production of virus, and transformation of cells with vectors were described previously (46). Interfering shRNAs were delivered by transduction of cells with lentivirus shRNA expression vectors. Double-stranded shRNAs for cloning into lentivirus vectors were directed to either the 5′ or 3′ UTRs of mRNAs targeted for gene silencing.
Clonogenic Survival Assays.
Cells were seeded in triplicate into 10-cm plates at 102 to 105 cells/plate according to the test condition and different cell lines. For IR experiments, a single dose of γ irradiation was applied once cells were attached (24 h). Cells were cultured up to 14 d. Colonies were fixed in 70% (vol/vol) methanol and stained with crystal violet. All colonies of 50 cells or greater were counted in quantitative assays. The survival fraction (SF) was estimated according to the formula: SF = number of colonies formed in test condition / (number of cells seeded × plating efficiency of control group).
SA–β-Gal Activity.
Cells were stained for β-gal activity using the Senescence β-Galactosidase Staining Kit (Cell Signaling). Briefly, cells were seeded in six-well plates containing coverslips. After the appropriate exposure, the cells were washed twice with PBS, fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS, and washed twice in PBS. Cells were stained overnight in X-gal staining solution [1 mg/mL X-gal, 40 mM citric acid/sodium phosphate (pH 6.0), 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 150 mM NaCl, 2 mM MgCl2]. Staining was visualized and digitally photographed on an inverted Zeiss phase-contrast microscope.
Reporter DNA Constructs and Assays.
The pRemcvF reporter was generated by insertion of the EMCV IRES from pBMN-IRES-EGFP (G. Nolan, Stanford University, Stanford, CA) into the pRF plasmid (EcoRI-NcoI). The XIAP 5′-UTR reporter construct, pβgal/XIAP/CAT, was provided by M. Holcik (University of Ottawa, Canada) (47). The GADD45a 5′ UTR reporter construct, pRLG45aFL (5′UTR200-318), was provided by F. Chen (West Virginia University, Morgantown, VA) (28). Plasmid pEGFP-LC3 was provided by T. Yoshimori (National Institute for Basic Biology, Okazaki, Japan). Other 5′-UTR test constructs were prepared using the pRF vector. Details are available upon request. For transiently transfected constructs, reporter expression was assayed 24 and 72 h posttransfection. Luciferase assays were performed using the Dual Luciferase Reporter Assay System (Promega).
Statistical Analysis.
Statistical analyses used the two-tailed Student’s t test, with P < 0.05 taken as significance.
Gene Expression Data.
Gene expression microarray data have been deposited in minimum information about a microarray experiment (MIAME)-compliant format online in the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo).
Additional Methods.
Additional information can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank T. Venuto for completing certain studies. This work was supported by grants from the Breast Cancer Research Foundation, the Avon Foundation for Women, the Manhasset Women's Coalition Against Cancer, and the Department of Defense Breast Cancer Research Program (to R.J.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE41627).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203853109/-/DCSupplemental.
References
- 1.Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27(4):589–605. doi: 10.1038/emboj.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29(21):5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powley IR, et al. Translational reprogramming following UVB irradiation is mediated by DNA-PKcs and allows selective recruitment to the polysomes of mRNAs encoding DNA repair enzymes. Genes Dev. 2009;23(10):1207–1220. doi: 10.1101/gad.516509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 6.Holcik M, Sonenberg N, Korneluk RG. Internal ribosome initiation of translation and the control of cell death. Trends Genet. 2000;16(10):469–473. doi: 10.1016/s0168-9525(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 7.de Breyne S, Yu Y, Unbehaun A, Pestova TV, Hellen CU. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc Natl Acad Sci USA. 2009;106(23):9197–9202. doi: 10.1073/pnas.0900153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hundsdoerfer P, Thoma C, Hentze MW. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc Natl Acad Sci USA. 2005;102(38):13421–13426. doi: 10.1073/pnas.0506536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramírez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol. 2008;181(2):293–307. doi: 10.1083/jcb.200710215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braunstein S, et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28(3):501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Shatsky IN, Dmitriev SE, Terenin IM, Andreev DE. Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol Cells. 2010;30(4):285–293. doi: 10.1007/s10059-010-0149-1. [DOI] [PubMed] [Google Scholar]
- 12.Clarkson BK, Gilbert WV, Doudna JA. Functional overlap between eIF4G isoforms in Saccharomyces cerevisiae. PLoS ONE. 2010;5(2):e9114. doi: 10.1371/journal.pone.0009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park EH, Zhang F, Warringer J, Sunnerhagen P, Hinnebusch AG. Depletion of eIF4G from yeast cells narrows the range of translational efficiencies genome-wide. BMC Genomics. 2011;12:68. doi: 10.1186/1471-2164-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franklin-Dumont TM, Chatterjee C, Wasserman SA, Dinardo S. A novel eIF4G homolog, Off-schedule, couples translational control to meiosis and differentiation in Drosophila spermatocytes. Development. 2007;134(15):2851–2861. doi: 10.1242/dev.003517. [DOI] [PubMed] [Google Scholar]
- 15.Baker CC, Fuller MT. Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog. Development. 2007;134(15):2863–2869. doi: 10.1242/dev.003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imataka H, Olsen HS, Sonenberg N. A new translational regulator with homology to eukaryotic translation initiation factor 4G. EMBO J. 1997;16(4):817–825. doi: 10.1093/emboj/16.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marash L, et al. DAP5 promotes cap-independent translation of Bcl-2 and CDK1 to facilitate cell survival during mitosis. Mol Cell. 2008;30(4):447–459. doi: 10.1016/j.molcel.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Silvera D, et al. Inflammatory breast cancer pathogenesis mediated by translation initiation factor eIF4G overexpression and unorthodox protein synthesis. Nat Cell Biol. 2009;11(7):903–910. doi: 10.1038/ncb1900. [DOI] [PubMed] [Google Scholar]
- 19.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10(4):254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 20.Comtesse N, et al. Frequent overexpression of the genes FXR1, CLAPM1 and EIF4G located on amplicon 3q26-27 in squamous cell carcinoma of the lung. Int J Cancer. 2007;120(12):2538–2544. doi: 10.1002/ijc.22585. [DOI] [PubMed] [Google Scholar]
- 21.Albert JM, Kim KW, Cao C, Lu B. Targeting the Akt/mammalian target of rapamycin pathway for radiosensitization of breast cancer. Mol Cancer Ther. 2006;5(5):1183–1189. doi: 10.1158/1535-7163.MCT-05-0400. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg-Lerner A, Kimchi A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis. 2009;14(4):376–391. doi: 10.1007/s10495-008-0307-5. [DOI] [PubMed] [Google Scholar]
- 23.Chen JH, Hales CN, Ozanne SE. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007;35(22):7417–7428. doi: 10.1093/nar/gkm681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lavin MF, et al. ATM signaling and genomic stability in response to DNA damage. Mutat Res. 2005;569(1-2):123–132. doi: 10.1016/j.mrfmmm.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84(4):568–577. doi: 10.1139/o06-072. [DOI] [PubMed] [Google Scholar]
- 26.Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer. 2006;95(1):1–5. doi: 10.1038/sj.bjc.6603201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res. 2005;569(1-2):133–143. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Chang Q, et al. Incorporation of an internal ribosome entry site-dependent mechanism in arsenic-induced GADD45 alpha expression. Cancer Res. 2007;67(13):6146–6154. doi: 10.1158/0008-5472.CAN-07-0867. [DOI] [PubMed] [Google Scholar]
- 29.Kolupaeva VG, Pestova TV, Hellen CU, Shatsky IN. Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. J Biol Chem. 1998;273(29):18599–18604. doi: 10.1074/jbc.273.29.18599. [DOI] [PubMed] [Google Scholar]
- 30.Svitkin YV, et al. Eukaryotic translation initiation factor 4E availability controls the switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol Cell Biol. 2005;25(23):10556–10565. doi: 10.1128/MCB.25.23.10556-10565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali IK, McKendrick L, Morley SJ, Jackson RJ. Truncated initiation factor eIF4G lacking an eIF4E binding site can support capped mRNA translation. EMBO J. 2001;20(15):4233–4242. doi: 10.1093/emboj/20.15.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Gregorio E, Preiss T, Hentze MW. Translation driven by an eIF4G core domain in vivo. EMBO J. 1999;18(17):4865–4874. doi: 10.1093/emboj/18.17.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graff JR, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117(9):2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesole G, et al. Structural and functional features of eukaryotic mRNA untranslated regions. Gene. 2001;276(1-2):73–81. doi: 10.1016/s0378-1119(01)00674-6. [DOI] [PubMed] [Google Scholar]
- 35.Babendure JR, Babendure JL, Ding JH, Tsien RY. Control of mammalian translation by mRNA structure near caps. RNA. 2006;12(5):851–861. doi: 10.1261/rna.2309906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu SI, et al. MNK2 inhibits eIF4G activation through a pathway involving serine-arginine-rich protein kinase in skeletal muscle. Sci Signal. 2012;5(211):ra14. doi: 10.1126/scisignal.2002466. [DOI] [PubMed] [Google Scholar]
- 37.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290(5497):1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SB, Tong SY, Kim JJ, Um SJ, Park JS. Caspase-independent autophagic cytotoxicity in etoposide-treated CaSki cervical carcinoma cells. DNA Cell Biol. 2007;26(10):713–720. doi: 10.1089/dna.2007.0577. [DOI] [PubMed] [Google Scholar]
- 39.Cao C, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66(20):10040–10047. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 40.Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19(36):4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- 41.Hollander MC, Fornace AJ., Jr Genomic instability, centrosome amplification, cell cycle checkpoints and Gadd45a. Oncogene. 2002;21(40):6228–6233. doi: 10.1038/sj.onc.1205774. [DOI] [PubMed] [Google Scholar]
- 42.Wang B, Matsuoka S, Carpenter PB, Elledge SJ. 53BP1, a mediator of the DNA damage checkpoint. Science. 2002;298(5597):1435–1438. doi: 10.1126/science.1076182. [DOI] [PubMed] [Google Scholar]
- 43.Shuck SC, Short EA, Turchi JJ. Eukaryotic nucleotide excision repair: From understanding mechanisms to influencing biology. Cell Res. 2008;18(1):64–72. doi: 10.1038/cr.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cann KL, Hicks GG. 2007. Regulation of the cellular DNA double-strand break response. Biochem Cell Biol 85(6):663–674.
- 45.Gilbert WV, Zhou K, Butler TK, Doudna JA. Cap-independent translation is required for starvation-induced differentiation in yeast. Science. 2007;317(5842):1224–1227. doi: 10.1126/science.1144467. [DOI] [PubMed] [Google Scholar]
- 46.Connolly E, Braunstein S, Formenti S, Schneider RJ. Hypoxia inhibits protein synthesis through a 4E-BP1 and elongation factor 2 kinase pathway controlled by mTOR and uncoupled in breast cancer cells. Mol Cell Biol. 2006;26(10):3955–3965. doi: 10.1128/MCB.26.10.3955-3965.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1(3):190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.