Abstract
Human Langerhans cells (LCs) are highly efficient at priming cytolytic CD8+ T cells compared with dermal CD14+ dendritic cells (DCs). Here we show that dermal CD14+ DCs instead prime a fraction of naïve CD8+ T cells into cells sharing the properties of type 2 cytokine-secreting CD8+ T cells (TC2). Differential expression of the CD8-antagonist receptors on dermal CD14+ DCs, the Ig-like transcript (ILT) inhibitory receptors, explains the difference between the two types of DCs. Inhibition of CD8 function on LCs inhibited cytotoxic T lymphocytes (CTLs) and enhanced TC2 generation. In addition, blocking ILT2 or ILT4 on dermal CD14+ DCs enhanced the generation of CTLs and inhibited TC2 cytokine production. Lastly, addition of soluble ILT2 and ILT4 receptors inhibited CTL priming by LCs. Thus, ILT receptor expression explains the polarization of CD8+ T-cell responses by LCs vs. dermal CD14+ DCs.
Dendritic cells (DCs) are potent antigen-presenting cells (APCs) responsible for inducing Ag-specific immunity and tolerance (1). Several populations of DCs take up residence in different tissues and carry common as well as unique biological functions (2, 3). The healthy human skin displays at least three DC populations—Langerhans cells (LCs) in the epidermis and interstitial CD1a+ and CD14+ DCs in the dermis (4, 5). Each of the different skin DC subsets carries out specialized functions. CD14+ DCs that reside in the dermis are particularly efficient at controlling the differentiation of naïve B cells into plasma cells (6, 7). Epidermal LCs, conversely, are highly efficient at priming naïve CD8+ T cells into potent cytotoxic T lymphocytes (CTLs). Both DC subsets are equally efficient at inducing a secondary CD8+ T-cell response (7–9).
T lymphocytes are also composed of subsets. The CD4+ T-cell subsets, Th1 and Th2 (10), were the first to be characterized. Subsequently, other subsets have been identified and include Tregs, Th17, Tfh, and Th9 (11). CD8+ T-cell subsets have also been divided into subsets based on their cytokine production profile (12–14). Type 1 cytokine-producing T cells (TC1) express IFN-γ and TNF-α, whereas type 2 cytokine-producing T cells (TC2) produce IL-4, -5, and -13. Suppressor CD8+ T cells produce IL-10 and TGF-β and are characterized by low expression levels of CD8 and CD28 (15, 16). These findings are physiologically relevant because the balance between TC1 to TC2 and CD8+ suppressor T cell populations correlates with a patient’s ability to overcome tumor overgrowth or viral infections.
Studies with CD8-α or CD8-β gene-targeted mice have revealed that CD8 plays a key role in the maturation and function of MHC class I-restricted T lymphocytes (17, 18). Interacting with MHC in the immunological synapse, a specialized junction between a T lymphocyte and an APC, CD8 can enhance the affinity of T-cell receptor (TCR)–CD8 complexes for MHC–peptide (pMHC) complexes by ∼10-fold (19). The importance of CD8 is also demonstrated by the function of two inhibitory receptors, ILT2 and ILT4, that can inhibit the function of CD8 by competing for MHC class I binding and generate T regulatory cells (20–23).
The present study was designed to determine why dermal CD14+ DCs are less potent than epidermal DCs in priming effector CD8+ T-cell responses. We found that ILT2 and ILT4, which are expressed specifically on human dermal CD14+ DCs, inhibit the differentiation of naïve CD8+ T cells into cytotoxic T cells and instead promote the differentiation of TC2 cells. This finding suggests that expression of specific inhibitory receptors on dermal CD14+ DCs modulates their ability to prime and activate naïve CD8+ T cells.
Results
CD14+ DCs Prime CD8lo TC2 Cells.
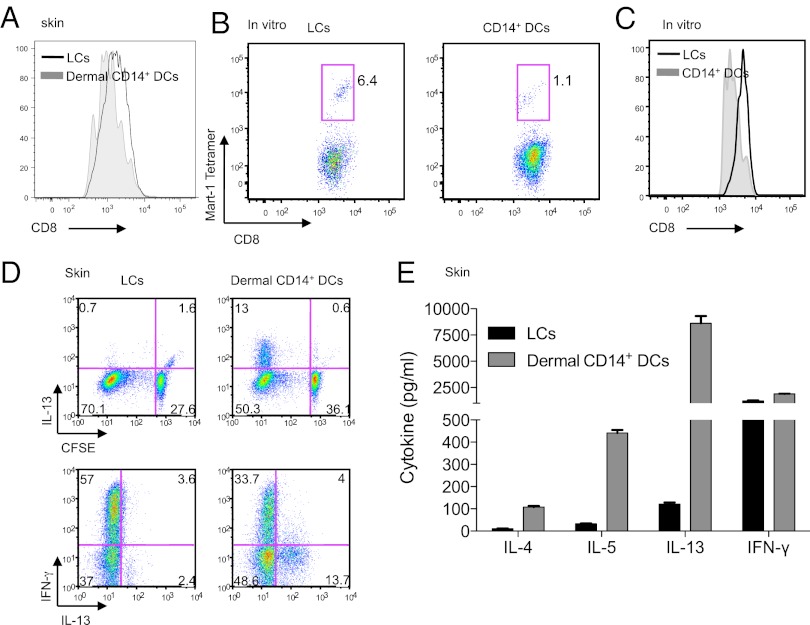
To analyze peptide-specific CD8+ T-cell priming, LCs and interstitial CD14+ DCs (CD14+ DCs) were generated by culturing HLA-A201+CD34+ hematopoietic progenitor cells (HPCs) in the presence of GM-CSF, Flt3-L, and TNF-α for 9 d. In vitro-cultured CD1a+CD14– LCs (in vitro LCs) and CD1a–CD14+ interstitial DCs (CD14+ DCs) were sorted, loaded with the HLA-A201–restricted melanoma peptide MART-1 (26-35), and cultured with autologous naïve CD8+ T cells for up to 10 d. Consistent with experiments using DCs isolated from skin (Fig. 1A), MART-1-specific CD8+ T cells expressed higher amounts of surface CD8 when primed by in vitro-generated LCs, compared with CD8+ T cells primed with CD14+ DCs (Fig. 1 B and C and Fig. S1A). In contrast, both of the in vitro-generated DC subsets loaded with the HLA-A201–restricted Flu Matrix peptide M1 Flu-MP (1 µM) (58-66), were equally efficient at expanding memory CD8+ T cells (7). In addition, the specific memory CD8+ T cells expressed comparable levels of surface CD8 (Fig. S1B).
Fig. 1.
Dermal CD14+ DCs prime CD8low TC2 cells. (A) Naïve CD8+ T cells were primed for 7 d by CD40L-activated skin isolated DC subsets: LCs (black) and dermal CD14+ DCs (gray). CFSElo cells were analyzed by flow cytometry for the expression of CD8 coreceptor. (B) In vitro CD40L-activated HLA-A201+ DC subsets were loaded with MART-1 peptide and used to prime naïve CD8+ T cells. Following two consecutive stimulations, cells were analyzed by flow cytometry for CD8 intensity and frequency of MART-1 tetramer-binding cells. (C) CD8 mean fluorescence intensity (MFI) expression of HLA-A201–MART-1 tetramer-binding CD8+ T cells, primed by in vitro CD40L-activated peptide-loaded autologous LCs and CD14+ DCs. Shown is 1 of 17 independent experiments. (D) CFSE-labeled naïve CD8+ T cells were primed for 7 d by CD40L-activated LCs and dermal CD14+ DCs. The cells were then expanded for 48 h with anti-CD3 and -CD28 mAbs and IL-2. Intracellular expression of IL-13 and IFN-γ was assessed by flow cytometry after additional 5-h stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin in the presence of monensin. Data are representative of three independent experiments. (E) CFSE-labeled naïve CD8+ T cells were primed for 7 d by skin CD40L-activated LCs and dermal CD14+ DCs. CFSElo cells were sorted at the end of the culture and restimulated for 48 h with anti-CD3 and -CD28 mAbs. The cytokines IL-13, -5, -4, and IFN-γ were measured in the culture supernatant by using Luminex.
To analyze T-cell cytokine secretion patterns, naïve CD8+ T cells were carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled and cultured with each of the skin DC subsets for 7 d. Flow cytometry analysis revealed that dermal CD14+ DCs, but not skin LCs, induced the generation of CFSElo CD8+ T cells that expressed IL-13 (13% vs. 0.7%). Both LCs and dermal CD14+ DCs induced CD8+ T cells to produce IFN-γ (Fig. 1D). To assess the secretion of cytokines using multiplex assays, CFSElo cells were sorted and stimulated with anti-CD3 and -CD28 mAbs for 48 h. In concordance with the flow cytometry data, CD8+ T cells primed by dermal CD14+ DCs secreted IL-13, as well as other type 2-associated cytokines (IL-4, -5). Both LCs and dermal CD14+ DCs induced CD8+ T cells that secreted IFN-γ (Fig. 1 D and E). Thus, dermal CD14+ DCs, but not LCs, induce a fraction of naïve CD8+ T cells to differentiate into TC2 cells.
Anti-CD8 mAb Inhibits Priming of Antigen-Specific CD8+ T Cells.
To test the role of CD8, we added anti-CD8 antibody to a mixed lymphocyte reaction (MLR) of skin LCs and naïve allogeneic T cells (Fig. S2A). Addition of anti-CD8, but not a control antibody, resulted in ∼80% inhibition of proliferation as assessed by [3H]thymidine incorporation (78% ± 12% reduction; n = 3; P < 0.0001). In MLRs performed with in vitro LCs and allogeneic naïve CD4+ and CD8+ T cells, addition of anti-CD8 also inhibited the proliferation of CD8+ T cells as assessed by CFSE dilution (16.6% vs. 56.7% CFSElo; Fig. S2B Upper) but did not affect proliferation of CD4+ T cells (75.7% vs. 74% CFSElo; Fig. S2B Lower).
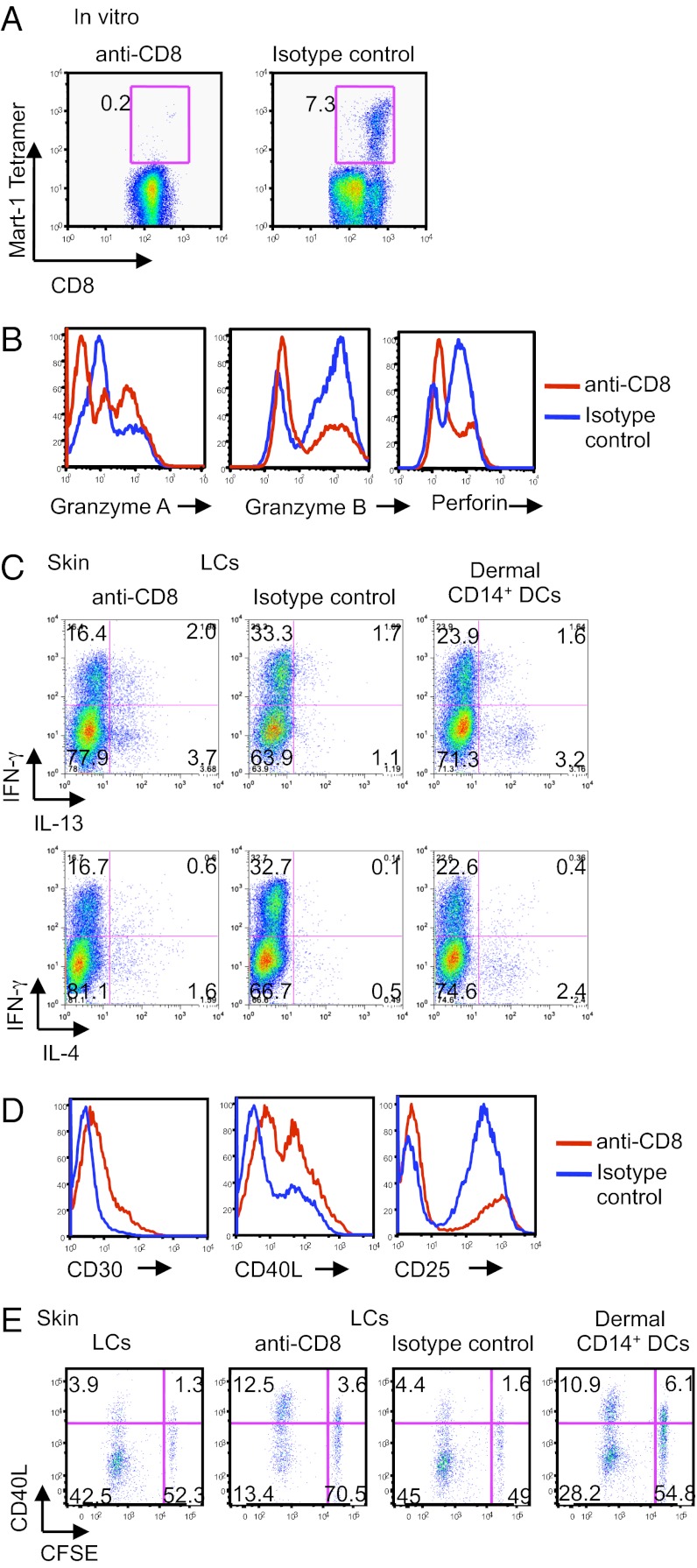
The anti-CD8 mAb also blocked the expansion of autologous MART-1-specific CD8+ T cells by MART-1-pulsed in vitro LCs (Fig. 2A). However, the addition of anti-CD8 mAb at concentrations as high as 2.5 µg/mL did not inhibit the proliferation of memory alloreactive CD8+ T cells induced by the in vitro LCs (Fig. S2C), or of autologous Flu-MP–specific CD8+ T cells (Fig. S2D). Together, these data demonstrate a critical role for CD8 in DC-mediated priming but not in the stimulation of memory CD8+ T cells.
Fig. 2.
Blocking CD8 inhibits CD8+ T cell priming and leads to the generation of TC2 cells. (A) Plots show the frequency of HLA-A201–MART-1–specific CD8+ T cells primed for 9 d by peptide-loaded in vitro CD40L-activated LCs and with 1 µg/mL anti-CD8 mAb or an isotype-matched control. Data are representative of five independent experiments. (B) Naïve CD8+ T cells were primed by allogeneic in vitro CD40L-activated LCs and with anti-CD8 mAb or an isotype-matched control for 7 d. The CD8+ T cells were analyzed by flow cytometry for the expression of intracellular granzymes A and B and perforin. (C) Allogeneic CFSE-labeled naïve CD8+ T cells were primed for 7 d by skin CD40L-activated DC subsets: LCs or dermal CD14+ DCs and with anti-CD8 or an isotype-matched control. The cells were then further expanded for 48 h with a combination of plate-bound anti-CD3 mAb, a soluble anti-CD28 mAb, and IL-2. Intracellular expression of IFN-γ, IL-4, and -13 was assessed by flow cytometry after additional 5-h stimulation with PMA and ionomycin in the presence of monensin. Plots show the production of the above cytokines by each of the CD8+ T-cell cultures. (D) Similar to B, cells were analyzed for the expression of CD30, CD40L, and CD25. (E) Skin LCs or dermal CD14+ DCs were activated with CD40L and cultured with CFSE-labeled naïve CD8+ T cells for 8 d. Anti-CD8 mAb or an isotype-matched control was added to the cultures as indicated. Cells were assessed for the dilution of CFSE dye and the expression of intracellular CD40L after overnight expansion with anti-CD3 and -CD28 mAbs and additional 5-h stimulation with PMA and ionomycin in the presence of monensin. Data are representative of two independent experiments.
CD8+ T Cells Primed with Anti-CD8 Differentiate to TC2 Cells.
Because the anti-CD8 mAb only partly blocked CD8+ T-cell proliferation, we wondered whether the remaining proliferating cells might show skewed differentiation. Indeed, addition of anti-CD8 mAb during priming of CD8+ T cells with allogeneic LCs yielded cells that expressed lower levels of the effector molecules, granzymes and perforin (Fig. 2B). To analyze cytokine secretion patterns using multiplexed cytokine assays, CFSE-labeled naïve CD8+ T cells were cocultured with in vitro LCs with or without anti-CD8 mAb for 7 d. CFSElo cells were sorted and stimulated with anti-CD3 and -CD28 mAbs for 48 h. Indeed, cells primed with anti-CD8 mAb produced higher amounts of IL-13, -5, -4, and -10, but similar levels of IL-2 and IFN-γ, compared with control cultures (Fig. S3); CD8+ T cells primed by skin LCs with anti-CD8 mAb, contained a higher frequency of IL-13–producing [Fig. 2C Left; 5.7 (2+3.7) vs. 2.8 (1.7+1.1)] and IL-4–producing cells [Fig. 2C Left; 2.2 (0.6+1.6) vs. 0.6 (0.1+0.5)] than did control cultures (Fig. 2C Center). Culturing LCs with naïve CD8+ T cells in the presence of anti-CD8 yielded as many IL-13–producing and IL-4–producing CD8+ T cells as did cultures of dermal CD14+ DCs with naïve CD8+ T cells [Fig. 2C Right; 4.8 (1.6+3.2) and 2.8 (0.4+2.4), respectively]. Consistent with TC2 cells (12, 24), the CD8+ T cells cultured with anti-CD8 mAb expressed higher levels of surface CD30 and CD40L, but lower levels of CD25 (Fig. 2D), compared with isotype controls. Furthermore, as observed by the dilution of CFSE dye and the expression level of CD40L (Fig. 2E), CD8+ T cells cultured with dermal CD14+ DCs resembled CD8+ T cells primed by LCs and anti-CD8 mAb, compared with control cultures (10.9% and 12.5% vs. 3.9% and 4.4%, respectively). Collectively, the data indicate that blocking CD8 on LCs converts the priming of naïve CD8+ T cells from cytolytic T cells to TC2 cells.
Dermal CD14+ DCs, but Not LCs, Express ILT Receptors.
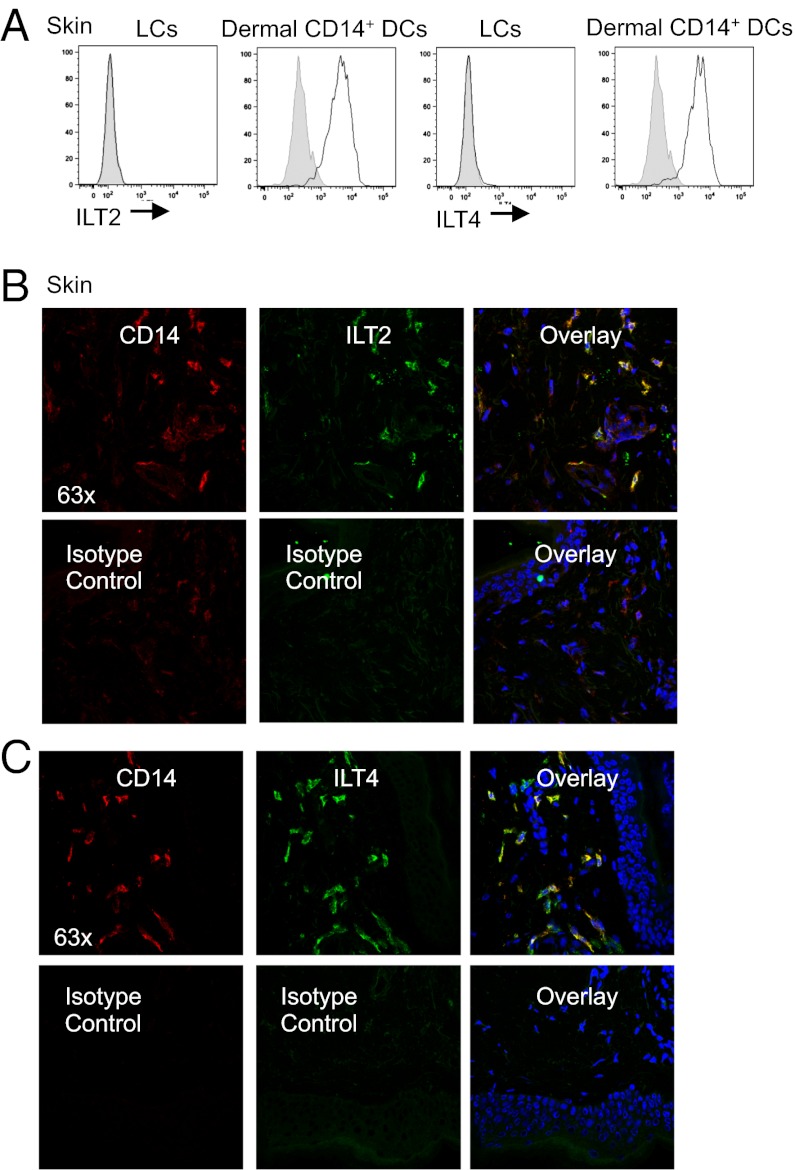
The results with anti-CD8 mAbs led us to hypothesize that dermal CD14+ DCs, but not LCs, might express CD8 antagonists such as the ILT receptors (21). ILT2 and ILT4 represent such candidates because they bind both classical and nonclassical MHC class I and compete with CD8 for its binding to MHC. Microarray analysis of freshly purified skin DCs indeed revealed that dermal CD14+ DCs, but not LCs, express ILT2 and ILT4, as well as ILT3 and ILT5 (Fig. S4A). Flow cytometric analysis of DCs that migrated out of skin confirmed that dermal CD14+ DCs express ILT2, ILT4, and ILT5 (Fig. 3A and Fig. S4B). Immunofluorescence staining of frozen skin sections also confirmed expression of ILT2 and ILT4 on dermal CD14+ DCs (Fig. 3 B and C). Activation of purified DC subsets through CD40, with or without Toll-like receptor (TLR) agonists, did not alter their ILT receptor expression (Fig. S4C). Thus, the expression of ILT2, ILT4, and ILT5 is restricted to dermal CD14+ DC.
Fig. 3.
Expression analysis of the ILT family receptors by the skin DC subsets. (A) Flow cytometry analysis of the ILT2 and ILT4 receptors on the surface of LCs and dermal CD14+ DCs (black histogram); gray histogram represents isotype control. Data are representative of at least four independent experiments. (B and C) Immunofluorescence staining of ILT2 (B) and ILT4 (C) receptors on sections of human dermis. ILT is visualized in green, CD14 in red, and cell nuclei in blue.
Soluble ILT4 Inhibit the Generation of Polyfunctional CD8+ T Cells by LCs.
To establish that ILT2 and ILT4 might actually prevent CD14+ DCs from generating polyfunctional T cells, soluble forms of ILT2 and ILT4 proteins (extracellular domains fused to an Fc fragment) were generated and then added to cocultures of in vitro LCs (that do not express ILTs) and naïve CD8+ T cells. As shown in Fig. S5A, naïve CD8+ T cells exposed to autologous in vitro LCs differentiated into cells expressing granzymes A and B. In contrast, addition of soluble ILT4, and to a lower extent ILT2, yielded T cells expressing low levels of granzymes, whereas an irrelevant Fc fusion protein had no effect (Fig. S5A). Addition of the ILT4, but not the ILT2, fusion protein, resulted in an increased frequency of IL-4–producing CD8+ T cells (Fig. S5A). Similar results were also observed when the ILT2 and ILT4 fusion proteins were added to cocultures of skin LCs and naïve CD8 T cells (Fig. S5 B and C). Concomitantly, the ILT4 fusion protein induced increased production of IL-13 by the LC-primed CD8+ T cells (520 vs. 173 pg/mL; Fig. S5D) and a decrease in the frequency of cells producing both TNF-α and IFN-γ (27% vs. 45%) (Fig. S5E). Thus, soluble ILT2- and ILT4-Fc inhibited the development and generation of effector CD8+ T cells induced by LCs.
Limited Amounts of ILT2 and ILT4 Receptor Expression Enable Dermal CD14+ DCs to Generate Polyfunctional CD8+ T Cells.
We next generated polyclonal Abs against ILT molecules to assess whether blocking their function would enhance the generation of effector CD8+ T cells by dermal CD14+ DCs. To this end, mice were immunized with soluble ILT2 and ILT4 proteins. Dermal CD14+ DCs were cultured with naïve CD8+ T cells, and anti-ILT2 or -ILT4 sera were added. Serum from mice immunized with only the Fc portion of the ILT molecule served as a control. After 9 d, the cultured cells were stimulated with phorbol 12-myristate 13-acetate (PMA) and ionomycin for 5 h. In line with our previous study, both skin LCs (Fig. S6A, right column) and dermal CD14+ DCs (Fig. S6A, left column) induced naïve CD8+ T cells to proliferate and secrete IFN-γ, whereas dermal CD14+ DCs primed CD8+ T cells to produce IL-13 (Fig. S6A). Blocking ILT4 during priming of naïve CD8+ T cells with dermal CD14+ DCs using polyclonal Abs resulted in a reduction (7.4–1.5%) of IL-13-producing CD8+ T cells (Fig. S6A). In addition, blocking ILT4 enhanced the expression of granzyme B by CFSElo cells (Fig. S6B, blue histogram) compared with cocultures performed with control serum or no serum at all (Fig. S6B, orange or cyan histogram). Addition of polyclonal ILT2 Abs or control serum to cocultures of dermal CD14+ DCs and naïve CD8+ T cells altered neither the level of proliferation nor the production of cytokines (Fig. S6A). However, we did find that a commercially available ILT2 mAb (clone 3F1; Fig. S6C) was able to mildly enhance the priming of polyfunctional CD8+ T cells, but this result was not statistically significant.
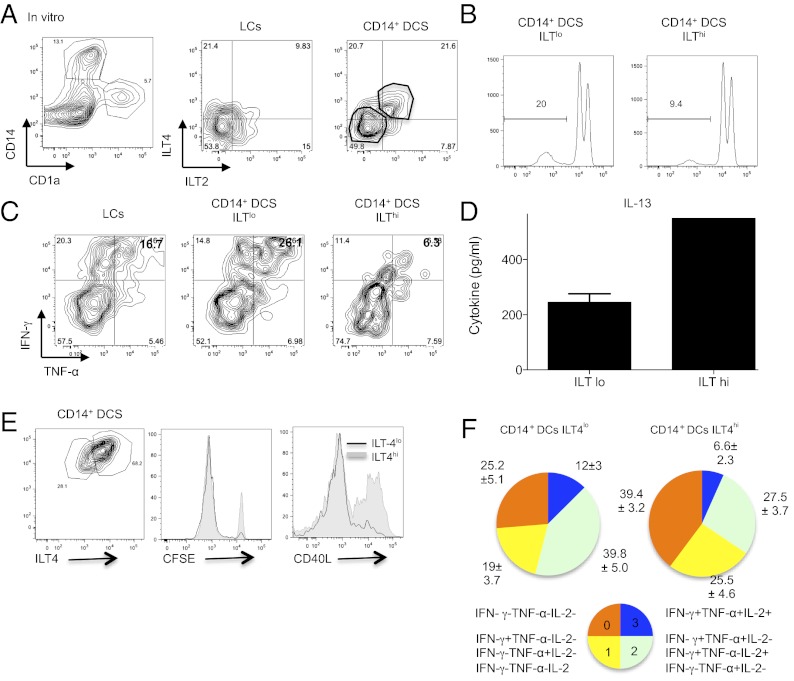
We next took advantage of the partial expression of ILT2 and ILT4 on in vitro-generated CD34+-differentiated DCs. Thus, we used cell sorting to separate dermal CD14+ DCs based on high and low ILT receptor expression (Fig. 4A). Consistent with our previous blocking experiments, low amounts of ILT expression on the CD14+ DCs allowed greater naïve CD8+ T-cell proliferation (20% vs. 9.4%) and effector cytokine (IFN-γ and TNF-α) production (Fig. 4 B and C, respectively). In addition, CD8+ T cells primed by ILTlo DCs produced lower amounts of IL-13 compared with ILThi DCs-primed T cells (Fig. 4D). Interestingly, separating the cells based on ILT4 expression alone was sufficient to drive greater CTL response, as observed by enhanced proliferation, reduced CD40L expression (Fig. 4E), and increased percentage of cells coexpressing two (green) or three (blue) effector cytokines (Fig. 4F).
Fig. 4.
ILT2 and ILT4 receptor density controls the generation of effector CD8+ T cells by CD14+ DCs. (A) CD34+-differentiated DCs were labeled with CD1a (LCs) and CD14 (CD14+ DCs) (Left), and each subset was analyzed for ILT2 or ILT4 expression by flow cytometry (Right). (B) In vitro CD14+ DCs expressing low or high amounts of ILT receptors (ILTlo or ILThi) were activated with CD40L and cultured with allogeneic CFSE-labeled naïve CD8+ T cells for 10 d. Histogram shows the fraction of cells that diluted CFSE dye at the end of the coculture. (C) As in B, primed CD8+ T cells were expanded with anti-CD3 and -CD28 mAbs and IL-2 for 48 h and assessed following 5 h of reactivation with PMA and ionomycin for the intracellular expression of IFN-γ and TNF-α by flow cytometry. In vitro LCs were used as a control. (D) CD8+ T cells primed by ILTlo or ILThi in vitro CD14+ DCs were sorted as CFSEloCD11c−CD4− cells, plated at 1.5 × 105 cells per mL, and activated for 48 h with anti-CD3 and -CD28 mAbs. IL-13 was measured in the culture supernatant by using Luminex. Graph shows mean ± SEM (n = 2). (E, Left) Plot shows gating strategy for separating ILT4lo- or ILT4hi-expressing CD14+ DCs. (Right) CD40-activated ILT4lo- or ILT4hi CD14+ DCs were cultured with CFSE-labeled naïve CD8+ T cells for 8 d. Primed cells were expanded with anti-CD3 and -CD28 mAbs and IL-2 and analyzed for the dilution of CFSE dye and intracellular expression of CD40L. (F) Similar to E, after 9 d of culture, primed CD8+ T cells were assessed following 5 h of reactivation with PMA and ionomycin for the intracellular expression of IFN-γ, TNF-α, and IL-2. Graphs show the relative populations based on their cytokine expression profile.
Anti-ILT4 Enhances Generation of Polyfunctional CD8+ T Cells.
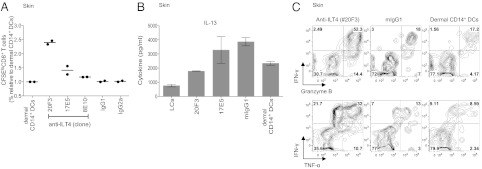
To further assess the biological significance of ILT4 in priming CTL responses, we selected and tested three purified ILT4-specific mAbs for their ability to enhance naïve CD8+ T-cell proliferation and effector molecule production (Fig. 5). As shown in Fig. 5A, clone 20F3 was able to induce robust CD8+ T-cell proliferation, compared with clones 17E5 and 8E10, which showed only moderate or no effect compared with control mAb. Moreover CD8+ T cells primed by dermal CD14+ DCs and the anti-ILT4 mAb clone 20F3 produced substantially lower amounts of IL13 compared with control cultures (Fig. 5B). Finally, in agreement with our other data, blocking ILT4 during primary CD8+ T-cell responses promoted the expansion of granzyme B (Fig. 5C Upper; 52.4% vs. 18% and 17.2%), as well as IFN-γ and TNF-α (Fig. 5C Lower; 32% vs. 13% vs. 8.6%) producing cells. Together, antagonists of ILT2 and ILT4 increase the capacity of dermal CD14+ DCs to generate polyfunctional effector CD8+ T cells.
Fig. 5.
Anti-ILT4 enhances the generation of polyfunctional CD8+ T cells. (A) Dermal CD14+ DCs were cultured at a ratio of 1:40 with allogeneic CFSE-labeled naïve CD8+ T cells and 20 µg/mL indicated anti-ILT4 mAb (clones 20F3, 17E5, and 8E10) or an isotype-matched control. After 9 d, the cells were assessed for their proliferation based on the dilution of CFSE dye. Graph shows mean results of two independent experiments. (B) CD8+ T cells primed for 11 d by dermal CD14+ DCs in the presence of indicated anti-ILT4 mAb or an isotype-matched control were sorted as CFSEloCD11c−CD4− cells and cultured at 1.5 × 105 cells per mL. IL-13 production was measured in the culture supernatant by using Luminex following 48-h stimulation with anti-CD3 and -CD28 mAbs. (C) CD8+ T cells primed for 9 d by dermal CD14+ DCs in the presence of anti-ILT4 (20F3) were expanded with anti-CD3 and -CD28 mAbs and IL-2 for 48 h and assessed following 5 h of reactivation with PMA and ionomycin for the intracellular expression of IFN-γ, TNF-α, and granzyme B by flow cytometry.
Discussion
DCs are composed of several subsets endowed with distinct immunological functions. The molecular basis for these functional differences remains poorly understood. The present study was initiated to define the mechanisms conferring LCs with a stronger potency for priming CD8+ T-cell responses than CD14+ DCs. Indeed, whereas LCs effectively prime CD8high polyfunctional T cells, naïve CD8+ T cells proliferating in response to dermal CD14+ DCs display lower levels of surface CD8 and produce type 2-associated cytokines (IL-4, -5, and -13). Indeed, the homogenous levels of CD8 displayed by blood-isolated naïve T cells suggests that dermal CD14+ DCs can specifically induce low CD8 expression or induce CD8 down-regulation. Our observation for the role of dermal CD14+ DCs in preferentially driving the polarization of naïve CD8+ T cells into type 2-cytokine–secreting cells is further supported by mouse studies demonstrating a role for dermal DCs in biasing toward Th2 responses (25, 26).
Similar to our previous study (7), we used DC subsets that were isolated from skin as well as their in vitro counterparts that were differentiated from CD34+ HPCs. These two systems complemented each other, because using in vitro-differentiated permits us to study not only allogeneic, but also autologous T-cell responses induced by the various DC subsets. By using allogeneic responses, our study showed that limiting CD8 density on naïve T cells using an anti-CD8 mAb during coculture with LCs inhibited T-cell proliferation and altered the quality of the response. This process resulted in polarization toward TC2 phenotypes associated with lower frequency of granzyme- and perforin-expressing cells, lower surface CD8 and CD25, and high CD30 and CD40L expression (12, 24, 27).
These experiments suggest that dermal CD14+ DCs might express a cell-intrinsic receptor that interferes with CD8 function. Microarray analysis revealed that dermal CD14+ DCs expressed ILT4 and ILT2, known inhibitors of CD8 binding to MHC class I (21, 22, 28). Indeed, structural modeling demonstrated that ILT2 or ILT4 sterically interfere with CD8-α in binding to MHC class I (Fig. S7). ILT receptors are known to impair natural killer (NK) cell function, as well as to promote the generation of regulatory CD8+ and CD4+ T cells (29), and the mouse homolog PIR-B was recently shown to regulate the suppressive function of myeloid-derived suppressor cells (30). Using soluble-ILT fusion proteins and ILT antibodies that we generated, we confirmed that ILT4 and, to a lower extent, ILT2 function to regulate the generation of effector CD8+ T cells by dermal CD14+ DCs.
Although we used several different approaches to study the role of ILT2—including using a soluble ILT2 receptor, ILT2 blocking antibodies, and the sorting of ILT2 high- and low-expressing DCs—our results were never as robust as with the ILT4 blocking reagents. We also tried generating lentiviruses to selectively knock down these receptors in the DCs, but, unfortunately, we were not able to get significant knockdown in our cells. We are not sure why the effect seen with the ILT2 is less robust than with the ILT4. We suspect that it could be due to differences between our reagents; it also suggests that ILT2 and ILT4 may have distinct functions.
Although ILT3 can also inhibit CD8+ T-cell responses and promote the induction of suppressor CD8+ T cells (31, 32), dermal CD14+ DCs expressed only low levels of ILT3 transcripts. We also could not detect ILT3 expression either at steady state or after microbial stimulation. Addition of soluble ILT3–Fc fusion protein to cocultures of LCs and naïve CD8+ T cells did not result in any significant effects on effector CD8+ T cell priming.
In contrast, ILT5 was detected at high levels on the surface of purified CD14+ DCs and in situ in skin sections. Its role in dermal CD14+ DCs remains to be established. Other inhibitory receptors—such as the nonclassical MHC, HLA-G, that acts in conjunction with ILT4—can promote the generation of IL-10–producing suppressor CD8low T cells (33). Only primary, but not recall, responses against viral or allogeneic antigens, however, were sensitive to the inhibitory effect of anti-CD8. Differences in the interaction of Lck with CD8 within naïve and memory CD8+ T cells could explain this finding (34); indeed, whereas Lck associates with CD8 at low stoichiometry in naïve cells, the level of association is much higher in memory and effector cells. Alternatively, it has been proposed that CD8 molecules interact with pMHC in two distinct orientations, potentially explaining why memory cells, as opposed to naïve cells, were insensitive to anti-CD8 blockage (35). Several studies indicate a unique role for CD8 in fine-tuning the activation threshold of T cells (36, 37) and in compensating for lower numbers of antigen-specific pMHC complexes (38). Our study supports the importance of CD8 accessibility during primary responses of naïve CD8+ T cells.
The biological role of TC2 cells remains mostly unknown, although they might have a regulatory function (14). Patient studies show an expansion of TC2 populations in various disease conditions including cancer (39–41) and chronic viral infections (27, 42). In all of these cases, TC2 accumulation was associated with disease pathogenesis. In addition, CD40L-producing as well as IL-4–producing CD8+ T cells have a B-cell–helper function (27, 43, 44), which also might be consistent with a role for CD14+ DCs in controlling humoral responses (6, 7). Blocking CD8 may have potential for clinical applications, such as controlling the pathogenic effect of allogeneic CD8+ T cells in allograft rejection. This approach, as our study suggests, could still allow for productive recall responses, as contrast to general immunosuppressive therapies that increase susceptibility to viral infections.
In summary, we demonstrated that CD8 modulation during a primary response is a mechanism to regulate the balance between type 1 effector and type 2 responses. Our data suggest that by expressing the inhibitory receptors, ILT2 and ILT4, dermal CD14+ DCs block efficient CTL differentiation and, instead, enhance the generation of TC2 cells. Viruses such as CMV that express class-I–like proteins (45) might use this escape mechanism to inhibit induction of viral-specific CD8+ T cells and thus promote TC2 cell responses that have only a limited ability to clear the infected or malignant cells. Similarly, tumors might up-regulate surface ILT2 or ILT4 to attenuate the production of CTLs. Importantly, strategies to block ILT expression on DCs may be useful to augment DC function to enhance immune responses to chronic viral infections and cancer. Alternatively, mobilizing dermal CD14+ DCs may be a useful approach to attenuate effector responses to combat transplant rejection and chronic inflammatory diseases.
Materials and Methods
DC Subsets.
CD34+-differentiated DCs were generated in vitro from CD34+ HPCs. Epidermal LCs (skin LCs) and dermal CD14+ DCs were purified from normal human skin specimens as described (7). All protocols were reviewed and approved by the Baylor Research Institute or Washington University Institutional Review Board.
Monoclonal Antibodies.
Anti-ILT2 HP-F1, anti-ILT3 ZM3.8, anti-ILT4 42D1 (Immunotech), and ILT5 MKT5.7H5.1 (eBiosciences) were used to evaluate expression on DCs by flow cytometry. Anti-ILT2 (BioLegend) and anti-ILT4 [Baylor Institute for Immunology Research (BIIR) 9B11] were used to evaluate expression on human skin sections by using immunofluorescence. Anti-ILT4 mAbs (BIIR 20F3, 17E5, and 8E10) were used in functional assays.
See SI Materials and Methods for information on ILT staining, DC/T-cell cocultures, cloning of ILT–Fc molecules, the generation of ILT-specific mAbs, polyclonal anti-ILT serum binding ELISA, and ILT–CD8–MHC class I structure analysis.
Supplementary Material
Acknowledgments
We thank Elizabeth Trahan, Sebastien Coquery, Jennifer Shay, Jill Plants, Olivier Agouna-Deciat, Peter Klucar, Amy O’Bar, Dr. Dorothee Duluc, Florentina Marches, and Dr. Chun I. Yu (BIIR) and Drs. Marina Cella and Erica Maria Lantelme (Department of Pathology and Immunology, Washington University School of Medicine) for their help; Dr. Carson Harrod (BIIR); Jennifer Duncan, and the surgeons and nurses at the Baylor Medical Centre Plastic Surgery Department; Dr. Thomas Tung (Department of Surgery, Washington University School of Medicine) and surgeons, James Yip, nurses, and staff at the Barnes Jewish Hospital; Washington University School of Medicine for providing access to skin samples; and Dr. Michael Ramsay for continuous support. This work was supported by the Baylor Health Care System Foundation; National Institutes of Health Grants R0-1 CA78846, R0-1 CA85540, P0-1 CA84512, and U-19 AI-57234 (to J.B.); 5R01HL097805 (to M.C.); and the Department of Pathology and Immunology, Washington University School of Medicine (E.K.). J.B. held the W. W. Caruth, Jr. Chair for Transplantation Immunology Research.
Footnotes
Conflict of interest statement: J.B., K.A.P., G.Z., and E.K. are co-inventors on a patent filing related to this work.
This article is a PNAS Direct Submission. R.W.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1205785109/-/DCSupplemental.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Dudziak D, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315(5808):107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 3.Castro AG, et al. Anti-interleukin 10 receptor monoclonal antibody is an adjuvant for T helper cell type 1 responses to soluble antigen only in the presence of lipopolysaccharide. J Exp Med. 2000;192(10):1529–1534. doi: 10.1084/jem.192.10.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993;151(11):6535–6545. [PubMed] [Google Scholar]
- 5.Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117(9):2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caux C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90(4):1458–1470. [PubMed] [Google Scholar]
- 7.Klechevsky E, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29(3):497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klechevsky E, et al. Understanding human myeloid dendritic cell subsets for the rational design of novel vaccines. Hum Immunol. 2009;70(5):281–288. doi: 10.1016/j.humimm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratzinger G, et al. Mature human Langerhans cells derived from CD34+ hematopoietic progenitors stimulate greater cytolytic T lymphocyte activity in the absence of bioactive IL-12p70, by either single peptide presentation or cross-priming, than do dermal-interstitial or monocyte-derived dendritic cells. J Immunol. 2004;173(4):2780–2791. doi: 10.4049/jimmunol.173.4.2780. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 11.Li D, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209(1):109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vukmanovic-Stejic M, Vyas B, Gorak-Stolinska P, Noble A, Kemeny DM. Human Tc1 and Tc2/Tc0 CD8 T-cell clones display distinct cell surface and functional phenotypes. Blood. 2000;95(1):231–240. [PubMed] [Google Scholar]
- 13.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: Reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgame P, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 15.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172(3):921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodland DL, Dutton RW. Heterogeneity of CD4(+) and CD8(+) T cells. Curr Opin Immunol. 2003;15(3):336–342. doi: 10.1016/s0952-7915(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 17.Fung-Leung WP, et al. The lack of CD8 alpha cytoplasmic domain resulted in a dramatic decrease in efficiency in thymic maturation but only a moderate reduction in cytotoxic function of CD8+ T lymphocytes. Eur J Immunol. 1993;23(11):2834–2840. doi: 10.1002/eji.1830231117. [DOI] [PubMed] [Google Scholar]
- 18.Fung-Leung WP, et al. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell. 1991;65(3):443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 19.Garcia KC, et al. CD8 enhances formation of stable T-cell receptor/MHC class I molecule complexes. Nature. 1996;384(6609):577–581. doi: 10.1038/384577a0. [DOI] [PubMed] [Google Scholar]
- 20.Colonna M, et al. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med. 1997;186(11):1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiroishi M, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100(15):8856–8861. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endo S, Sakamoto Y, Kobayashi E, Nakamura A, Takai T. Regulation of cytotoxic T lymphocyte triggering by PIR-B on dendritic cells. Proc Natl Acad Sci USA. 2008;105(38):14515–14520. doi: 10.1073/pnas.0804571105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregori S, et al. Differentiation of type 1 T regulatory cells (Tr1) by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010;116(6):935–944. doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 24.Manetti R, et al. CD30 expression by CD8+ T cells producing type 2 helper cytokines. Evidence for large numbers of CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180(6):2407–2411. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11(7):608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stadel D, et al. TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin Cancer Res. 2010;16(23):5734–5749. doi: 10.1158/1078-0432.CCR-10-0985. [DOI] [PubMed] [Google Scholar]
- 27.Maggi E, et al. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180(2):489–495. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colonna M, et al. Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol. 1998;160(7):3096–3100. [PubMed] [Google Scholar]
- 29.Chang CC, et al. Tolerization of dendritic cells by T(S) cells: The crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3(3):237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 30.Ma G, et al. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34(3):385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vlad G, Cortesini R, Suciu-Foca N. CD8+ T suppressor cells and the ILT3 master switch. Hum Immunol. 2008;69(11):681–686. doi: 10.1016/j.humimm.2008.08.286. [DOI] [PubMed] [Google Scholar]
- 32.Vlad G, et al. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol. 2010;29(2):119–132. doi: 10.3109/08830180903281185. [DOI] [PubMed] [Google Scholar]
- 33.Naji A, et al. CD3+CD4low and CD3+CD8low are induced by HLA-G: Novel human peripheral blood suppressor T-cell subsets involved in transplant acceptance. Blood. 2007;110(12):3936–3948. doi: 10.1182/blood-2007-04-083139. [DOI] [PubMed] [Google Scholar]
- 34.Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci USA. 2006;103(44):16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HC, Tan K, Hsu YM. CD8alphabeta has two distinct binding modes of interaction with peptide-major histocompatibility complex class I. J Biol Chem. 2006;281(38):28090–28096. doi: 10.1074/jbc.M604931200. [DOI] [PubMed] [Google Scholar]
- 36.Willemsen RA, et al. CD8 alpha coreceptor to improve TCR gene transfer to treat melanoma: Down-regulation of tumor-specific production of IL-4, IL-5, and IL-10. J Immunol. 2006;177(2):991–998. doi: 10.4049/jimmunol.177.2.991. [DOI] [PubMed] [Google Scholar]
- 37.Demotte N, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28(3):414–424. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321(5892):1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minkis K, et al. Type 2 Bias of T cells expanded from the blood of melanoma patients switched to type 1 by IL-12p70 mRNA-transfected dendritic cells. Cancer Res. 2008;68(22):9441–9450. doi: 10.1158/0008-5472.CAN-08-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roberts WK, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest. 2009;119(7):2042–2051. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheu BC, et al. Predominant Th2/Tc2 polarity of tumor-infiltrating lymphocytes in human cervical cancer. J Immunol. 2001;167(5):2972–2978. doi: 10.4049/jimmunol.167.5.2972. [DOI] [PubMed] [Google Scholar]
- 42.Maggi E, et al. Functional characterization and modulation of cytokine production by CD8+ T cells from human immunodeficiency virus-infected individuals. Blood. 1997;89(10):3672–3681. [PubMed] [Google Scholar]
- 43.Nazaruk RA, Rochford R, Hobbs MV, Cannon MJ. Functional diversity of the CD8(+) T-cell response to Epstein-Barr virus (EBV): Implications for the pathogenesis of EBV-associated lymphoproliferative disorders. Blood. 1998;91(10):3875–3883. [PubMed] [Google Scholar]
- 44.Hermann P, Van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25(10):2972–2977. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 45.Beck S, Barrell BG. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.