Abstract
Both ghrelin and somatostatin (SST) inhibit glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells, but how these independent actions are regulated has been unclear. The mechanism must accommodate noncanonical ghrelin receptor (GHS-R1a)–G-protein coupling to Gαi/o instead of Gαq11 and dependence on energy balance. Here we present evidence for an equilibrium model of receptor heteromerization that fulfills these criteria. We show that GHS-R1a coupling to Gαi/o rather than Gαq11 requires interactions between GHS-R1a and SST receptor subtype 5 (SST5) and that in the absence of SST5 ghrelin enhances GSIS. At concentrations of GHS-R1a and SST5 expressed in islets, time-resolved FRET and bioluminescence resonance energy transfer assays illustrate constitutive formation of GHS-R1a:SST5 heteromers in which ghrelin, but not SST, suppresses GSIS and cAMP accumulation. GHS-R1a–G-protein coupling and the formation of GHS-R1a:SST5 heteromers is dependent on the ratio of ghrelin to SST. A high ratio enhances heteromer formation and Gαi/o coupling, whereas a low ratio destabilizes heteromer conformation, restoring GHS-R1a–Gαq11 coupling. The [ghrelin]/[SST] ratio is dependent on energy balance: Ghrelin levels peak during acute fasting, whereas postprandially ghrelin is at a nadir, and islet SST concentrations increase. Hence, under conditions of low energy balance our model predicts that endogenous ghrelin rather than SST establishes inhibitory tone on the β-cell. Collectively, our data are consistent with physiologically relevant GHS-R1a:SST5 heteromerization that explains differential regulation of islet function by ghrelin and SST. These findings reinforce the concept that signaling by the G-protein receptor is dynamic and dependent on protomer interactions and physiological context.
Keywords: GPCR oligomers, glucose homeostasis
The growth hormone secretagogue receptor type 1a (GHS-R1a), was identified in 1996 as an orphan G-protein–coupled receptor (GPCR) that regulates the action of a family of small synthetic molecules designed to rejuvenate the growth hormone (GH) axis in humans (1, 2). Three years later GHS-R1a was deorphanized by discovery of an endogenous agonist made in the stomach called “ghrelin” (3). Ghsr−/− mice are refractory to the GH-releasing and orexigenic properties of ghrelin, confirming that GHS-R1a is a physiologically relevant ghrelin receptor (4). The study we describe was prompted by the need for a model that includes endogenous ghrelin as well as somatostatin (SST) as a regulator of pancreatic islet function and that elucidates the paradox that ghrelin inhibits rather than enhances glucose-stimulated insulin secretion (GSIS).
Experiments in ghrelin−/− and ghsr−/− mice led to the conclusion that endogenous ghrelin is a physiologically important regulator of GSIS (5–7). In fed mice endogenous ghrelin concentrations are at a nadir; hence, the majority of GHS-R1a binding sites are unoccupied. In this context, treating WT mice with exogenous ghrelin suppresses GSIS. When WT mice are fasted, endogenous ghrelin levels reach a maximum, and the mice are refractory to exogenous ghrelin. In contrast, fasted ghrelin−/− mice are fully responsive to the suppression of GSIS by exogenous ghrelin. Collectively, these results indicate that in fasted WT mice GHS-R1a binding sites are fully occupied by endogenous ghrelin, suggesting that under conditions of negative energy balance endogenous ghrelin acts by establishing inhibitory tone on insulin secretion.
Endogenous ghrelin is fundamentally important for maintaining euglycemia and for survival under conditions of acute food restriction (7, 8). Mice lacking the essential enzyme ghrelin octanoylacyltransferase (GOAT), which converts inactive ghrelin peptide into its active octanoylated form, are severely compromised by a 60% reduction in dietary intake (9–11). However, the phenotype can be rescued by restoring ghrelin or GH to the concentrations measured in control WT mice subjected to 60% caloric restriction, indicating that ghrelin is critically important for preventing neuroglycopenia (11, 12). Ironically, expression cloning of GHS-R1a that led to the discovery of ghrelin was achieved using a synthetic molecule that augmented episodic GH release (2, 3).
GHS-R1a is expressed in pancreatic β-cells, and ghrelin inhibits GSIS (13, 14). Inhibition of insulin release appears paradoxical, because generally activation of GHS-R1a ghrelin results in coupling to Gαq11, which would enhance insulin secretion. However, in the islet and β-cells, instead ofGαq11-mediated signal transduction, ghrelin suppresses GSIS via GHS-R1a coupling to Gαi/o, thereby reducing cAMP accumulation (14). Traditionally; SST activation of somatostatin receptor subtype-5 (SST5) is considered the major inhibitor of insulin secretion from β-cells (15–18). SST released from islet δ-cells also inhibits glucagon secretion by activating SST2 on α-cells (19–22).
How and under what physiological conditions are the inhibitory actions of ghrelin and SST on insulin secretion independently controlled? Any model that explains ghrelin action on pancreatic β-cells in vivo must include a mechanism for the switch in canonical GHS-R1a–G-protein coupling from Gαq11 to Gαi/o, a role for SST5 and SST, and dependence on reciprocal changes in relative concentrations of ghrelin and SST as a consequence of changes in glucose concentrations. This report provides evidence for a model incorporating GHS-R1a:SST5 heteromers that meet these criteria.
Results
Ghrelin Inhibition of GSIS Is Dependent on both GHS-R1a and SST5.
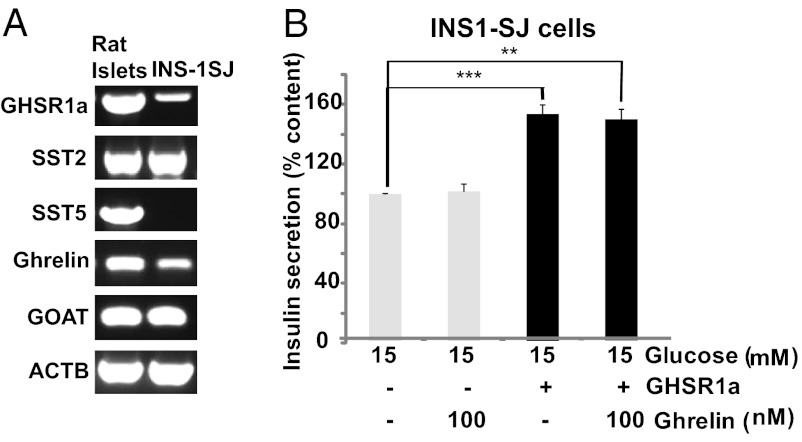
We first measured expression of ghrelin, GOAT, GHS-R1a, SST5, and SST2 in rat pancreatic islets (Fig. 1A) and used these results to develop a system to model ghrelin and SST signaling. Pharmacology studies indicate that SST5 rather than SST2 is primarily involved in inhibiting GSIS from β-cells (15–18). We hypothesized that noncanonical GHS-R1a coupling resulting in ghrelin inhibition of GSIS involves molecular interactions between GHS-R1a and SST5. To test the relative contributions of GHS-R1a and SST5, we sought a subclone of INS-1 cells that would allow independent manipulation of GHS-R1a and SST5 to match concentrations present in pancreatic islets. A subclone was identified, INS-1SJ, that expresses SST2, ghrelin, and GOAT at levels similar to those found in rat pancreatic islets with low expression of GHS-R1a and undetectable SST5 (Fig. 1A).
Fig. 1.
(A) Comparison of GHS-R1a, SST2, SST5, ghrelin, and GOAT expression in rat islets and INS-1SJ cells by PCR. (B) INS1-SJ cells transduced with GHS-R1a–expressing lentivirus causes augmentation of GSIS without suppression by ghrelin. Data represent means ± SEM (n = 4); **P < 0.01, ***P < 0.001.
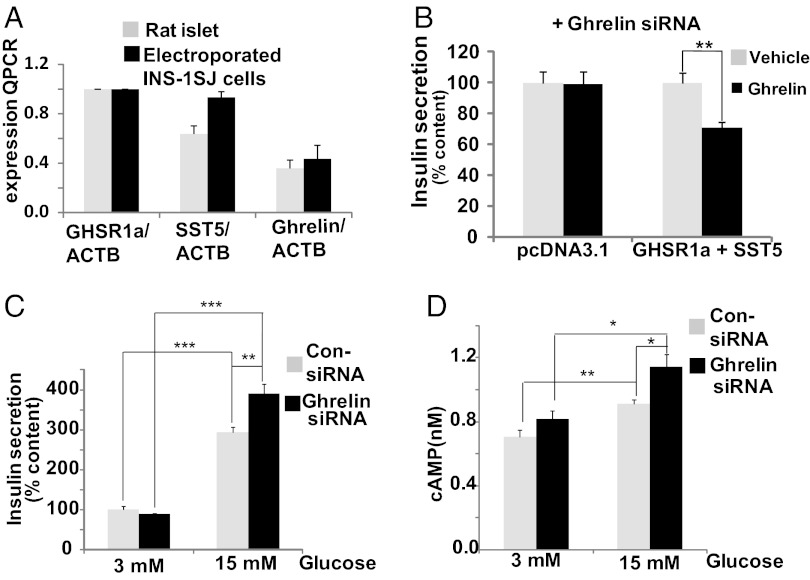
Increasing GHS-R1a levels alone in INS-1SJ cells by transducing the cells with a GHS-R1a–expressing lentivirus enhanced insulin secretion in either the presence or absence of ghrelin, consistent with GHS-R1a canonical Gαq11 signaling and production of endogenous ghrelin (Fig. 1B). Because INS1-SJ cells do not express SST5, this result suggested that SST5 is required for ghrelin suppression of GSIS. We then established conditions for expressing GHS-R1a and SST5 at levels closely matching those in pancreatic islets (Fig. 2A). INS1-SJ cells expressing GHS-R1a and SST5 (INS1SJ-GHSR-SST5) were treated with ghrelin siRNA to knockdown endogenous ghrelin production (Fig. S1). Under these conditions exogenous ghrelin inhibited insulin release induced by 15 mM glucose (Fig. 2B). When INS1SJ-GHSR-SST5 cells were treated with 3 mM and 15 mM glucose, high glucose increased GSIS, and expression of ghrelin siRNA to knock down production of endogenous ghrelin further enhanced GSIS (Fig. 2C); hence, ghrelin siRNA unmasks the suppression of GSIS caused by endogenous ghrelin. Importantly, enhanced GSIS is accompanied by an increase in cAMP (Fig. 2D), consistent with ghrelin regulation of GSIS reported in pancreatic islets by noncanonical Gαi/o coupling (14).
Fig. 2.
Suppression of insulin secretion by ghrelin is dependent on coexpression of GHS-R1a and SST5 in INS-1SJ cells electroporated with GHS-R1a and SST5 to match levels expressed in rat pancreatic islets. (A) Quantitation by real-time PCR of GHS-R1a, SST5, and ghrelin expression in rat islets and electroporated INS-1SJ cells. (B) Ghrelin inhibits insulin secretion induced by 15 mM glucose in INS-1SJ cells in the presence but not in the absence of GHS-R1a and SST5 following suppression of endogenous ghrelin production with ghrelin siRNA. (C) Knockdown of endogenous ghrelin production increases GSIS. (D) Knockdown of endogenous ghrelin production increases intracellular cAMP. Data represent means ± SEM (n = 4); *P < 0.05, **P < 0.01, ***P < 0.001.
Expression of Equivalent Concentrations of GHS-R1a Prevents SST5 Inhibition of GSIS.
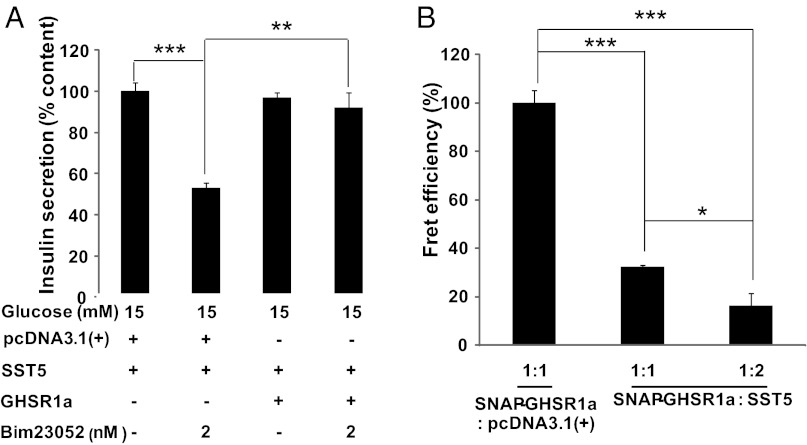
We next asked if coexpression of equivalent levels of GHS-R1a andSST5 modifies SST5 function. To avoid activating SST2 in INS-1SJ cells, we selected the SST5-selective agonist Bim23052. INS-1SJ cells expressing either SST5 alone or with an equal concentration of GHS-R1a were treated with Bim23052. When SST5 was expressed alone, Bim23052 inhibited GSIS, but coexpression of GHS-R1a blocked Bim23052 action (Fig. 3A). Native SST5 exists as a monomer, but signal transduction requires agonist-induced formation of homodimers (23); therefore, antagonism of SST5 signaling could be explained by constitutive formation of GHS-R1a:SST5 heteromers. To test this possibility, we used time-resolved (Tr)-FRET SNAP-tag technology (24, 25), which has the high sensitivity necessary to detect the association of GHS-R1a with SST5 at the concentrations found in pancreatic islets.
Fig. 3.
Expression of an equivalent concentration of GHS-R1a blocks SST5 agonist-induced inhibition of GSIS in INS-1SJ cells by a mechanism consistent with the formation of GHS-R1a:SST5 heteromers. (A) Coexpression of SST5 with an equivalent concentration of GHS-R1a blocks suppression of GSIS by the SST5 agonist Bim23052. (B) Tr-FRET using N-terminal SNAP-tagged GHS-R1a illustrates competitive inhibition of GHS-R1a:GHS-R1a homomer formation by equal (1/1) or excess (1/2) concentrations of SST5. In each case, data represent means ± SEM (n = 4); *P < 0.05, **P < 0.01, ***P < 0.001.
In isolation, GHS-R1a exists as homodimers. Previously, we showed by Tr-FRET that dopamine receptor-2 (DRD2) is a competitive inhibitor of GHS-R1a homodimerization, resulting in the formation of GHS-R1a:DRD2 heteromers (26). Using similar methods employing SNAP-GHS-R1a, Tr-FRET illustrates the formation of GHS-R1a:GHS-R1a homodimers on the plasma membrane of INS-1SJ cells (Fig. 3B). Concentration-dependent inhibition of the Tr-FRET signal is observed when SST5 is coexpressed with SNAP-GHS-R1a at ratios of 1/1 and 2/1 (Fig. 3B). Control experiments confirmed that attenuation of the Tr-FRET signal was not a consequence of coexpressed SST5 inhibiting transport of SNAP-GHS-R1a to the plasma membrane (Fig. S2); therefore, the marked inhibition of GHS-R1a homodimerization by SST5 is a consequence of high-affinity interactions between GHS-R1a and SST5.
SST5-Dependent Modification of GHS-R1a–G-protein Coupling Is Controlled by the [Ghrelin]/[SST] Ratio.
Both ghrelin and SST are capable of inhibiting insulin secretion by a mechanism requiring Gαi/o suppression of cAMP accumulation. Above we showed that ghrelin inhibition of GSIS and suppression of cAMP accumulation, as well as GHS-R1a–dependent antagonism of inhibition of GSIS by a selective SST5 agonist, are dependent on both GHS-R1a and SST5. To elucidate the mechanism more completely and to deduce the relative contributions of SST2 and SST5, a cell system was needed in which the relative concentrations of ghrelin, SST, GHS-R1a, SST5, and SST2 could be controlled. Precise control requires a null background; therefore, we selected HEK293 cells. Because inhibition of GSIS by ghrelin and SST in pancreatic β-cells and in the INS-1SJ β-cell line is dependent on the inhibition of cAMP production, we selected cAMP accumulation as a surrogate for GSIS.
We next tested whether interactions between GHS-R1a and SST5 affected SST suppression of cAMP accumulation. In HEK293 cells expressing SST5, SST dose-dependently suppresses forskolin-induced cAMP accumulation with maximum inhibition at 1–10 nM (Fig. S3A). Remarkably, as in the INS-1SJ cells, coexpressing GHS-R1a with SST5 made the cells refractory to SST (Fig. S3B); however, treatment with ghrelin, but not with des-acyl ghrelin (Fig. S3C), inhibits forskolin-induced cAMP accumulation by a pertussis toxin (PTX)-sensitive mechanism (Fig. S3D). Hence, the noncanonical GHS-R1a–G-protein coupling to Gαi/o observed in pancreatic β-cells and INS-1SJ β-cells is recapitulated in HEK293 cells coexpressing GHS-R1a and SST5.
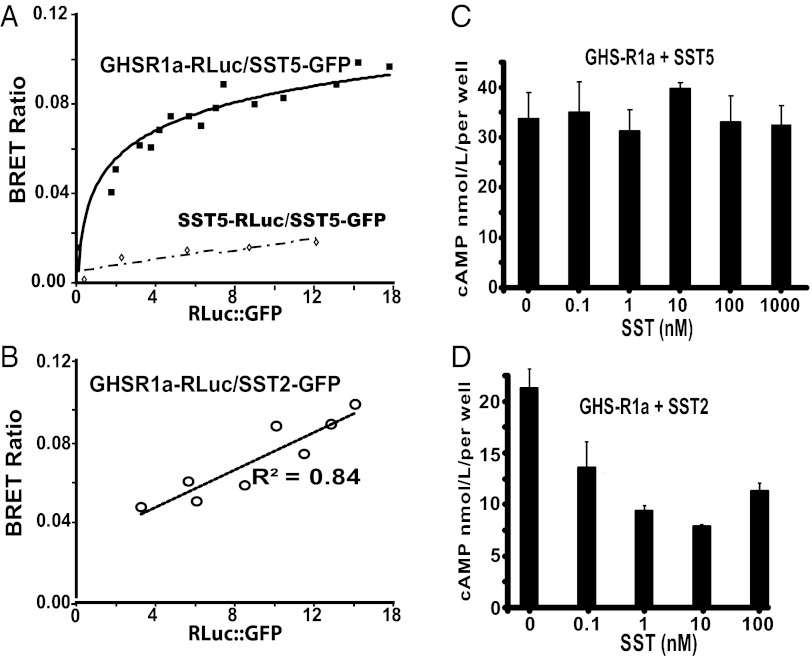
Tr-FRET analysis in INS-1SJ cells used fluorophor tagging at the GHS-R1a N terminus. As an additional test of heteromer formation, we used bioluminescence resonance energy transfer (BRET) with GHS-R1a and SST5 tagged at the C terminus with either the energy donor luciferase or GFP as acceptor (27). A fixed DNA concentration of donor and increasing concentrations of acceptor at ratios of 1/1–1/18 were transfected into HEK293 cells, and the BRET ratio was plotted as a function of the relative concentrations of donor and acceptor. The hyperbolic function obtained with the GHS-R1a-Rluc and SST5-GFP pair and the BRET50 ratio indicates high-affinity interactions (upper curve in Fig. 4A; BRET50 ∼2). An identical result is obtained when donor- and acceptor-tagged receptors are reversed (SST5-Rluc and GHS-R1a-GFP). In contrast, titration of SST5-Rluc + SST5-GFP (lower curve in Fig. 4A) and GHS-R1a-Rluc + SST2-GFP (Fig. 4B) produces linear BRET functions indicating random collisions rather than heteromer formation. We next asked whether heteromer formation correlated with resistance to SST inhibition of cAMP accumulation. Cells coexpressing GHS-R1a with either SST5 or SST2 were treated with forskolin and SST. With SST5 coexpression, SST did not inhibit cAMP accumulation (Fig. 4C), whereas with SST2 coexpression SST inhibited forskolin-induced cAMP accumulation (Fig. 4D). Hence, as observed in INS1SJ-GHSR-SST5 cells, formation of GHS-R1a:SST5 heteromers blocks SST responsiveness in favor of ghrelin responsiveness.
Fig. 4.
BRET analyses and cAMP assays illustrating that GHS-R1a forms constitutive heteromers with SST5 but not with SST2 and blocks SST/SST5-induced suppression of cAMP accumulation. The BRET ratio is expressed as a function of the acceptor/donor DNA ratio and is defined as [(emission at 515 nm) − (background emission at 515 nm)]/[(emission at 410 nm) − (background emission at 410 nm)]. (A) The BRET ratio in HEK293 cells cotransfected with 0.1 μg GHS-R1a-Rluc and increasing amounts (0.1–1.8 μg) of SST5-GFP (upper curve; BRET50 = 2) or 0.1 μg SST5-Rluc DNA and increasing amounts (0.1–1.8 μg) of SST5-GFP DNA (lower curve). (B) The BRET ratio measured in HEK293 cells cotransfected with 0.1 μg GHS-R1a-Rluc and 0.1–1.8 μg ST2-GFP. (C) HEK293 cells coexpressing GHS-R1a and SST5 are refractory to SST suppression of forskolin-induced cAMP accumulation. (D) SST inhibition of forskolin-induced cAMP accumulation in HEK293 cells coexpressing GHS-R1a and SST2.
[Ghrelin]/[SST] Ratio Modifies GHS-R1a–G Protein Coupling and Is Associated with Heteromer Formation.
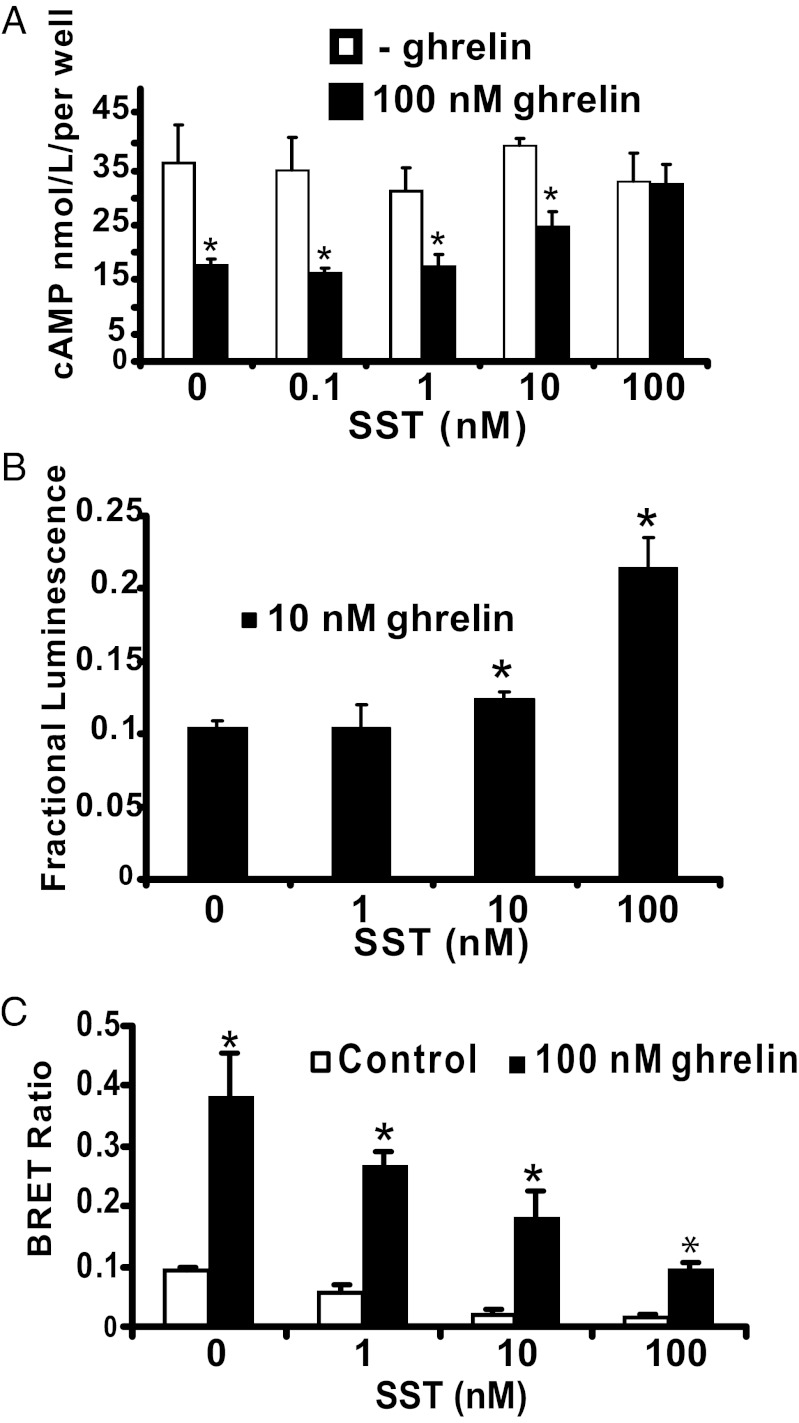
In vivo, the concentration of ghrelin is increased during an acute fast and is suppressed after a meal. The postprandial increase in blood glucose suppresses ghrelin and releases SST in pancreatic islets. To determine if GHS-R1a coupling to Gαi/o vs. Gαq11 is dependent on the relative concentrations of ghrelin and SST, we asked if the [ghrelin]/[SST] ratio (1,000/1–1/1) influences signal transduction. High [ghrelin]/[SST] ratios result in ghrelin suppression of cAMP accumulation, whereas lowering the ratio antagonizes ghrelin inhibition of cAMP accumulation (Fig. 5A). To test if SST antagonism of ghrelin inhibition of cAMP accumulation is accompanied by restoration of canonical GHS-R1a–Gαq11 coupling, HEK293 cells expressing GHS-R1a, SST5, and aequorin were used (1, 28). Consistent with an equilibrium between GHS-R1a coupling to Gαi/o and Gαq11, lowering the [ghrelin]/[SST] ratio produces a dose-dependent increase in the internal calcium concentration ([Ca2+]i) mobilization as measured by aequorin bioluminescence (Fig. 5B). Next we performed BRET assays in the absence or presence of ghrelin with increasing SST concentrations to test whether the specificity of GHS-R1a–G-protein coupling correlates with heteromer formation. Ghrelin markedly increased the BRET ratio which was dose-dependently attenuated by SST (Fig. 5C). These results are consistent with agonist concentration-dependent equilibrium between GHS-R1a:SST5 and GHS-R1a:GHS-R1a dimers, which in turn regulate noncanonical and canonical GHS-R1a signal transduction, respectively.
Fig. 5.
In HEK293 cells coexpressing GHS-R1a and SST5, GHS-R1a coupling to Gαi/o vs. Gαq11 is dependent on the [ghrelin]/[SST] ratio, which correlates with GHS-R1a:SST5 heteromer formation. (A) Decreasing the [ghrelin]/[SST] ratio antagonizes ghrelin suppression of forskolin-induced cAMP accumulation. (B) HEK293-AEQ17 cells coexpressing GHS-R1a and SST5. Decreasing the [ghrelin]/[SST] ratio increases ghrelin-induced [Ca2+]i. (C) HEK293 cells cotransfected with GHS-R1a-Rluc (0.2 μg) and SST5-GFP (1.8 μg). A high [ghrelin]/[SST] ratio increases the BRET ratio; a low [ghrelin]/[SST] ratio decreases the BRET ratio. The data represent the means ± SD of three independent experiments, each performed in triplicate. *P < 0.05 compared with absence of both agonists.
Discussion
Based on our results, we propose a model of regulation of pancreatic islet function incorporating endogenous ghrelin and SST. Although GHS-R1a normally couples to Gαq11, both ghrelin and SST inhibit GSIS secretion via GHS-R1a and SST5 coupling to Gαi/o. To test conclusively for GHS-R1a and SST5 molecular interactions and to deduce how combinations of ghrelin and SST might regulate signal transduction differentially, we identified a clone of INS-1 cells (INS-1SJ) that formed a basis for our studies. By matching concentrations of GHS-R1a and SST5 in INS-1SJ cells to those expressed in pancreatic islets, we conclude that, contrary to current belief, ghrelin rather than SST suppresses GSIS and cAMP accumulation through a PTX-sensitive mechanism. This outcome is surprising, because canonical ghrelin signaling through GHS-R1a (via Gαq11) would mobilize [Ca2+]i and stimulate rather than inhibit insulin secretion. Indeed, transducing INS-1SJ cells with lentivirus encoding GHS-R1a enhanced insulin secretion. However, when GHS-R1a is coexpressed with SST5 in INS-1SJ cells at concentrations and relative ratios commensurate with those measured in native rat islets, ghrelin inhibits insulin secretion, supporting our hypothesis of physiologically relevant molecular interactions between GHS-R1a and SST5.
To determine the mechanism by which ghrelin inhibits insulin secretion via noncanonical GHS-R1a–G-protein coupling, we tested for GHS-R1a:SST5 heteromer formation using highly sensitive Tr-FRET and BRET assays. Tr-FRET used receptors tagged at the N terminus, and for BRET receptors were tagged at the C terminus. Each method used relative and absolute expression levels of GHS-R1a and SST5 closely matching those in rat pancreatic islets. The results of the two different experimental methods are in complete agreement and illustrate high-affinity interactions between GHS-R1a and SST5 at equimolar concentrations. Indeed, these results support our hypothesis that the atypical GHS-R1a–G-protein coupling essential for ghrelin suppression of GSIS is a consequence of the formation of GHS-R1a:SST5 heteromers. Most importantly, we show that differential GHS-R1a–G-protein coupling and signal transduction are influenced by the relative concentrations of ghrelin and SST. In cells coexpressing GHS-R1a and SST5, when the relative [ghrelin]/[SST] ratio is high, ghrelin suppresses cAMP accumulation; when the ratio is low, the cells are refractory to ghrelin inhibition of cAMP production, and ghrelin increases [Ca2+]i mobilization, consistent with restoration of GHS-R1a–Gαq11 coupling. Furthermore, reducing the [ghrelin]/[SST] ratio lowers the BRET ratio, consistent with dissociation of GHS-R1a:SST5 heteromers, thereby implicating an agonist concentration-dependent equilibrium model of heteromerization. Of course, we cannot preclude the possibility that changes in the BRET ratio also might be explained by a change in conformation of the GHS-R1a:SST5 heteromer resulting in altered G-protein coupling.
Constitutive formation of GHS-R1a:SST5 heteromers explains why GHS-R1a antagonizes SST5 suppression of GSIS and cAMP accumulation. Because SST-induced formation of SST5:SST5 homomers is required for signal transduction (23), the insensitivity to SST argues that formation of SST5:SST5 or GHS-R1a:GHS-R1a homomers is energetically unfavorable compared with GHS-R1a:SST5 formation. Indeed, we speculate that the heteromer acts as a buffer preventing ghrelin-induced insulin secretion by GHS-R1a–Gαq coupling and oversuppression of insulin release by unopposed SST5 coupling to Gαi/o.
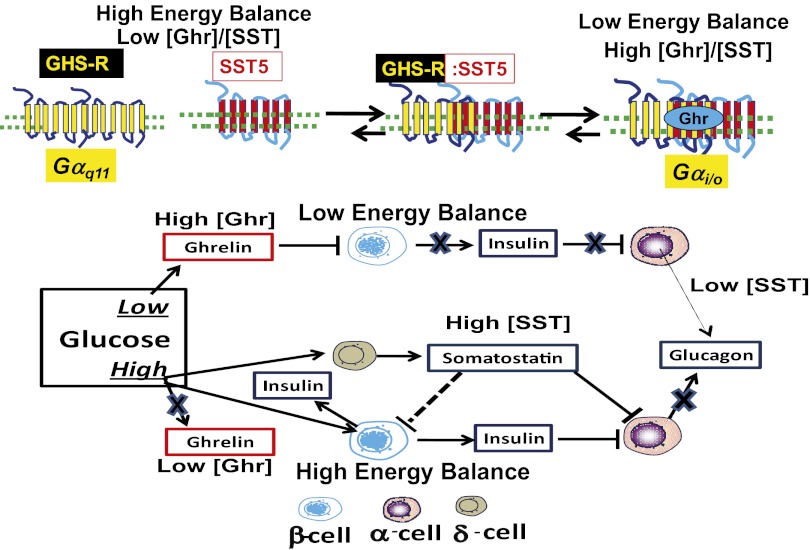
We propose a model that defines a role for ghrelin and GHS-R1a for controlling glucose homeostasis according to energy balance (Scheme 1). We show that when GHS-R1a and SST5 are coexpressed, they are functionally and physically associated, resulting in coupling to Gαi/o. The physical association of GHS-R1a and SST5 allows signal transduction to be regulated tightly according to the relative concentrations of ghrelin and SST. Under conditions of low energy balance, ghrelin concentrations are elevated, resulting in GHS-R1a:SST5–mediated inhibition of insulin secretion. Ghrelin also stimulates glucagon secretion by direct effects on α-cells and indirectly by disinhibiting insulin suppression of α-cell activity (29–31). As well as inhibiting GSIS, ghrelin inhibits arginine-induced SST secretion (32). In the context of positive energy balance, high glucose lowers circulating ghrelin (14, 33), thereby relieving the ghrelin suppression of insulin release from β-cells and stimulating the release of SST from δ-cells (34); SST, in turn, activates SST2 on α-cells, suppressing glucagon secretion (34). Therefore, only in states of low energy balance does ghrelin play a dominant role in regulating glucose homeostasis.
Scheme 1.
Equilibrium model of ghrelin regulation of islet function mediated by GHS-R1a:SST5 heteromers. Our data support a model of ghrelin signaling dependent on the ratio of ghrelin to SST according to energy balance. Low glucose increases ghrelin concentrations; ghrelin suppresses insulin secretion from β-cells and increases glucagon release from α-cells. High glucose reduces ghrelin but increases SST release from islet δ-cells. Hence, when glucose is low, the [ghrelin]/[SST] ratio is high. In this context, GHS-R1a activation by ghrelin results in noncanonical coupling to Gαi/o that lowers cAMP accumulation and inhibits insulin secretion, which is dependent on GHS-R1a:SST5 heteromer formation. Conversely, by lowering the [ghrelin]/[SST] ratio, high glucose destabilizes GHS-R1a:SST5 heteromers, reducing GHS-R1a coupling to Gαi/o; as a consequence, insulin secretion no longer is regulated by ghrelin.
Recent studies show that glucose inhibits glucagon secretion in WT mice but not in sst−/− mice, as is consistent with a direct role for glucose on SST secretion (33). The rapid cessation of insulin release upon glucose removal is the same in WT and sst−/− mouse islets, indicating that termination of the insulin-secretory response is not regulated by SST from δ-cells. This result is consistent with our model, which proposes that when glucose levels fall in vivo, it is ghrelin rather than SST that establishes an inhibitory set point on the β-cell to control insulin secretion (Scheme 1). This effect is not observed in isolated mouse islets after glucose removal because, unlike rat and human islets, mouse islets do not produce ghrelin.
In summary, we provide evidence for an equilibrium model in which the formation of GHS-R1a:SST5 heteromers is controlled by the [ghrelin]/[SST] ratio; hence, ghrelin and SST differentially establish a set point for β-cell insulin secretion. Of course, implicit in our equilibrium model is dependence on the relative concentrations of GHS-R1a and SST5. In rat islets, we show both are expressed at approximately equal concentrations. Clearly the results obtained from model systems do not necessarily equate to primary β-cells; nevertheless, the proposed mechanism is conceptually consistent with observations made in isolated islets. Finally, our results reinforce the notion that regulation of intracellular signaling by GPCRs is not simply a linear relationship but is dependent on modulation via receptor signaling networks that can be modified according to relative agonist concentrations and relative GPCR concentrations.
Experimental Procedures
Expression Constructs.
GHS-R1a-GFP was constructed as described previously (27). The GHS-R1a-Rluc:human GHS-R1a cDNA fragment was inserted in-frame into the EcoRI/EcoRV sites of the pRluc-N1 vector (Perkin Elmer). Human SST5 and SST2 cDNA constructs were purchased from Missouri S&T cDNA Resource Center (www.cdna.org). The SST5-GFP:SST5 cDNA fragment was inserted in-frame into the EcoRI/EcoRV sites of the pGFP-N3 vector (Perkin Elmer). The SST5-Rluc:SST5 cDNA fragment was inserted in-frame into the EcoRI/EcoRV sites of the pRluc-N1vector (Perkin Elmer). The integrity of all tagged GHS-R1a, SST5, and SST2 expression constructs was confirmed by nucleotide sequencing and tested to check that they displayed functional characteristics identical to those of WT receptors in transfected cells. SNAP-GHS-R1a was prepared as described previously (26).
INS-1SJ Cell Line and GSIS.
INS-1 832/13 cells that exhibit robust GSIS derived from a rat insulinoma were a kind gift from Chris Newgard (Duke University Medical Center, Winston Salem, NC). A subclone of these cells, which we named “INS-1SJ,” was selected specifically for our experiments because it allowed testing of the hypothesis that ghrelin attenuation of GSIS is dependent on GHS-R1a and SST5 by independently matching expression levels of GHS-R1a and SST5 to match levels in rat pancreatic islets. Eighteen hours before GSIS experiments, the standard tissue culture medium for INS-1 cells containing 11.1 mM glucose was changed to fresh medium containing 5 mM glucose (35). Insulin secretion was assayed in HBSS (114 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 116 mM MgSO4, 20 mM Hepes, 2.5 mM CaCl2, 25.5 mM NaHCO3, and 0.2% BSA, pH 7.2). At confluence, cells were washed with 1 mL HBSS containing 3 mM glucose followed by a 2-h preincubation in 2 mL of the same buffer. Then insulin secretion was measured after static incubation or 2 h in 0.8 mL of HBSS containing the glucose concentrations and/or ghrelin, SST, or Bim23052 as indicated in the figures. Insulin was measured using the rat/mouse insulin ELISA kit (Linco Research). Acid alcohol was added to extract total protein, which was assayed using the Coomassie protein assay reagent kit (Pierce Biotechnology). Secreted insulin was normalized to total protein.
Measurements of Intracellular cAMP.
cAMP was measured in INS-1SJ cells using the cAMP dynamic 2 kit (Cisbio) using homogeneous time-resolved fluorescence technology. cAMP levels were calculated by fluorescence ratio (665 nm/620 nm). Forty-eight hours after transfection, the transfected HEK293 cells were detached using nonenzymatic cell dissociation solution (Sigma), were washed with HBSS, and were resuspended in stimulation buffer [1× PBS with 0.04% BSA (pH 7.4) and 0.5 mmol/L 3-isobutyl-1-methylxanthine]. cAMP accumulation also was determined using the Lance cAMP 384 kit from Perkin Elmer.
Live-Cell Tr-FRET and BRET.
Cells were labeled 48 h after being transfected by electroporation as described previously (26). Briefly, SNAP-GHS-R1a–transfected cells were incubated in the presence of 25 nM of donor benzyl guanine-conjugated terbium cryptate (BG-TbK) (SNAP-Lumi4-Tb; Cisbio) and 250 nM of acceptor benzyl guanine-conjugated d2 fluorophore (BC-647) (SNAP-surface 647; New England BioLabs) for 1 h at 37 °C in 0.5% FBS containing RPMI medium. Tr-FRET signals were measured at 665 nm and 620 nm after excitation at 337 nm with 50-μs delay and integration time of 400 μs by using an EnVision plate reader (Perkin Elmer). The ratio of fluorescence (665 nm/620 nm) was calculated for each sample. BRET experiments were based on previous studies (27). Details are given in SI Experimental Procedures.
Aequorin Bioluminescence Assay.
The aequorin bioluminescence assay was carried out as described in ref. 28. Human ghrelin and SST (Phoenix) were diluted with modified HBSS (25 mM Hepes at pH 7.3) and distributed into 96-well plates. Assays were performed with the Luminoskan Luminometer (ThermoFisher Labsystems). The fractional luminescence for each well was calculated by taking the ratio of the integrated response to the initial challenge to the total integrated luminescence, as well as the Triton X-100 lysis response. Fractional luminescence data for each point represent the average of triplicate measurements. Statistical analyses were performed using the Student t test.
Additional methods are described in SI Experimental Procedures.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Aging Grant R01 AG019230 (to R.G.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.O.T. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209590109/-/DCSupplemental.
References
- 1.Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- 2.Smith RG, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18(5):621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA. 2004;101(13):4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006;3(5):379–386. doi: 10.1016/j.cmet.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Asnicar M, Smith RG. Central and peripheral roles of ghrelin on glucose homeostasis. Neuroendocrinology. 2007;86(3):215–228. doi: 10.1159/000109094. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology. 2008;149(2):843–850. doi: 10.1210/en.2007-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szentirmai E, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc Natl Acad Sci USA. 2009;106(33):14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez JA, et al. Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci USA. 2008;105(17):6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132(3):387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhao TJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA. 2010;107(16):7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nass RM, Gaylinn BD, Rogol AD, Thorner MO. Ghrelin and growth hormone: Story in reverse. Proc Natl Acad Sci USA. 2010;107(19):8501–8502. doi: 10.1073/pnas.1002941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gromada J, Franklin I, Wollheim CB. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- 14.Dezaki K, Kakei M, Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: Novel signal transduction of ghrelin. Diabetes. 2007;56(9):2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- 15.Brazeau P, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 16.Patel YC, Greenwood MT, Warszynska A, Panetta R, Srikant CB. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem Biophys Res Commun. 1994;198(2):605–612. doi: 10.1006/bbrc.1994.1088. [DOI] [PubMed] [Google Scholar]
- 17.Mitra SW, et al. Colocalization of somatostatin receptor sst5 and insulin in rat pancreatic beta-cells. Endocrinology. 1999;140(8):3790–3796. doi: 10.1210/endo.140.8.6937. [DOI] [PubMed] [Google Scholar]
- 18.Hoyer D, et al. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16(3):86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 19.Rossowski WJ, Coy DH. Specific inhibition of rat pancreatic insulin or glucagon release by receptor-selective somatostatin analogs. Biochem Biophys Res Commun. 1994;205(1):341–346. doi: 10.1006/bbrc.1994.2670. [DOI] [PubMed] [Google Scholar]
- 20.Rohrer SP, et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282(5389):737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, et al. Synthesis and biological activities of potent peptidomimetics selective for somatostatin receptor subtype 2. Proc Natl Acad Sci USA. 1998;95(18):10836–10841. doi: 10.1073/pnas.95.18.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambre Y, et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem Pharmacol. 1999;57(10):1159–1164. doi: 10.1016/s0006-2952(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 23.Rocheville M, et al. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000;275(11):7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 24.Gautier A, et al. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15(2):128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Maurel D, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: Application to GPCR oligomerization. Nat Methods. 2008;5(6):561–567. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang H, Betancourt L, Smith RG. Ghrelin amplifies. dopamine signaling by crosstalk involving formation of GHS-R/D1R heterodimers. Mol Endocrinol. 2006;20(8):1772–1785. doi: 10.1210/me.2005-0084. [DOI] [PubMed] [Google Scholar]
- 28.Feighner SD, et al. Structural requirements for the activation of the human growth hormone secretagogue receptor by peptide and nonpeptide secretagogues. Mol Endocrinol. 1998;12(1):137–145. doi: 10.1210/mend.12.1.0051. [DOI] [PubMed] [Google Scholar]
- 29.Qader SS, Lundquist I, Ekelund M, Håkanson R, Salehi A. Ghrelin activates neuronal constitutive nitric oxide synthase in pancreatic islet cells while inhibiting insulin release and stimulating glucagon release. Regul Pept. 2005;128(1):51–56. doi: 10.1016/j.regpep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Chuang JC, et al. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol Endocrinol. 2011;25(9):1600–1611. doi: 10.1210/me.2011-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamori D, et al. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab. 2009;9(4):350–361. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauge-Evans AC, et al. Somatostatin secreted by islet delta-cells fulfills multiple roles as a paracrine regulator of islet function. Diabetes. 2009;58(2):403–411. doi: 10.2337/db08-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briatore L, Andraghetti G, Cordera R. Acute plasma glucose increase, but not early insulin response, regulates plasma ghrelin. Eur J Endocrinol. 2003;149(5):403–406. doi: 10.1530/eje.0.1490403. [DOI] [PubMed] [Google Scholar]
- 34.Egido EM, Rodriguez-Gallardo J, Silvestre RA, Marco J. Inhibitory effect of ghrelin on insulin and pancreatic somatostatin secretion. Eur J Endocrinol. 2002;146(2):241–244. doi: 10.1530/eje.0.1460241. [DOI] [PubMed] [Google Scholar]
- 35.Hohmeier HE, et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.