Abstract
Although it is well established that the plant host encodes and synthesizes the apoprotein for leghemoglobin in root nodules, the source of the heme moiety has been uncertain. We recently found that the transcript for coproporphyrinogen III oxidase, one of the later enzymes of heme synthesis, is highly elevated in soybean (Glycine max L.) nodules compared with roots. In this study we measured enzyme activity and carried out western-blot analysis and in situ hybridization of mRNA to investigate the levels during nodulation of the plant-specific coproporphyrinogen oxidase and four other enzymes of the pathway in both soybean and pea (Pisum sativum L.). We compared them with the activity found in leaves and uninfected roots. Our results demonstrate that all of these enzymes are elevated in the infected cells of nodules. Because these are the same cells that express apoleghemoglobin, the data strongly support a role for the plant in the synthesis of the heme moiety of leghemoglobin.
The major tetrapyrrole synthesized in plants is chlorophyll, which in leaf tissue may be present at 2.5 μmol/g fresh weight. In contrast, the levels of other tetrapyrroles such as siroheme, phytochromobilin, and heme are much lower, with an estimated 2 nmol/g fresh weight for mitochondrial heme. However, in leguminous root nodules, levels of heme may be elevated a few hundred-fold. Nodules are unique, specialized organs that are the result of a symbiotic association between plants of the family Leguminosae and soil bacteria of the genera Sinorhizobium, Rhizobium, Bradyrhizobium, and Azorhizobium. The bacteria are present in the infected plant cell surrounded by the peribacteroid membrane, which is derived from the plant cell membrane. There they differentiate into bacteroids and express the enzyme nitrogenase, which enables them to fix atmospheric dinitrogen, thus allowing the plant host to grow without external reduced nitrogen. Nitrogenase is oxygen-sensitive, but the vigorously respiring bacteroids require an adequate supply of oxygen. This is achieved by the presence of leghemoglobin, which facilitates oxygen diffusion to the endosymbiont. Leghemoglobin has been immunolocalized to the cytosol of the infected plant cell, and is absent from the bacteroid and peribacteroid space (for review, see Appleby, 1984).
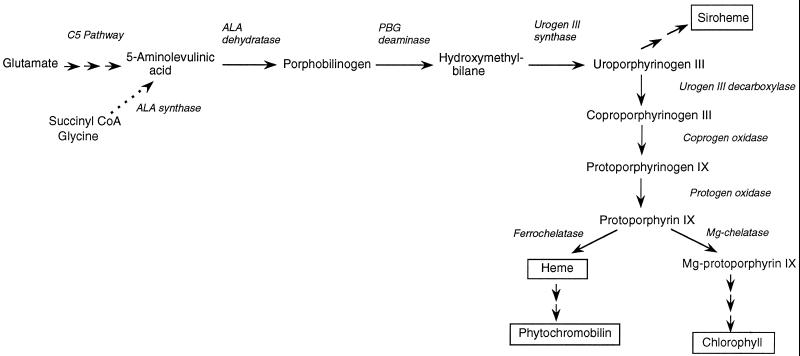
It is well established that the plant host encodes the gene for the leghemoglobin apoprotein (Jensen et al., 1981), but the source of the heme moiety has been the subject of much debate. Early biochemical work found that plant nodule tissue could not synthesize heme, whereas the rhizobia both made and exported heme (Cutting and Schulman, 1969). Similarly, the bacteroid fraction of soybean (Glycine max L.) nodules contained detectable levels of the enzyme ALA synthase, but the plant cytosol had none (Nadler and Avissar, 1977). ALA is the first committed precursor of all cellular tetrapyrroles (Fig. 1). The conclusion drawn from these studies was that the bacterial endosymbiont was responsible for the synthesis of the heme group of leghemoglobin, leading to proposals that leghemoglobin synthesis is a means of intimate contact between the plant and bacterial host (Appleby, 1984; Haaker, 1988).
Figure 1.
Pathway of tetrapyrrole synthesis in higher plants, showing the major end products (boxed) and relevant intermediates and enzymes. In plants, algae, and most bacteria, the first committed precursor ALA is synthesized from glutamate in the C5 pathway in three steps. However, in rhizobial species (as well as in certain other bacteria, animals, and fungi) ALA is synthesized in a single step by ALA synthase (dotted line). Urogen, Uroporphyrinogen.
However, the conclusions were based on erroneous assumptions about plant heme biosynthesis (for review, see O'Brian, 1996). In particular, it is now known that plant cells synthesize ALA by a completely different route, namely from glutamate via the so-called C5 pathway involving three enzymes (for review, see Kannangara et al., 1988). Glutamate-dependent ALA synthesis has been shown to be present at much higher levels in soybean nodules than in uninfected roots (Sangwan and O'Brian, 1992), whereas bacterial ALA synthase activity was essentially the same in nodules and in free-living bacteria. Furthermore, the plant gene for one of the C5 pathway enzymes, GSA aminotransferase, is induced during nodulation, concomitantly with the increase in enzyme activity (Sangwan and O'Brian, 1993; Frustaci et al., 1995). The same group also investigated the next enzyme in the pathway, ALA dehydratase. They found that, although message levels were relatively high in roots, there was no detectable protein. However, in nodules both protein level and enzyme activity are markedly increased (Kaczor et al., 1994). The results of both these studies suggest that the plant host responds during nodulation to the need for increased heme biosynthesis.
We have reported the isolation and characterization of a soybean cDNA that is strongly induced during nodulation, with a time course comparable to the increase in leghemoglobin (Madsen et al., 1993). This cDNA was identified as encoding coprogen oxidase, a later enzyme of tetrapyrrole synthesis (Fig. 1), because it showed considerable sequence similarity to the same enzyme isolated from yeast (Zagorec et al., 1988) and was able to complement a yeast mutant with a deletion in the coprogen oxidase gene (Madsen et al., 1993). It has since been shown to encode a protein with coprogen oxidase activity (M.A. Santana and A.G. Smith, unpublished data). The fact that it is induced in root nodules suggests, as for GSA aminotransferase and ALA dehydratase, that it plays a role in the requirement for increased heme synthesis in these organs. To investigate this further, we carried out a careful biochemical and molecular analysis of the levels of coprogen oxidase enzyme during nodulation and also examined four other enzymes of the pathway, namely ALA dehydratase, PBG deaminase, protogen oxidase, and ferrochelatase (Fig. 1). This paper presents our findings.
MATERIALS AND METHODS
Growth of Plant Material and Nodulation
Soybean (Glycine max L., cv Merrill) seeds were surface sterilized in hypochlorite and then soaked in water overnight. They were planted in trays containing sterile, lightweight, expanded clay aggregate. Pea (Pisum sativum L., cv Feltham First) seeds were surface sterilized and soaked in water for 1 to 2 h. They were then sown in Levington compost (Fisons, Beverly, MA). Plants were maintained in a greenhouse at approximately 25°C under a 16-h light, 8-h dark cycle or, alternatively, in complete darkness for the production of etiolated plants. After 2 weeks of growth, soybean plants were inoculated with Bradyrhizobium japonicum USDA110 and pea plants were inoculated with Rhizobium leguminosum bv viciae strain 3841. For growth under anaerobic conditions, 2-week-old soybean plants were placed in pots in which the roots were submerged in water continuously bubbled with nitrogen to saturate it. A set of control plants was maintained under the same conditions but bubbled with air.
Tissue extracts for enzyme assays and western analysis were prepared by grinding fresh material in 50 mm Hepes-NaOH, pH 8.2, 6 mm MgCl2, and 5 mm 2-mercaptoethanol in a mortar with a pestle and a small amount of acid-washed sand and centrifuging at 13,000g for 10 min. They were stored at −70°C until needed.
Enzyme Assays and Analytical Methods
ALA dehydratase and PBG deaminase assays were carried out as described by Smith (1988), and coprogen oxidase and protogen oxidase were measured fluorimetrically as described by Smith et al. (1993). Ferrochelatase was assayed spectrofluorimetrically using deuteroporphyrin IX as the substrate by the method of Porra and Lascelles (1968). Alcohol dehydrogenase was assayed as described by Mohanty et al. (1993). All assays were carried out two to four times on at least two independent extractions. Chlorophyll was measured by the method of Arnon (1949). Protein was determined with a protein estimation kit (Bio-Rad) according to the manufacturer's instructions, using BSA as the standard. Heme was measured with a hemoglobin kit (Sigma), following the manufacturer's instructions.
Western Analysis
For western analysis, protein extracts (10–50 μg protein) were subjected to electrophoresis, as described by Laemmli (1970), on 12.5% polyacrylamide gels in the presence of SDS (1% [w/v] in the gel, 0.1% [w/v] in the electrode buffer), and then transferred to nitrocellulose membranes (Schleicher & Schuell) using a semidry blotting apparatus (Atto Corp., Tokyo). Proteins were visualized with Ponceau S and washed in TBS (20 mm Tris-HCl, pH 7.5, and 500 mm NaCl), and the nonspecific protein sites were blocked overnight with 3% nonfat, powdered milk in TBS. The blots were then challenged with antiserum raised against soybean coprogen oxidase (M.A. Santana and A.G. Smith, unpublished data) at a 1:2000 dilution in TBS containing 0.05% Tween 20 and 1% nonfat milk. Bound antibodies were visualized with goat anti-rabbit antibodies conjugated to alkaline phosphatase (Bio-Rad) according to the manufacturer's instructions.
In Situ Hybridization
Pea and soybean root nodules of various sizes were harvested 26 d after inoculation and fixed with FAA (3.7% formaldehyde, 5% acetic acid, and 50% ethanol) or with 4% para-formaldehyde and 0.25% glutaraldehyde in 50 mm sodium phosphate buffer (pH 7.2) for 4 h, dehydrated in graded ethanol and xylene series, and embedded in Paraplast. Sections (7 μm) were attached to poly-l-Lys-coated slides, deparaffinized with xylene, and rehydrated through a graded ethanol series. Cross-sections of nodules were hybridized with 35S-labeled antisense or sense RNAs (see below), as described by van de Wiel et al. (1990) from a modification of the method of Cox and Goldberg (1988). Sections were pretreated with proteinase K, dehydrated, dried under a vacuum until they were coated with NTB2 nuclear emulsion (Kodak), and exposed for 7 to 40 d at 4°C. Slides were developed in D19 developer (Kodak) and fixed in Unifix (Kodak). Sections were stained with 0.25% toluidine blue, dehydrated, and mounted with DPX (BDH, Toronto, Ontario, Canada). They were viewed and photographed with an axioscope (Zeiss) equipped with dark-field and epipolarization optics on Fujicolour HG Super 100 film.
Sense and antisense RNA probes were prepared from the pea ALA dehydratase cDNA clone pALAD209 (Boese et al., 1991; a gift from Dr. M. Timko, University of Virginia, Charlottesville), the pea PBG deaminase cDNA clone pPD1 (Witty et al., 1993), and the soybean coprogen oxidase cDNA clone pCOF (Madsen et al., 1993). RNA was transcribed in vitro from each of these clones using SP6 or T7 polymerase, as appropriate, in the presence of 35S-UTP (1250 Ci/mmol, Amersham) and was degraded to about 150-nucleotide-long fragments before hybridization.
RESULTS
Induction of Coprogen Oxidase during Nodulation
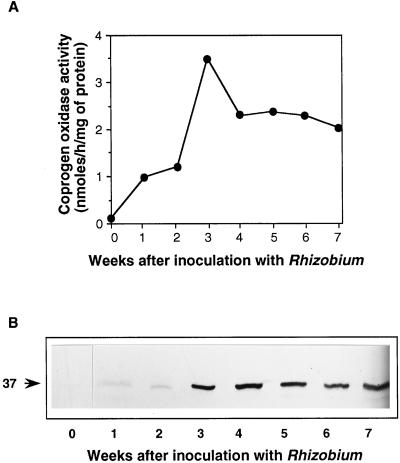
The maximum level of coprogen oxidase mRNA was seen at about 3 weeks after infection (Madsen et al., 1993), just as the number of observable nodules starts to increase. This is also the time at which the rate of heme synthesis is maximal (Nadler and Avissar, 1977; Sangwan and O'Brian, 1992). However, the amount of transcript is not necessarily a direct indication of the amount of enzyme; therefore, we investigated this with western-blot analysis and a determination of enzyme activity over the same time course of nodulation. Figure 2 shows the results of a representative experiment. The levels of activity were virtually undetectable in uninfected roots when the fluorimetric assay for coprogen oxidase was used (Labbe et al., 1985), although with the more sensitive radioactive assay (Elder and Evans, 1978), it was possible to measure activity reproducibly (data not shown). However, within 1 week of inoculation with B. japonicum, coprogen oxidase activity started to increase markedly, reaching a maximum at 3 weeks (Fig. 2A). The increase in specific activity was 27-fold compared with uninfected roots. Because there was also an increase in protein during the development of nodules, if the activity is expressed per gram fresh weight, then the increase is even more dramatic at 250-fold, although the shape of the curve over the whole time course is essentially the same (data not shown). Thereafter, coprogen oxidase activity declined, but there was considerable activity even at 7 weeks (when the nodules are effectively senescent), more in fact than was found in green leaves (see below).
Figure 2.
Changes in coprogen oxidase during the development of soybean root nodules. A, Coprogen oxidase activity measured with the fluorimetric assay. Once determinate nodules were visible (after 2–3 weeks), these were removed from the roots, but the samples before this included some root material. The sample before inoculation was root alone. Each point is the mean of three assays of the same sample extract; the variation between assays was less than 10%. B, Western-blot analysis of coprogen oxidase on the same samples assayed in A. Soluble proteins (10 μg) were separated by SDS-PAGE, blotted onto nitrocellulose, and then challenged with polyclonal antibodies raised against recombinant soybean coprogen oxidase. Bound antibodies were visualized with alkaline phosphatase-linked second antibody followed by colorimetric detection. The arrow indicates the 37-kD coprogen oxidase protein.
Because the assays were carried out on extracts from whole, unfractionated nodules, it was not possible to determine whether the enzyme activity was associated with the plant host or the bacterial symbiont. We therefore carried out western-blot analysis. Samples of the soluble protein extracts were subjected to electrophoresis and transferred to nitrocellulose. The blot was then challenged with antibodies raised against the 37-kD soybean coprogen oxidase (M.A. Santana and A.G. Smith, unpublished data). These antibodies do not cross-react with a protein of similar size in extracts of free-living B. japonicum (data not shown). It can be seen (Fig. 2B) that during the first 4 weeks there was a parallel increase in the levels of enzyme protein, although there was not such an obvious decline in the older nodules. The amount of coprogen oxidase protein was estimated by densitometry to be 14 times greater in 3-week-old nodules than in uninfected roots. Although this figure shows that no immunoreactive protein was visible in the uninfected roots, when 3 times more protein was used (30 μg), a band was detected (Fig. 3, lane 0). From these experiments, it would appear that the nodule-induced increase in expression of the soybean coprogen oxidase gene results directly in an increase in plant-specific coprogen oxidase protein, with a concomitant increase in enzyme activity within the nodule. Similar time courses of induction were observed after western-blot analysis of two of the earlier enzymes of the pathway, GSA aminotransferase (Sangwan and O'Brian, 1993; Frustaci et al., 1995) and ALA dehydratase (Kaczor et al., 1994).
Figure 3.
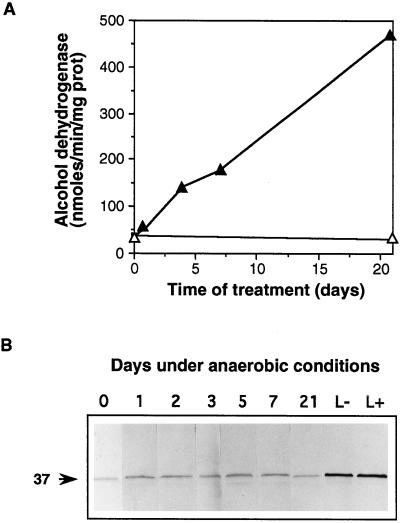
Effect of anaerobiosis on soybean roots. Two-week-old soybean plants were placed in pots so that the roots were submerged in water through which either air or nitrogen was bubbled. A, Activity of alcohol dehydrogenase in roots under aerobic (▵) or anaerobic (▴) conditions. The increase in alcohol dehydrogenase in the latter samples confirmed that the roots were anaerobic (Russell et al., 1990). Each point is the mean of two assays carried out on the same extract. Levels of coprogen oxidase activity remained essentially undetectable in these roots throughout the treatment. B, Western analysis of coprogen oxidase. Soluble protein (30 μg) from roots grown under anaerobic conditions (0–21 d) or leaves from plants grown aerobically (L−) or anaerobically (L+) for 21 d were challenged with coprogen oxidase antibodies as described in the legend of Figure 2. prot, Protein.
Correlation between Coprogen Oxidase Activity and Leghemoglobin Synthesis
The observation that plant coprogen oxidase increases during nodulation suggests that it has a role in enhanced heme biosynthesis, presumably for leghemoglobin. To investigate this further, we determined the levels of heme in different-aged nodules and calculated the rate of heme synthesis over the period 3 to 4 weeks postinoculation to be 0.73 nmol h−1 g−1 fresh weight (Table I). For comparison, the rate of chlorophyll synthesis in soybean leaves was determined, both during greening of dark-grown etiolated plants and during leaf expansion of light-grown plants, and both were found to be much higher than that of heme synthesis in nodules. In contrast, coprogen oxidase activity was much greater in the nodules than in the leaves. This was also seen in the western analysis, in which the amount of coprogen oxidase per unit protein was estimated by densitometry to be 5 times greater in 3-week-old nodules (Fig. 2B, lane 3) compared with light-grown leaves (Fig. 3B, lane L).
Table I.
Rates of tetrapyrrole synthesis and levels of tetrapyrroles in soybean tissues
| Tissue | Coprogen Oxidase | Rate of Tetrapyrrole Synthesis | Heme | Chlorophyll |
|---|---|---|---|---|
| nmol h−1 g−1 fresh wt | nmol g−1 fresh wt | |||
| Nodules 21 d after infection | 108.2 ± 9.2 | — | 58.6 ± 8.9 | |
| Nodules 28 d after infection | 81.4 ± 6.2 | 0.73 | 181.5 ± 10.3 | |
| 24-h Greened leaves | 35.0 ± 4.8 | — | 388.9 ± 23.0 | |
| 48-h Greened leaves | 15.9 ± 1.6 | 26.9 | 955.6 ± 16.5 | |
| Newly emerged leaves | 38.9 ± 1.8 | — | 2330.0 ± 58.9 | |
| Fully expanded leaves | 16.6 ± 0.2 | 3.3 | 2890.0 ± 89.5 | |
Soybean plants were grown as described in Methods. For the measurement of heme synthesis during nodulation, they were grown in a greenhouse for 14 d before infection with B. japonicum. For chlorophyll synthesis during greening, plants were grown in complete darkness for 7 d and then exposed to continuous illumination. The rate was determined between 24 and 48 h, in the exponential phase. For chlorophyll synthesis during leaf expansion of light-grown plants, samples of leaves were taken at the newly emerged stage and then again when they were fully expanded (7 d later). The enzyme activity values are the means ± se of four samples, and those for heme and chlorophyll are the means of three samples.
The fact that the activity of coprogen oxidase in nodules was much greater than that required to account for the rate of heme synthesis might mean that the induction of coprogen oxidase activity is unrelated to the need to synthesize more heme and is due to some other factor. One possibility is that the low free-oxygen content within the nodule is responsible for the induction. Coprogen oxidase uses molecular oxygen as one of its substrates, and in yeast the enzyme is induced by anaerobiosis (Zagorec and Labbe-Bois, 1986). It has been proposed that this induction in yeast is to ensure a large excess of the enzyme to scavenge any available oxygen so that heme synthesis may continue. We therefore investigated the effect of anaerobiosis on levels of coprogen oxidase by growing soybean roots in water through which either air or nitrogen was continuously bubbled. Root samples were taken after 1, 3, 5, 7, and 21 d of treatment, when plants under anaerobic conditions showed severe chlorosis of young leaves, growth retardation, and early senescence of mature leaves. There was considerable induction of alcohol dehydrogenase activity in the roots compared with those grown in air-saturated water (Fig. 3A), demonstrating that they were indeed under anaerobic stress (Russell et al., 1990). In contrast, coprogen oxidase activity remained essentially undetectable with the fluorimetric assay. This is also reflected in the western blot (Fig. 3B), in which the levels of enzyme protein remained unchanged, considerably less than that present in leaves, either those from anaerobically treated plants (L+) or from controls (L−). In nodules there was 3 to 10 times more coprogen oxidase activity and protein than in green leaves (Table I). Similar results were obtained when a suspension culture of soybean cells was grown under anaerobic conditions. Although alcohol dehydrogenase activity was induced from 63 to 1385 nmol min−1 mg−1 protein after 8 d, as observed previously by Mohanty et al. (1993), coprogen oxidase activity remained essentially undetectable throughout the anaerobic treatment. Therefore, it is unlikely that the induction of coprogen oxidase in nodules is due to the anaerobic conditions but rather is due to a response to the need for increased heme synthesis.
Levels of Other Tetrapyrrole Synthesis Enzymes in Root Nodules
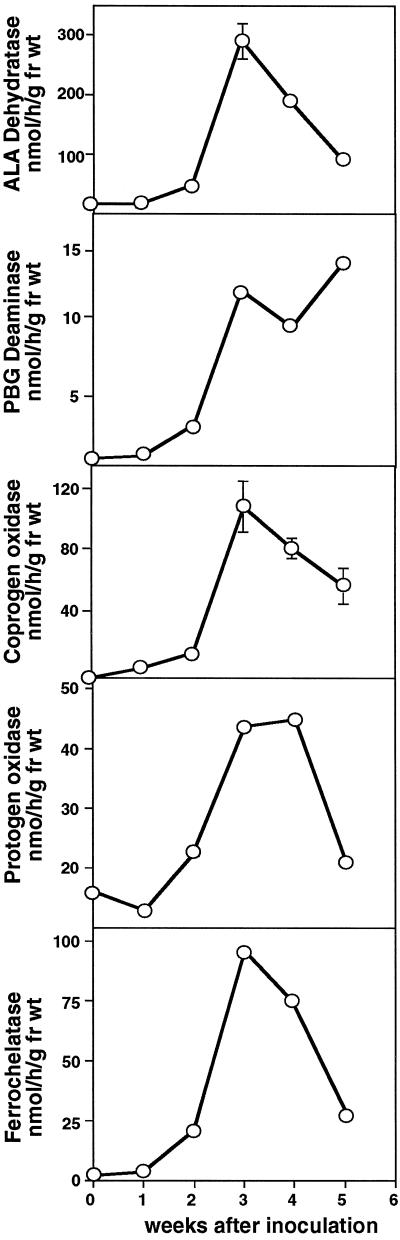
To investigate this further, the activities of other heme synthesis enzymes during the nodulation process were determined. Figure 4 shows the results of a representative experiment expressed per gram fresh weight; essentially similar shaped curves were seen for enzyme-specific activity (data not shown). The activities of all of the enzymes followed very similar time courses over the 5-week period: in each case there was an increase in activity starting at about 14 d, which was when the first nodules were visible on the roots. The levels peaked at 21 d, concomitantly with the initial appearance of leghemoglobin, and were more than sufficient to account for the rate of heme synthesis in nodules during this period. The stimulation of the first three enzymes, ALA dehydratase, PBG deaminase, and coprogen oxidase, was about 17-fold, whereas it was only 3- to 4-fold for protogen oxidase and ferrochelatase, respectively, probably because the activities of the latter two enzymes were relatively high, even in uninfected roots. This increase in enzyme activity was specific to the nodule tissue, because the activities in the roots of the nodulated plants were essentially unaltered compared with uninfected plants (Table II). When the levels in nodules were compared with those in expanded green leaves, differences between the enzymes were apparent. Both ALA dehydratase and PBG deaminase activities were higher in leaves than in nodules, whereas for the two oxidases and ferrochelatase the reverse was true.
Figure 4.
Activity of heme biosynthesis enzymes during the development of soybean root nodules. Samples of root nodule were taken as described in the legend of Figure 2 and assayed for the different enzyme activities. Each value is the mean of four replicate assays, except ferrochelatase, which is the mean of two replicates. The variation in assay values were in general less than 10%. This is a representative experiment; essentially similar time courses were observed on several occasions. fr wt, Fresh weight.
Table II.
Activities of heme synthesis enzymes in soybean tissues
| Heme Synthesis Enzyme | Before Inoculation
|
Three
Weeks after Inoculation
|
||
|---|---|---|---|---|
| Expanded leaves | Roots (uninfected) | Roots (infected) | Nodules | |
| nmol h−1 g−1 fresh wt | ||||
| ALA dehydratase (3) | 317.0 ± 19.0 | 16.7 ± 2.8 | 18.0 ± 2.0 | 291.0 ± 3.3 |
| PBG deaminase (3) | 34.0 ± 2.71 | 0.594 ± 0.04 | 0.624 ± 0.04 | 10.9 ± 0.38 |
| Coprogen oxidase (4) | 16.6 ± 0.21 | n.d.a | n.d. | 108.2 ± 9.2 |
| Protogen oxidase (4) | 15.9 ± 0.11 | 15.9 ± 2.2 | 18.4 ± 0.25 | 43.5 ± 6.0 |
| Ferrochelatase (2) | 55.7 | 22.8 | 39.2 | 95.8 |
The levels of heme synthesis enzymes were determined in roots and expanded leaves of 2-week-old soybean plants and then again in nodules and roots 3 weeks after inoculation with B. japonicum. The values are the means ± se of the number of samples indicated in parentheses. Because four molecules of PBG are required for each tetrapyrrole molecule, in general, in most plant tissues examined the ALA dehydratase rate is much higher than that of the other enzymes of the pathway (Smith, 1986, 1988).
n.d., Not detectable.
To determine whether this increase in heme synthesis enzymes was a universal feature of nodule physiology or unique to soybean, enzyme activities were determined during nodulation of pea roots with R. leguminosarum bv viciae. Table III presents the activity of ALA dehydratase, PBG deaminase, coprogen oxidase, and protogen oxidase in uninfected pea roots and in nodules 21 d after infection. For each enzyme, the activity was enhanced in the nodules, and as in soybean, both coprogen and protogen oxidase activities were greater in nodules than in green leaves.
Table III.
Activity of heme synthesis enzymes in pea tissues
| Heme Synthesis Enzyme | Expanded Leaves | Uninfected Roots | Three-Week-Old Nodules |
|---|---|---|---|
| nmol h−1 g−1 fresh wt | |||
| ALA dehydratase | 2400.0 | 30.1 | 225.0 |
| PBG deaminase | 191.8 | 2.99 | 23.5 |
| Coprogen oxidase | 11.2 | n.d. | 55.5 |
| Protogen oxidase | 17.1 | n.d. | 42.5 |
Pea plants were grown under a 16-h light, 8-h dark cycle as described in Methods. After 14 d, root and leaf samples were taken for assay, and the plants were inoculated with R. leguminosarum bv. viciae. Nodules (approximately 2 mm in diameter) were harvested after 3 weeks. The activities are the means of two determinations. The variation between samples was less than 10%. n.d., Not detectable.
In Situ Localization of Transcripts for Heme Synthesis Enzymes
Although the measurement of enzyme activities in nodules showed that the heme synthesis enzymes were elevated, as for coprogen oxidase, it is not possible to distinguish between the bacterial and plant enzymes when measuring enzyme activity alone. However, plant coprogen oxidase transcripts are increased in nodules (Madsen et al., 1993), as are transcripts of plant ALA dehydratase (Kaczor et al., 1994) and plant GSA aminotransferase (Sangwan and O'Brian, 1993; Frustaci et al., 1995). We have found that transcripts of PBG deaminase also increase during nodule development (data not shown). We have now studied the distribution of transcripts by in situ hybridization of three enzymes for which we had suitable cDNA clones. As well as demonstrating the increase in plant-specific message levels, it also provided information about the spatial location of the transcripts within the nodule.
The probes used were the cDNAs for soybean coprogen oxidase (Madsen et al., 1993), pea PBG deaminase (Witty et al., 1993), and pea ALA dehydratase (Boese et al., 1991). Antisense RNAs for each of these clones were prepared and hybridized to sections of soybean root nodules of 1 to 2 mm (3–4 weeks after infection), where the maximum induction of enzyme activity had been observed. In nodules of this size, the meristematic region has already differentiated into nodular tissue because soybean nodules are of the determinate type. The largest part of such a nodule is composed of central tissue containing mainly infected cells, which are surrounded by the nodule inner cortex and the outermost cortex (outer cortex). Sense RNAs were used as controls.
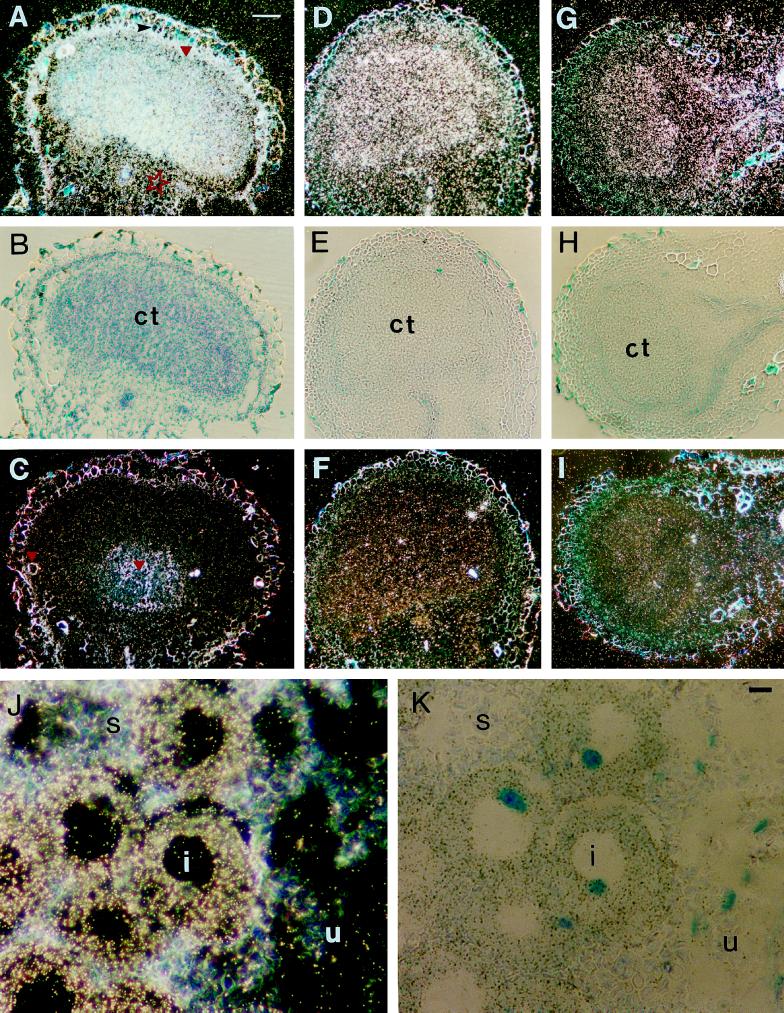
With a 35S-labeled antisense probe for ALA dehydratase, the transcript was localized in the central tissue of the nodule (Fig. 5, A and B), mainly in infected cells, with a gradient of expression in the infected zone of the central tissue. An increased signal was also present in the nodule inner cortex (Fig. 5, A and B, red triangle), but a weak signal was visible in the uninfected cells of the outer cortex (Fig. 5A, red star). This was concluded from the light-microscope observations made at a higher magnification. No hybridization was obtained when the 35S-labeled sense probe was used (Fig. 5C). Some thick-walled schlerenchyma cells, lignified xylem cells, and starch grains were refractive in dark-field microscopy (Fig. 5C, red triangles).
Figure 5.
Localization of ALA dehydratase (A–C), PBG deaminase (D–F), and coprogen oxidase (G–I) transcripts in longitudinal sections of soybean nodules using 35S-labeled probes. A, C, D, F, G, and I, Dark-field micrographs in which silver grains are visible as white dots. B, E, and H, Bright-field micrographs of the same sections that show the antisense RNAs pictured in A, D, and G. An intense signal in the infected cells of the central tissue (ct) is observed in A, D, and G. Increased expression in the nodule inner cortex (red triangle in A) and weak expression in the outer cortex (red star in A) are apparent. A black arrowhead points to the endodermis in A. No specific signal was detectable in the micrographs probed with the sense transcripts (C, F, and I). Thick-walled schlerenchyma cells, lignified xylem cells, and starch grains appear yellowish-white (red triangles in C). Bar = 100 μm. J and K, Localization of PBG deaminase transcripts in longitudinal section of the root nodule of pea using the 35S-labeled probe. Dark-field micrograph (J) shows that transcripts are localized in the cytoplasm of infected cells (i), whereas only few silver grains are present in uninfected cells (u). Starch (s) is visible as grayish grains in uninfected cells. K, Bright-field micrograph of section shown in J. Nuclei are stained blue. Bar = 10 μm.
The intensity of the signals obtained after hybridization with the PBG deaminase (Fig. 5, D and E) or with the coprogen oxidase (Fig. 5, G and H) probes was not as intense as with ALA dehydratase. However, the distribution of both PBG deaminase (Fig. 5D) and coprogen oxidase (Fig. 5G) transcripts resembled that of ALA dehydratase, i.e. they were present in the central tissue, although there was no gradient of expression or an increased expression in the nodule inner cortex. No hybridization signal was obtained with sense probes for either of these two enzyme clones (Fig. 5, F and I).
In similar experiments on pea nodules, the results were the same. The transcripts of all three enzymes appeared even more distinct in infected cells of pea than in those of soybean because of the larger size of the pea nodule cells (Fig. 5, J and K). In both pea and soybean the signal intensities of the three enzyme transcripts were not significantly above background levels in root tissues adjacent to the nodule.
Leghemoglobin gene transcripts are located mainly in infected cells (de Billy et al., 1991; Tate et al., 1994). The plant heme synthesis enzymes are therefore increased in the same type of cells where the protein moiety of leghemoglobin is synthesized.
DISCUSSION
In this study we determined the expression of five heme synthesis enzymes during the nodulation of soybean and pea roots. All of these enzymes, ALA dehydratase, PBG deaminase, coprogen oxidase, protogen oxidase, and ferrochelatase, increase during nodulation, with a maximum at 3 weeks after infection with the appropriate inoculant (Fig. 4). The time courses of induction were virtually identical for each enzyme and paralleled the increase of both heme and holo-leghemoglobin in the nodule (Nadler and Avissar, 1977; Sangwan and O'Brian, 1992). A similar observation was made for GSA aminotransferase, one of the enzymes of the C5 pathway (Sangwan and O'Brian, 1993), which was undetectable in uninfected roots, and increased to levels greater than in leaves 20 d after infection. This was paralleled by increases in transcript and enzyme protein (Frustaci et al., 1995). The same workers found that ALA dehydratase activity and message were high in nodules but were completely absent from uninfected roots (Kaczor et al., 1994). This differs somewhat from our results, in which ALA dehydratase was present before infection. Because the synthesis of heme is cell autonomous, the enzymes of the pathway would be expected to be present in all tissues of the plant to provide heme for cytochromes and other hemoproteins. The discrepancy might be explained by the fact that we used roots from 14-d-old plants, whereas Kaczor et al. (1994) measured activity in much older plants (23 d postinoculation). The detectable activity of ALA dehydratase and PBG deaminase in pea roots was found to be a maximum 5 d after germination and thereafter steadily declined (Smith, 1986). Additionally, maximum PBG deaminase activity was found in root tips compared with the older tissue in the elongation zone (Witty et al., 1993). Therefore, it is most likely that enzyme activity is present in roots but at levels too low to be assayed accurately. Similarly, we were unable to detect coprogen oxidase activity reproducibly in uninfected roots, most likely because of the limitations of the fluorimetric assay, which suffers from high background (Smith and Griffiths, 1993). In contrast, protein was detectable on western blots of uninfected roots (Fig. 3), and coprogen activity was measurable in roots (data not shown) using a more sensitive radiochemical assay (Elder and Evans, 1978).
Our assays of enzyme activity were carried out on extracts of whole, unfractionated nodules; therefore, it is not possible to distinguish whether the enzymes were plant or bacteria derived. However, support for the conclusion that it is the plant enzymes that are stimulated comes from the results of cell-fractionation studies. Sangwan and O'Brian (1991) found that ALA dehydratase and PBG deaminase levels were the same in free-living and symbiotic B. japonicum cells, and Jacobs et al. (1990) found increased protogen oxidase activity in the peribacteroid membrane (which is plant derived) compared with uninfected roots. We have obtained further evidence by investigating the levels of enzyme protein with antibodies and transcripts with the available cDNA probes. These molecular probes are specific for the plant enzymes and do not detect those from the symbiont. For coprogen oxidase, in situ hybridization confirmed earlier results (Madsen et al., 1993) that there is a specific increase in mRNA levels in nodules, and with western analysis we found a concomitant increase in enzyme protein, which correlates with the increase in enzyme activity. The similarly large increase in ALA dehydratase and PBG deaminase transcripts detected by northern analysis and in situ hybridization also provides evidence that the increase in enzyme activity is due to the plant-specific enzyme. Furthermore, the increased level of transcripts for the plant enzymes is specifically in the infected cells, where the apoprotein of leghemoglobin is exclusively synthesized (de Billy et al., 1991; Tate et al., 1994).
Thus, our results provide additional evidence for the involvement of the plant heme synthesis enzymes in the production of heme in the mature nodule. Because the major hemoprotein by far is leghemoglobin, the conclusion to be drawn is that the plant host provides some if not all of its prosthetic heme. At first this might seem contradictory to results obtained from the study of rhizobial mutants of heme synthesis. For instance, although hemA mutants (defective in ALA synthase) of Rhizobium sp. NGR234 (Stanley et al., 1988), R. meliloti (Leong et al., 1982, 1985; Mohapatra and Puhler, 1986; de Bruijn et al., 1989), and Azorhizobium caulinodans (Pawlowski et al., 1993) are able to elicit nodules on their corresponding host roots, the nodules are unable to fix nitrogen. Similarly, B. japonicum mutants defective in ALA dehydratase (hemB; Chauhan and O'Brian, 1993) and ferrochelatase (hemH; Frustaci and O'Brian, 1992) elicit such so-called Fix− nodules. In all of these examples, there is no apoleghemoglobin. Another B. japonicum mutant lacking protogen oxidase activity, which also forms Fix− nodules, nonetheless contains the leghemoglobin apoprotein (O'Brian et al., 1987) despite the lack of bacterial heme. In this case, the primary mutation has been demonstrated to be in a gene involved in the biosynthesis of c-type cytochromes (Ramseier et al., 1991). In contrast, a hemA mutant of B. japonicum MLG1, which has no detectable heme in the free-living form, induces the formation of functional nodules containing holo-leghemoglobin and other bacterial hemes (Guerinot and Chelm, 1986; Chauhan and O'Brian, 1993). This discrepancy has been explained by the proposal that ALA can be provided to the bacterial symbiont by the soybean host but that later intermediates cannot be transported across the peribacteroid membrane (Sangwan and O'Brian, 1991). Further evidence for this model comes from the observation that those Rhizobium species that require the hemA gene for symbiosis are severely deficient in ALA uptake activity (McGinnis and O'Brian, 1995).
Further work on the hemA mutants of R. meliloti using ultrastructural analysis, translation in vitro of plant RNA, and northern blots found that there was (a) atypical nodule morphology and infection thread development, (b) arrest early in development, and (c) only early nodulin genes expressed (Dickstein et al., 1991). Three of the mutants initiated nodules that did not contain any intracellular bacteria. A reduction in viable bacterial cells is observed in the hemB and hemH mutants as well (Frustaci and O'Brian, 1992; Chauhan and O'Brian, 1993). Thus, the Fix− phenotype may not be a direct consequence of the bacteria's inability to supply heme for leghemoglobin but rather is a pleiotropic effect of the failure of nodule development.
Although we have clearly demonstrated induction in the nodule of plant-specific enzymes of heme biosynthesis, it remains uncertain as to what signal leads to this induction. Although the time course of increase in the level of the coprogen oxidase transcript was the same as that for leghemoglobin, there were no significant sequence similarities between the coprogen oxidase promoter and those of other late nodulin genes (Madsen et al., 1993). The induction is also unlikely to be in direct response to anoxia, which increases expression of the yeast coprogen oxidase gene (Zagorec and Labbe-Bois, 1986), because we could detect no increase in the soybean enzyme in roots subjected to anaerobiosis. This is perhaps not surprising, because although the nodule environment has a low free-oxygen content, this is mediated by leghemoglobin, which requires coprogen oxidase to provide its prosthetic group. Therefore, the requirement for increased coprogen oxidase activity would be before the production of a hypoxic environment. One interesting observation is that the coprogen oxidase gene appears to be induced in nodules to a much higher level than in leaves, even though the rates of tetrapyrrole synthesis are much greater in the latter (Table I). Similarly, protogen oxidase activity is greater in nodules than in leaves (Tables II and III). It is conceivable that the efficiency of extraction of the enzymes from the two tissues is different, although intuitively it would be expected to be easier from the softer leaf tissue. The present study is the first, to our knowledge, in which the activity of several of the tetrapyrrole synthesis enzymes has been determined in different tissues of the same plant, so this question must remain unresolved. Nonetheless, in nodules all of the enzymes are in vast excess over that required to sustain the observed rate of heme, even ALA dehydratase and PBG deaminase, which appear to be higher in leaves than in nodules.
An explanation for this apparent anomaly would be that there is considerable heme turnover; therefore, the rate we have calculated is an underestimate. Evidence for the ability of plants to turn over heme comes from the study of tetrapyrrole synthesis during the greening of etiolated seedlings, when there is massive flux through the chlorophyll branch of the pathway. When [14C]ALA was administered to greening barley seedlings after 5 h of illumination, the specific radioactivities of extractable protoheme and pheophorbide (a chlorophyll derivative) were essentially the same, indicating that both were synthesized during the period. However, heme did not accumulate (Castelfranco and Jones, 1975). If a similar situation prevails in the nodule, the next obvious question is for what purpose is the heme synthesized? One possibility is that heme is not just required as a prosthetic group for leghemoglobin and respiratory cytochromes but that it is also involved in the regulation of nodule formation and/or function. Jensen et al. (1986) found that a heme-specific regulatory system in yeast modulated the translation of chimeric genes containing 5′ flanking sequences from the soybean leghemoglobin c3 gene. More intriguingly, the FixL protein of R. meliloti has been shown to be a hemoprotein (Monson et al., 1992). FixL and FixJ form the two-component regulatory system involved in sensing and transducing the low-oxygen signal, which leads to expression of the rhizobial genes required for nitrogen fixation.
In summary, the evidence presented in this paper supports that of earlier studies (Jacobs et al., 1990; Madsen et al., 1993; Sangwan and O'Brian, 1993; Kaczor et al., 1994, Frustaci et al., 1995; O'Brian, 1996), and together provide strong evidence that the plant cell is responsible for making at least some, if not all, of the heme moiety of leghemoglobin. Thus, the hypothesis that the plant host makes the apoprotein and the bacterial endosymbiont makes the prosthetic group (Appleby, 1984) is no longer tenable. Nonetheless, the fact that several rhizobial heme-synthesis mutants are unable to form functional nodules suggests the possibility that heme or some other tetrapyrrole is required as a signal for the induction of certain plant genes that are necessary for the later stages of nodule development and/or function.
ACKNOWLEDGMENTS
K.P.-M. thanks Dr. Ton Bisseling, Dr. Wei-Cai Yang, and Jan-Elo Jørgensen for helpful discussion of in situ-hybridization techniques. We are grateful to Dr. Mike Timko (University of Virginia, Charlottesville) for the gift of the cDNA clone for pea ALA dehydratase.
Abbreviations:
- ALA
5-aminolevulinic acid
- coprogen oxidase
coproporphyrinogen III oxidase
- GSA
glutamate-1-semialdehyde
- PBG
porphobilinogen
- protogen
protoporphyrinogen IX
Footnotes
This work was supported by a studentship from the Venezuelan National Academy of Science (M.A.S.), and by a short-term fellowship from the European Molecular Biology Organization and grants from the Joint Committee of the Nordic Natural Science Research Councils (K.P.-M.).
LITERATURE CITED
- Appleby CA. Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol. 1984;35:443–478. [Google Scholar]
- Arnon DI. Copper enzymes in chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese QF, Spano AJ, Li J, Timko MP. Aminolevulinic acid dehydratase in pea (Pisum sativum L.)—identification of an unusual metal-binding domain in the plant enzyme. J Biol Chem. 1991;266:17060–17066. [PubMed] [Google Scholar]
- Castelfranco PA, Jones OTG. Protoheme turnover and chlorophyll synthesis in greening barley tissue. Plant Physiol. 1975;55:485–490. doi: 10.1104/pp.55.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S, O'Brian MR. Bradyrhizobium japonicum delta-aminolevulinic acid dehydratase is essential for symbiosis with soybean and contains a novel metal-binding domain. J Bacteriol. 1993;175:7222–7227. doi: 10.1128/jb.175.22.7222-7227.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KH, Goldberg RB. Analysis of plant gene expression. In: Shaw CH, editor. Plant Molecular Biology: A Practical Approach. Oxford, UK: IRL Press; 1988. pp. 1–34. [Google Scholar]
- Cutting JA, Schulman HM. The site of heme synthesis in soybean root nodules. Biochim Biophys Acta. 1969;192:486–493. doi: 10.1016/0304-4165(69)90398-5. [DOI] [PubMed] [Google Scholar]
- de Billy F, Barker DG, Gallusci P, Truchet G. Leghaemoglobin gene transcription is triggered in a single cell layer in the indeterminate nitrogen-fixing root nodule of alfalfa. Plant J. 1991;1:27–35. [Google Scholar]
- de Bruijn FJ, Rossbach S, Schneider M, Ratet P, Messmer S, Szeto WW, Ausubel FM, Schell J. Rhizobium meliloti 1021 has 3 differentially regulated loci involved in glutamine biosynthesis, none of which is essential for symbiotic nitrogen-fixation. J Bacteriol. 1989;171:1673–1682. doi: 10.1128/jb.171.3.1673-1682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein R, Scheirer DC, Fowle WH, Ausubel FM. Nodules elicited by Rhizobium meliloti heme mutants are arrested at an early stage of development. Mol Gen Genet. 1991;230:423–432. doi: 10.1007/BF00280299. [DOI] [PubMed] [Google Scholar]
- Elder GH, Evans JO. A radiochemical method for the measurement of coproporphyrinogen oxidase and the utilization of substrates other than coproporphyrinogen III by the enzyme from rat liver. Biochem J. 1978;169:205–214. doi: 10.1042/bj1690205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci JM, O'Brian MR. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J Bacteriol. 1992;174:4223–4229. doi: 10.1128/jb.174.13.4223-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci JM, Sangwan I, O'Brian MR. Gsa1 is a universal tetrapyrrole synthesis gene in soybean and is regulated by a GAGA element. J Biol Chem. 1995;270:7387–7393. doi: 10.1074/jbc.270.13.7387. [DOI] [PubMed] [Google Scholar]
- Guerinot ML, Chelm BK. Bacterial δ-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci USA. 1986;83:1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker H. Biochemistry and physiology of nitrogen-fixation. Bioessays. 1988;9:112–117. [Google Scholar]
- Jacobs JM, Jacobs NJ, Borotz SE, Guerinot ML. Effects of the photobleaching herbicide, acifluorofen-methyl, on protoporphyrinogen oxidation in barley organelles, soybean root mitochondria, soybean root nodules and bacteria. Arch Biochem Biophys. 1990;280:369–375. doi: 10.1016/0003-9861(90)90344-x. [DOI] [PubMed] [Google Scholar]
- Jensen EO, Marcker KA, Villadsen IS. Heme regulates the expression in Saccharomyces cerevisiae of chimaeric genes containing 5′-flanking soybean leghemoglobin sequences. EMBO J. 1986;5:843–847. doi: 10.1002/j.1460-2075.1986.tb04293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EO, Paludan K, Hyldig-Nielsen JJ, Jørgensen P, Marcker KA. The structure of a chromosomal leghaemoglobin gene from soybean. Nature. 1981;291:677–679. [Google Scholar]
- Kaczor CM, Smith MW, Sangwan I, O'Brian MR. Plant δ-aminolevulinic acid dehydratase. Expression in soybean root nodules and evidence for a bacterial lineage of the Alad gene. Plant Physiol. 1994;104:1411–1417. doi: 10.1104/pp.104.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannangara CG, Gough SP, Bruyant P, Hoober JK, Kahn A, von Wettstein D. Transfer RNA-Glu as a cofactor in delta-aminolevulinate biosynthesis—steps that regulate chlorophyll synthesis. Trends Biochem Sci. 1988;13:139–143. doi: 10.1016/0968-0004(88)90071-0. [DOI] [PubMed] [Google Scholar]
- Labbe P, Camadro J-M, Chambon H. Fluorometric assays for coproporphyrinogen oxidase and protoporphyrinogen oxidase. Anal Biochem. 1985;149:248–260. doi: 10.1016/0003-2697(85)90502-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leong SA, Ditta GS, Helinski DR. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for δ-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982;257:8724–8730. [PubMed] [Google Scholar]
- Leong SA, Williams PH, Ditta GS. Analysis of the 5′ regulatory region of the gene for delta-aminolevulinic acid synthetase of Rhizobium meliloti. Nucleic Acids Res. 1985;13:5956–5976. doi: 10.1093/nar/13.16.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen O, Sandal L, Sandal N, Marcker KA. A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules. Plant Mol Biol. 1993;23:35–43. doi: 10.1007/BF00021417. [DOI] [PubMed] [Google Scholar]
- McGinnis SD, O'Brian MR. The rhizobial hemA gene is required for symbiosis in species with deficient delta-aminolevulinic acid uptake activity. Plant Physiol. 1995;108:1547–1552. doi: 10.1104/pp.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty B, Wilson PM, ap Rees T. Effects of anoxia on growth and carbohydrate-metabolism in suspension-cultures of soybean and rice. Phytochemistry. 1993;34:75–82. [Google Scholar]
- Mohapatra SS, Puhler A. Detection of nodule-specific polypeptides from effective and ineffective root-nodules of Medicago sativa L. J Plant Physiol. 1986;126:269–281. [Google Scholar]
- Monson EK, Weinstein M, Ditta GS, Helinski DR. The FixL protein of Rhizobium meliloti can be separated into a heme-binding oxygen sensing domain and a functional C-terminal kinase domain. Proc Natl Acad Sci USA. 1992;89:4280–4284. doi: 10.1073/pnas.89.10.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler KD, Avissar YJ. Heme synthesis in soybean root nodules. On the role of bacteroid δ-aminolevulinic acid synthase and δ-aminolevulinic acid dehydratase in the synthesis of the heme of leghemoglobin. Plant Physiol. 1977;60:433–436. doi: 10.1104/pp.60.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian MR. Heme synthesis in the Rhizobium-legume symbiosis—a palette for bacterial and eukaryotic pigments. J Bacteriol. 1996;178:2471–2478. doi: 10.1128/jb.178.9.2471-2478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian MR, Kirshbom PM, Maier RJ. Bacterial heme-synthesis is required for expression of the leghemoglobin holoprotein but not the apoprotein in soybean root-nodules. Proc Natl Acad Sci USA. 1987;84:8390–8393. doi: 10.1073/pnas.84.23.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski K, Gough SP, Kannangara CG, Debruijn FJ. Characterization of a 5-aminolevulinic acid synthase mutant of Azorhizobium caulinodans Ors571. Mol Plant-Microbe Interact. 1993;6:35–44. [Google Scholar]
- Porra RJ, Lascelles J. Studies on ferrochelatase: the enzymatic formation of haem in proplastids, chloroplasts and plant mitochondria. Biochem J. 1968;108:343–348. doi: 10.1042/bj1080343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseier TM, Winteler HV, Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991;266:7793–7803. [PubMed] [Google Scholar]
- Russell DA, Wong DML, Sachs MM. The anaerobic response of soybean. Plant Physiol. 1990;92:401–407. doi: 10.1104/pp.92.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I, O'Brian MR. Evidence for an inter-organismic heme biosynthetic pathway in symbiotic soybean root nodules. Science. 1991;251:1220–1222. doi: 10.1126/science.251.4998.1220. [DOI] [PubMed] [Google Scholar]
- Sangwan I, O'Brian MR. Characterization of delta-aminolevulinic acid formation in soybean root nodules. Plant Physiol. 1992;98:1074–1079. doi: 10.1104/pp.98.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I, O'Brian MR. Expression of the soybean (Glycine max) glutamate 1-semialdehyde aminotransferase gene in symbiotic root nodules. Plant Physiol. 1993;102:829–834. doi: 10.1104/pp.102.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG (1986) Enzymes for chlorophyll synthesis in developing peas. In G Akoyunoglou, H Senger, eds, Regulation of Chloroplast Differentiation. Alan R. Liss, New York, pp 49–54
- Smith AG. Subcellular localization of two porphyrin-synthesis enzymes in Pisum sativum (pea) and Arum (cuckoo-pint) species. Biochem J. 1988;249:423–428. doi: 10.1042/bj2490423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Griffiths WT. Enzymes of heme and chlorophyll synthesis. Methods Plant Biochem. 1993;9:299–343. [Google Scholar]
- Smith AG, Marsh O, Elder GH. Investigation of the subcellular location of the tetrapyrrole-biosynthesis enzyme coproporphyrinogen oxidase in higher plants. Biochem J. 1993;292:503–508. doi: 10.1042/bj2920503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J, Dowling DN, Broughton WJ. Cloning of hemA from Rhizobium sp NGR234 and symbiotic phenotype of a gene-directed mutant in diverse legume genera. Mol Gen Genet. 1988;215:32–37. [Google Scholar]
- Tate R, Patriarca EJ, Riccio A, Defez A, Iaccarino M. Development of Phaseolus vulgaris root-nodules. Mol Plant Microbe Interact. 1994;7:582–589. [Google Scholar]
- van de Wiel C, Scheres B, Franssen H, van Lierop MJ, van Lammeren A, van Kammen A, Bisseling T. The early nodulin transcript enod2 is located in the nodule parenchyma (inner cortex) of pea and soybean root-nodules. EMBO J. 1990;9:1–7. doi: 10.1002/j.1460-2075.1990.tb08073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty M, Wallace-Cook ADM, Albrecht H, Spano AJ, Michel H, Shabanowitz J, Hunt DF, Timko MP, Smith AG. Structure and expression of chloroplast-localized porphobilinogen deaminase from pea (Pisum sativum L.) isolated by redundant PCR. Plant Physiol. 1993;103:139–147. doi: 10.1104/pp.103.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorec M, Buhler JM, Treich I, Keng T, Guarente L, Labbe-Bois R. Isolation, sequence, and regulation by oxygen of the yeast HEM13 gene coding for coproporphyrinogen oxidase. J Biol Chem. 1988;263:9718–9724. [PubMed] [Google Scholar]
- Zagorec M, Labbe-Bois R. Negative control of yeast coproporphyrinogen oxidase synthesis by heme and oxygen. J Biol Chem. 1986;261:2506–2509. [PubMed] [Google Scholar]