Abstract
Most animal studies using passive administration of HIV broadly neutralizing monoclonal antibodies (bnMAbs) have associated protection against high-dose mucosal viral challenge with relatively high serum concentrations of antibody. We recently identified several bnMAbs remarkable for their in vitro potency against HIV. Of these bnMAbs, PGT121 is one of the most broad and potent antibodies isolated to date and shows 10- to 100-fold higher neutralizing activity than previously characterized bnMAbs. To evaluate the protective potency of PGT121 in vivo, we performed a protection study in rhesus macaques. Animals were i.v. administered 5 mg/kg, 1 mg/kg, or 0.2 mg/kg PGT121 24 h before being vaginally challenged with a single high dose of chimeric simian-human immunodeficiency virus (SHIV)SF162P3. Sterilizing immunity was achieved in all animals administered 5 mg/kg and 1 mg/kg and three of five animals administered 0.2 mg/kg PGT121, with corresponding average antibody serum concentrations of 95 µg/mL, 15 µg/mL, and 1.8 µg/mL, respectively. The results suggest that a protective serum concentration for PGT121 is in the single-digit µg/mL for SHIVSF162P3, showing that PGT121 can mediate sterilizing immunity at serum concentrations that are significantly lower than those observed in previous studies and that may be achievable through vaccination with the development of a suitable immunogen.
Keywords: passive transfer, animal model, antibody prophylaxis
Elicitation of broad and potent neutralizing antibodies with multiple specificities is likely to be a crucial property of an effective HIV vaccine because neutralization is widely considered to be the best correlate for antibody-mediated protection against HIV (1–5). Indeed, studies using the chimeric simian/HIV (SHIV)/macaque model have repeatedly shown that passive transfer of broadly neutralizing monoclonal antibodies (bnMAbs), such as 2G12, b12, 2F5, and 4E10, can induce protection against mucosal challenge (6–13). In the so-called high-dose challenge model, to ensure the infection of control animals, the challenge dose is typically several orders of magnitude higher than that encountered in most human exposures (14). Under such conditions, protection has required relatively high serum antibody concentrations (6–13). Comparison between protection and neutralization has suggested that approximately 50% of the exposed animals are protected against high-dose viral challenge when the serum bnMAb levels are of the order of tens to several thousands times the neutralizing IC50 values measured in vitro. The numbers depend on a number of factors, including the neutralization assay used to estimate IC50 values (8, 12, 15). Studies using repeated low-dose challenges and the bnMAb b12 suggest that protection may be achieved at notably lower serum antibody concentrations and neutralizing titers than are required for high-dose viral challenge (16, 17).
In recent years, we have seen the emergence of a new class of remarkably potent neutralizing antibodies such as PG9, VRC01, 3BNC117, NIH45-46, PGV04, PGT121, and PGT128 (18–22). Of these bnMAbs, PGT121 is one of the most potent and broad neutralizing anti-HIV antibodies isolated to date. Using a 162-pseudovirus panel, representing all major circulating clades, PGT121 was demonstrated to have 10-fold higher neutralizing potency than PG9, VRC01, and PGV04 and a 100-fold higher potency than the earlier described bnMAbs 2G12, b12, and 4E10 (19). The breadth of PGT121 was shown to match or exceed that of most other bnMAbs at low antibody concentration because it neutralized 44% of the 162-virus panel with an IC50 below 0.1 µg/mL. This percentage is almost twice that neutralized under the same conditions by PG9, VRC01, and PGV04 and 20–40 times that neutralized by the bnMAbs 2G12, b12, and 4E10, previously investigated in passive protection studies (19). A crystal structure of PGT121 in complex with its epitope has not yet been solved. However, epitope mapping studies have shown that PGT121 competes with V3 loop antibodies for binding to gp120 and does not bind to gp120deltaV3, suggesting that its epitope is in proximity to or contiguous with the V3 loop (19). Interestingly, PGT121 was also shown to compete with the glycan-specific bnMAb 2G12 and to be sensitive to an N332A substitution, which removes the glycan at this position, suggesting that it belongs to the group of antibodies that bind to a high-mannose patch on the glycan shield of gp120 (19, 23).
Given the remarkable in vitro neutralization potency of PGT121, we wished to determine to what degree this potency would be translated into enhanced protection in vivo. We speculated that protection against SHIV challenge might be achieved at serum concentrations well below those previously determined for other HIV-specific bnMAbs. To investigate in detail, we designed a three-dose passive protection titration study in rhesus macaques. The challenge virus was the R5-specific SHIVSF162P3, which PGT121 was shown to neutralize with an IC50 of 0.005 µg/mL in the TZM-bl/pseudovirus assay and with an IC50 of 0.002 µg/mL in the rhesus macaque peripheral blood mononuclear cell (PBMC)/replication competent virus assay. We describe here that prophylactically administered PGT121 provided sterilizing immunity to all animals given antibody doses of 5 and 1 mg/kg. A dose of only 0.2 mg/kg protected three of five animals. At this dose, the animals had, at the time of challenge, serum concentrations of the order of 1.2–2.6 µg/mL. In comparison, more than a 100-fold higher serum antibody concentration is required to achieve a comparable level of protection for 2G12 against SHIVSF162P3 and for b12 against the related SHIVSF162P4 isolate (8, 11). Our results demonstrate that the high in vitro neutralization potency of PGT121 translates into enhanced protection against viral mucosal challenge in macaques and suggests that protection against mucosal exposure by systemic vaccination in humans may be achievable if the appropriate antibody specificities can be elicited.
Results
PGT121 Potently Neutralizes the Challenge Virus SHIVSF162P3.
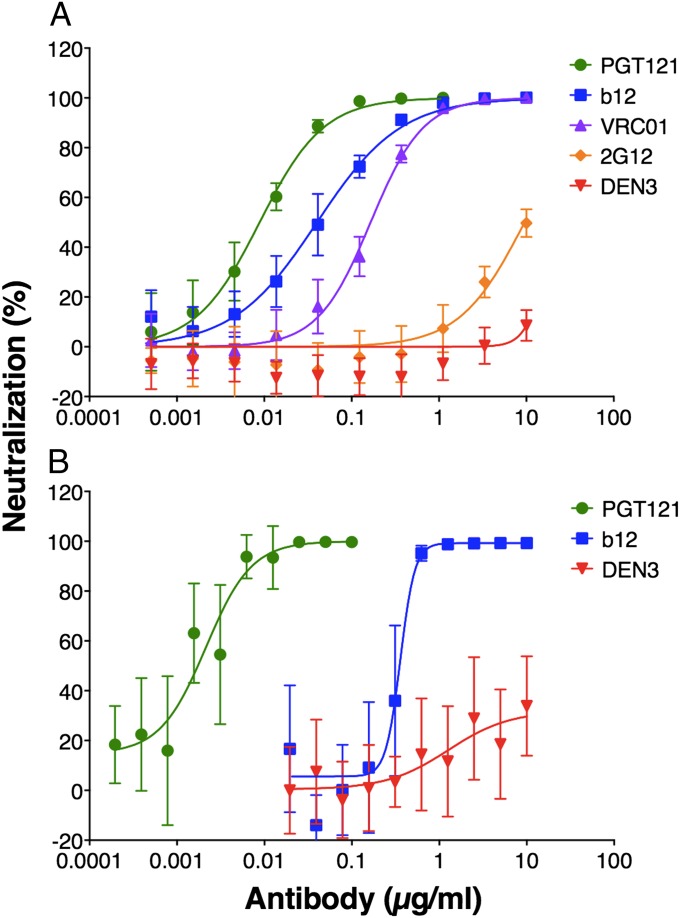
PGT121 has been shown to have greater neutralization potency in comparison with other bnMAbs such as PG9, VRC01, PGV04, b12, and 2G12 (19). In protection studies using rhesus macaques, a much-used challenged virus is the HIV-1 SF162-derived R5 tier 2 virus SHIVSF162P3 and, to evaluate the in vitro potency of PGT121 against this virus, we performed two neutralization assays. BnMAbs, previously tested in protection studies against SHIVSF162P3, i.e., b12 and 2G12, as well as VRC01, were included in a TZM-bl–based pseudovirus assay (9, 11). The IC50 for PGT121 was 0.005 µg/mL, whereas the IC50 values for b12, 2G12, and VRC01 were 0.04, 8.8, and 0.10 µg/mL, respectively (Fig. 1A). We also compared PGT121 to b12 in a rhesus macaque PBMC-based assay using replication competent SHIVSF162P3. The IC50 for PGT121 was 0.002 µg/mL, whereas the IC50 for b12 was 0.36 µg/mL (Fig. 1B). No neutralization was observed in either assay by using the antidengue antibody DEN3. The assays demonstrate that PGT121 readily neutralizes SHIVSF162P3 and with notably higher potency than previously tested bnMAbs.
Fig. 1.
Neutralization of SHIVSF162P3 by bnMAb PGT121. Neutralization was measured by two assays: SHIVSF162P3 pseudovirus using TZM-bl cells (A) and replication competent SHIVSF162P3 using rhesus macaque PBMCs (B). Both assays show potent neutralization of SHIVSF162P3 by PGT121 compared with other bnMAbs. In the TZM-bl cell-based assay the following IC50s were obtained, 0.005 µg/mL PGT121, 0.04 µg/mL b12, 8.8 µg/mL 2G12, and 0.10 µg/mL VRC01 and in the rhesus macaque PBMC-based assay 0.002 µg/mL PGT121 and 0.36 µg/mL b12. Values are means and SDs of five wells. The assays were performed three times with similar results.
PGT121 Protects Against Mucosal SHIVSF162P3 Challenge at Low Administered Dose.
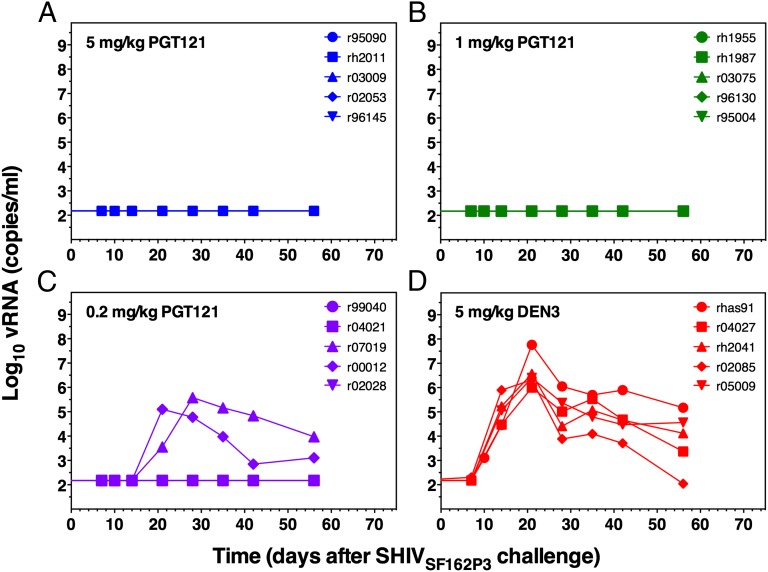
To evaluate the activity of PGT121 in vivo, we designed a three-dose antibody titration protection study by using the SHIV/macaque model. A total of 20 animals were used, and all animals were pretreated with medroxy-progesterone (Depo-Provera) to synchronize menstrual cycles and thin the vaginal mucosa. The study included four treatment groups of five animals each; one group received 5 mg/kg PGT121, one group received 1 mg/kg PGT121, one group received 0.2 mg/kg PGT121, and one group received 5 mg/kg of an isotype control anti-dengue antibody, DEN3. The antibodies were administered i.v. 24 h before the animals were vaginally challenged with a single high dose of SHIVSF162P3 (300 TCID50). All animals treated with the isotype control antibody became infected with a peak viremia at week 3 of 106 to 108 viral copies per mL (Fig. 2D). In contrast, no detectable viremia (detectable limit was 150 RNA copies per mL) was observed for all animals given 5 mg/kg and 1 mg/kg PGT121 (Fig. 2), suggesting sterilizing immunity. In the group receiving 0.2 mg/kg PGT121, three of five animals were fully protected and the two animals that became infected showed lower level of peak viremia (less than 106 viral copies per mL) and one animal also showed a delayed peak viremia (week 4) compared with control animals (Fig. 2C).
Fig. 2.
Plasma viremia in macaques pretreated with different doses of the bnMAbs PGT121 and challenged with SHIVSF162P3. Plasma viral loads are shown for animals administered 5 mg/kg PGT121 (A), 1 mg/kg PGT121 (B), 0.2 mg/kg PGT121 (C), and 5 mg/kg DEN3 (D). Sterilizing immunity was observed for all animals given 5 mg/kg and 1 mg/kg PGT121, and in three of five animals given 0.2 mg/kg. One animal (r95090) administered 5 mg/kg PGT121 was euthanized 3 wk after challenge because of unrelated illness. Necropsy showed no sign of viral infection. All animals treated with the control antibody DEN3 became infected. The assay sensitivity limit was 150 RNA copies per mL.
Pharmacokinetics of PGT121 in the Protection Study.
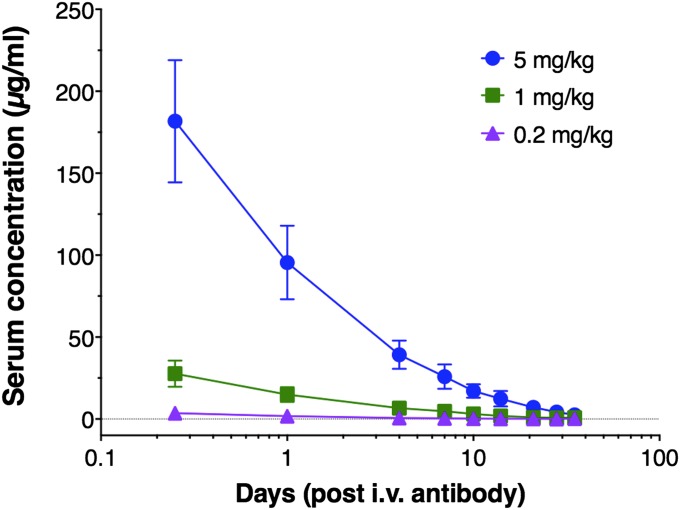
Serum samples were obtained throughout the study, and concentrations of PGT121 were determined by an HIV-1 gp120-specific ELISA. The results show that the animals administered 5 mg/kg, 1 mg/kg, and 0.2 mg/kg PGT121 had average serum concentrations of 95 µg/mL, 15 µg/mL, and 1.8 µg/mL at the time of challenge (Fig. 3). Based on the level of serum antibody decay (one-phase exponential decay), starting at day 4 to account for the initial distribution phase, the half-life of PGT121 was determined to be 5.8 d (Fig. 3) (10). In addition, serum-neutralizing titers were determined by using a pseudovirus/TZM-bl assay for samples up to 10 d after antibody administration (Table 1). Serum concentrations and serum neutralizing titers showed good correlation as calculation of a serum IC50 concentration of PGT121 (serum concentration divided by the serum dilution resulting in 50% inhibition) showed an average serum IC50 of 0.01 µg/mL, which is very close to the IC50 (0.005 µg/mL) obtained from purified antibody (Fig. 1). We finally determined the concentration of PGT121 in vaginal secretions after antibody administration. As observed previously for b12 and 2G12 (8, 11), only a small fraction of the administered antibody reached the vaginal surface. Animals administered 5 mg/kg and 1 mg/kg had average concentrations of 0.9 µg/mL and 0.2 µg/mL at the time of challenge (Table 2). The vaginal concentration of PGT121 in animals administered 0.2 mg/kg was below the detection limit (∼0.1 µg/mL) of the assay (Table 2).
Fig. 3.
Serum concentration of PGT121 in antibody-treated animals. PGT121 concentrations were determined throughout the study. The results show average serum concentrations of 95 µg/mL, 15 µg/mL, and 1.8 µg/mL at the time of challenge in the 5 mg/kg group, 1 mg/kg group, and 0.2 mg/kg group, respectively. A serum half-life for the elimination phase was calculated to be 5.8 d. Values are means and SDs.
Table 1.
Neutralizing antibody titers in sera
| Group | Animal | Neutralizing titers*,†, IC50 |
|||
| Day 1 | Day 4 | Day 7 | Day 10 | ||
| 5 mg/kg | r95090 | 6,880 | 2,238 | 1,762 | 989 |
| rh2011 | 7,310 | 5,111 | 2,835 | 1,967 | |
| r03009 | 7,977 | 3,253 | 3,670 | 1,958 | |
| r02053 | 5,893 | 2,397 | 1,240 | 657 | |
| r96145 | 7,349 | 2,823 | 2,259 | 1,344 | |
| 1 mg/kg | rh1955 | 1,938 | 641 | 469 | 154 |
| rh1987 | 1,804 | 904 | 508 | 291 | |
| r03075 | 1,326 | 391 | 206 | 130 | |
| r96130 | 1,166 | 454 | 599 | 192 | |
| r95004 | 1,242 | 514 | 333 | 190 | |
| 0.2 mg/kg | r99040 | 200 | 88 | 80 | 44 |
| r04021 | 206 | 99 | 95 | 62 | |
| r07019 | 213 | 87 | 96 | 57 | |
| r00012 | 309 | 113 | 107 | 48 | |
| r02028 | 495 | 271 | 154 | 119 | |
*Numbers represent reciprocal dilution of serum producing 50% neutralization.
†Days after antibody administration, day 1 is the day of challenge.
Table 2.
PGT121 concentration in vaginal fluid
| Group | Antibody concentration*, µg/mL |
||
| 6 h | 24 h† | 96 h | |
| 5 mg/kg | 1.4 | 0.9 | 1.1 |
| 1 mg/kg | 0.3 | 0.2 | 0.3 |
| 0.2 mg/kg‡ | — | — | — |
*Time points after infusion of antibody.
†Time of viral challenge.
‡Below detection level of assay.
Discussion
Here, we report that passive transfer of the highly potent neutralizing anti-HIV gp120 antibody PGT121 efficiently protects against high-dose challenge of SHIV in macaques. Sterilizing immunity was observed for all animals administered 5 mg/kg and 1 mg/kg and for three of five animals administered 0.2 mg/kg. The corresponding average serum concentrations at the time of challenge for the three treatment groups were 95 µg/mL, 15 µg/mL, and 1.8 µg/mL, demonstrating protection against high-dose viral challenge at relatively low single-digit µg/mL serum antibody concentrations.
Protection against SHIVSF162P3 or the related SHIVSF162P4 isolate has been described for 2G12 and b12 by using vaginal challenge in the macaque model (8, 9, 11). Comparison of the average serum concentration at the time of challenge necessary for protection of 50% of the challenged animals shows that PGT121 (1.8 µg/mL) is effective at serum concentrations ∼600-fold lower than for 2G12 (1,000 µg/mL) and 100-fold lower than for b12 (200 µg/mL, challenge virus was SHIVSF162P4) (8, 11). Therefore, the superior protective activity of PGT121 correlates with its more potent neutralizing activity against the challenge virus (19).
Our results report on protection against a single virus (SHIVSF162P3) by a single antibody (PGT121). Cross-checking the neutralization breadth and potency of PGT121 against the previously tested panel of 162 pseudoviruses shows that 25 viruses (15%) are neutralized with comparable potency (IC50 less than 0.01 µg/mL) to that of SHIVSF162P3 (19). Therefore, by simple extrapolation from the macaque model to humans, these data would indicate that PGT might offer sterilizing immunity against 15% of global isolates at serum concentrations of the order of 10 µg/mL, which other studies suggest should be readily achievable by vaccination (24). However, the SHIV/macaque model uses a high-dose challenge (300 TCID50), whereas the viral inoculum found in most human exposures has been estimated to be at least 100-fold less (lower than 3 TCID50) (14, 17). In addition, the animals are treated with Depo-Provera to synchronize the menstrual cycle and to thin the vaginal epithelium, which further increases the susceptibility to infection (25, 26). The high-dose SHIV challenge of macaques can thus be regarded as an informative but highly stringent model system for determining the relative protective potency of bnMAbs, and we find it reasonable to speculate that lower serum antibody levels may be protective against the viral inoculum found in most human exposures. Indeed, macaque studies support this notion (16, 17). For example, serum b12 concentrations in the range of 13–60 µg/mL afforded protection to the extent that 108 challenges of 10 TCID50 SHIVSF162P3 were required to infect four of five Depo-Provera–treated animals compared with 10 challenges to infect five of five control animals (17). If the lower viral challenge dose associated with human exposure results in a 10-fold decrease in the ratio between neutralization IC50 in vitro and protection EC50 in vivo, then simple extrapolation would suggest that PGT121 at approximately 10 µg/mL in serum would protect against 44% of the viruses from the 162-virus panel (19). A 100-fold differential would suggest a corresponding figure of 57%. Clearly these figures are based on simplistic assumptions but also suggest that even a single specificity might provide substantial protection at achievable concentration (24). Of course, the aim of a vaccine would be to induce multiple Ab specificities of high neutralizing potency and complementary breadth to increase viral coverage (19). Furthermore, it is plausible that a potent vaccine-induced cellular response might contain an infection that breaks through an antibody response, either to abort an infection or to contain it to such an extent that disease did not ensue (27). Such scenario would further increase the efficiency of a vaccine-induced Ab response.
A general correlation of the serum concentration of neutralizing antibodies necessary for protection of 50% of animals against a single high-dose challenge has been suggested to be in the range of tens to several thousands times the in vitro neutralizing IC50 (8, 12, 15, 28). For PGT121, we found the 50% protective serum concentration to be ∼300-fold the in vitro neutralizing IC50 (1.8 µg/mL / 0.005 µg/mL), which is within the typical range.
Finally, it should be noted that we are using a human IgG1 antibody in macaques and that our results may underestimate the efficacy of the human antibody in humans. However, the effects of this species mismatch would be expected to be limited in terms of protection against a single high-dose viral challenge. First, human antibodies may have reduced serum half-life in macaques, but the so-called “window of opportunity” for inhibiting infection after viral challenge has been estimated to be in the first 24–48 h (4, 13, 29), which is well within the half-life of PGT121 (approximately 6 d). Second, Fc receptor interactions can influence the protective ability of antibody in vivo (9, 17). However, we and others have provided evidence that interaction between human IgG and macaque Fc gamma receptors results in efficient Fc-mediated effector functions in vitro and in vivo (9, 30, 31). Further, a recent study has suggested that human and cynomolgus IgG1 bind human and cynomolgus Fc gamma receptors with similar affinity (32).
In summary, our results are direct evidence that protection against viral challenge can be achieved at low serum antibody concentration and support that sterilizing immunity may be achievable through vaccination if immunogens can be developed that elicit HIV-neutralizing antibody responses with comparable breadth and potency as PGT121.
Materials and Methods
Macaques.
This study was carried out in strict accordance with the recommendations in the “Guide for the Care and Use of Laboratory Animals” of the National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin (Animal Welfare Assurance A3368-01), and all efforts were made to minimize pain and distress. Antibody administration and viral challenge protocols are more fully described (8, 33). The antibody dose was administered i.v. 24 h before the animals were atraumatically challenged vaginally with 300 TCID50 of SHIVSF162P3 (corresponding to 10 animal infectious dose 50%, AID50) diluted in 0.5 mL of PBS. Animals were given 30 mg of medroxyprogesterone (Depo-Provera) intramuscularly at day −28 before viral challenge to synchronize the menstrual cycle and thin the vaginal epithelium. All animals were experimentally naive and were negative for antibodies against HIV-1, SIV, and type D retrovirus at the start of the experiment. Serum samples for antibody detection and serum neutralization titers were obtained at day −6, 0 (6 h after antibody administration), 1, 4, 7, 10, 14, 21, 29, 35, 42, and 56. Plasma samples for viral load determination were obtained at day −27, 7, 10, 14, 21, 29, 35, 42, and 56. Day 0 is the day of antibody administration.
Challenge Virus.
The challenge virus was SHIVSF162P3 (34, 35) propagated in phytohemagglutinin-activated rhesus macaque PBMCs. The quantity of SHIV viral RNA (vRNA) genomic copy equivalents (vRNA copy Eq/mL) in EDTA-anticoagulated plasma was determined by using a quantitative reverse-transcription PCR assay as described (36).
Antibody Production.
Antibodies were produced as described and purified by using Protein A affinity matrix (GE Healthcare) (9, 19). DEN3, a dengue virus NS1-specific human IgG1 antibody, was used as the isotype control antibody in this study. Antibodies used for the passive transfer experiments were endotoxin free (less than 0.03 EU/mg).
ELISA.
We determined PGT121 concentration in macaque serum and vaginal samples by ELISA using recombinant HIV-1JRFL gp120 (Progenics Pharmaceuticals) as described (8). Briefly, microtiter plates were coated with 2 µg/mL HIV-1JRFL gp120 and incubated overnight at 4 °C. The plates were washed with PBS/0.05% Tween-20 and blocked with 3% (vol/vol) BSA. After blocking, serial dilution of serum samples, vaginal samples, or monoclonal antibodies were added to the plate and incubated for 2 h at 37 °C. Binding was detected with a goat anti-human IgG F(ab)2 fragments coupled to alkaline phosphatase (Pierce) and visualized with p-nitrophenyl phosphate substrate (Sigma-Aldrich).
Antibody Isolation from Vaginal Secretion.
Antibody was isolated from vaginal secretions as described (8). Briefly, vaginal secretions were absorbed to cellulose wicks (Solan Weck-Cel surgical spears; Xomed Surgical Products) by gently placing it into the vaginal fornix. After 5 min, wicks were collected and frozen at −70 °C. The antibody was extracted from the wicks by adding 2 × 200 µL of PBS, 1% FBS, and a protease inhibition mixture and spinning it through a 0.45-µm pore size filter (Spin-X). Vaginal secretion samples were obtained at day −6, 0 (6 h after antibody administration), 1, and 4. Day 0 is the day of antibody administration.
Neutralization.
In vitro neutralization was assessed by two different methods. (i) Neutralization of pseudovirus was evaluated by using TZM-bl cells as described (37). Briefly, pseudovirus was generated in 293T cells and used to infect TZM-bl cells seeded in a 96-well plate. Pseudovirus was preincubated with the antibody for 1 h at 37 °C, and DEAE-Dextran (Sigma-Aldrich) was added to the TZM-bl cells at a final concentration of 10 µg/mL. Luciferase expression was quantified 48 h after infection upon cell lysis and the addition of luciferase substrate (Promega). (ii) Neutralization of PBMC grown replication competent virus was evaluated by using rhesus macaque PBMCs as described (8). Briefly, PBMCs was isolated and stimulated overnight in the presence of phytohemagglutinin (Sigma-Aldrich) and IL-2 (Hoffmann–La Roche Inc.). Antibody and virus were preincubated for 1 h at 37 °C before being added to the stimulated PBMCs in a 96-well plate. The cells were incubated for 2 d, washed three times, and incubated for an additional 5 d. The amount of SHIV was quantified in the cell supernatant by a p27-specific ELISA (Advanced BioScience Laboratories).
Acknowledgments
We are grateful for the assistance provided by the Virology group, Wisconsin National Primate Research Center. The following reagents were obtained through the AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health: Original stock of SHIVSF162P3 from Drs. Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal; and the Division of AIDS, NIAID, and human rIL-2 from Dr. Maurice Gately, Hoffmann–La Roche Inc. This work was supported by the International AIDS Vaccine Initiative through the Neutralizing Antibody Consortium (D.R.B. and P.P.); National Institutes of Allergy and Infectious Diseases Grants R01 AI33292 (to D.R.B.) and U19AI090970 (to P.P.), Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery Grant UM1AI100663 (to D.R.B.); The Ragon Institute of MGH, MIT, and Harvard (D.R.B.); and an Alfred Benzon Fellowship (to B.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: Good news for an HIV-1 vaccine? Nat Med. 2009;15(8):866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 2.Montefiori DC, Mascola JR. Neutralizing antibodies against HIV-1: Can we elicit them with vaccines and how much do we need? Curr Opin HIV AIDS. 2009;4(5):347–351. doi: 10.1097/COH.0b013e32832f4a4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 5.Hoxie JA. Toward an antibody-based HIV-1 vaccine. Annu Rev Med. 2010;61:135–152. doi: 10.1146/annurev.med.60.042507.164323. [DOI] [PubMed] [Google Scholar]
- 6.Mascola JR, et al. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73(5):4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mascola JR, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 8.Parren PW, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75(17):8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 10.Hessell AJ, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84(3):1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hessell AJ, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog. 2009;5(5):e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura Y, et al. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76(5):2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimura Y, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: Implications for HIV-1 vaccine development. Proc Natl Acad Sci USA. 2003;100(25):15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3(9):e351. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willey R, Nason MC, Nishimura Y, Follmann DA, Martin MA. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res Hum Retroviruses. 2010;26(1):89–98. doi: 10.1089/aid.2009.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moldt B, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86(11):6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessell AJ, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15(8):951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker LM, et al. Protocol G Principal Investigators Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326(5950):285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker LM, et al. Protocol G Principal Investigators Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477(7365):466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheid JF, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458(7238):636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 21.Scheid JF, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333(6049):1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329(5993):856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanlan CN, et al. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1—>2 mannose residues on the outer face of gp120. J Virol. 2002;76(14):7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martell BA, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: A randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx PA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 26.Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 27.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320(5877):760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 28.Gauduin MC, et al. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3(12):1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 29.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 30.Lazar GA, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci USA. 2006;103(11):4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardarelli PM, et al. A nonfucosylated human antibody to CD19 with potent B-cell depletive activity for therapy of B-cell malignancies. Cancer Immunol Immunother. 2010;59(2):257–265. doi: 10.1007/s00262-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warncke M, et al. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol. 2012;188(9):4405–4411. doi: 10.4049/jimmunol.1200090. [DOI] [PubMed] [Google Scholar]
- 33.Burton DR, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci USA. 2011;108(27):11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harouse JM, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3) J Virol. 2001;75(4):1990–1995. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan RC, Harouse JM, Gettie A, Cheng-Mayer C. In vivo adaptation of SHIV(SF162): Chimeric virus expressing a NSI, CCR5-specific envelope protein. J Med Primatol. 1999;28(4-5):164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 36.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: Practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34(5-6):303–312. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 37.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]