Abstract
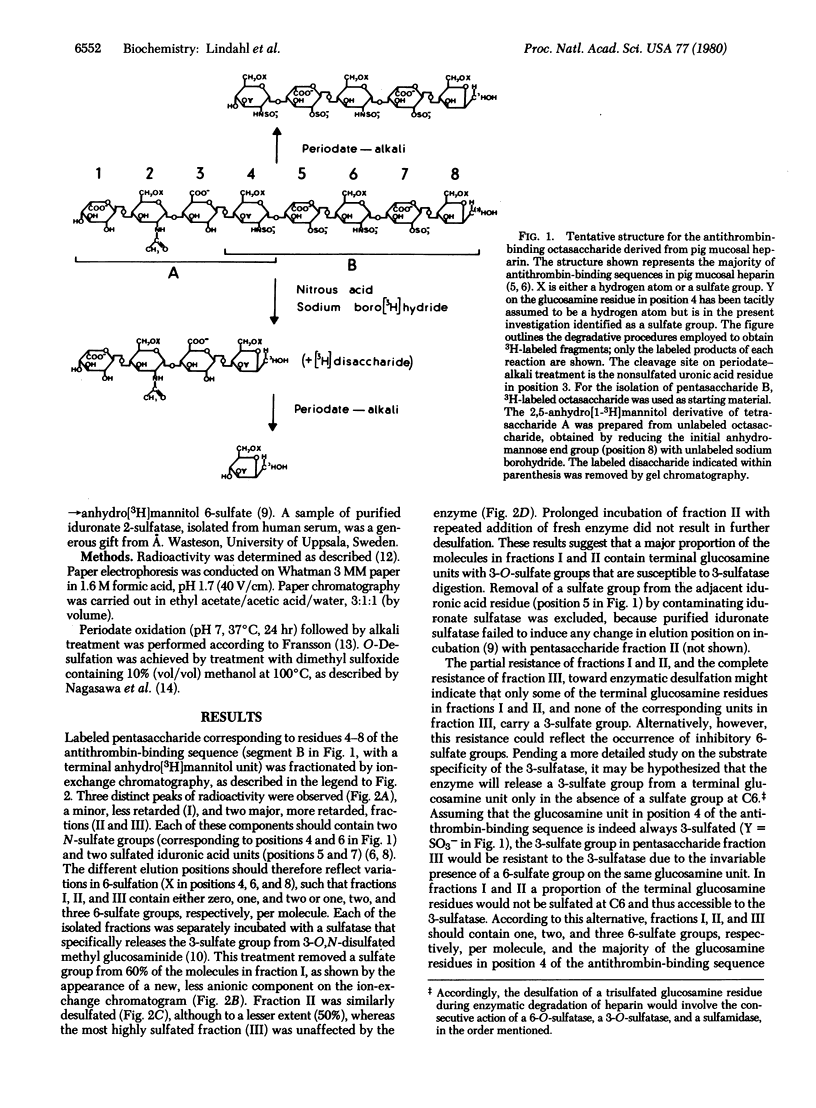
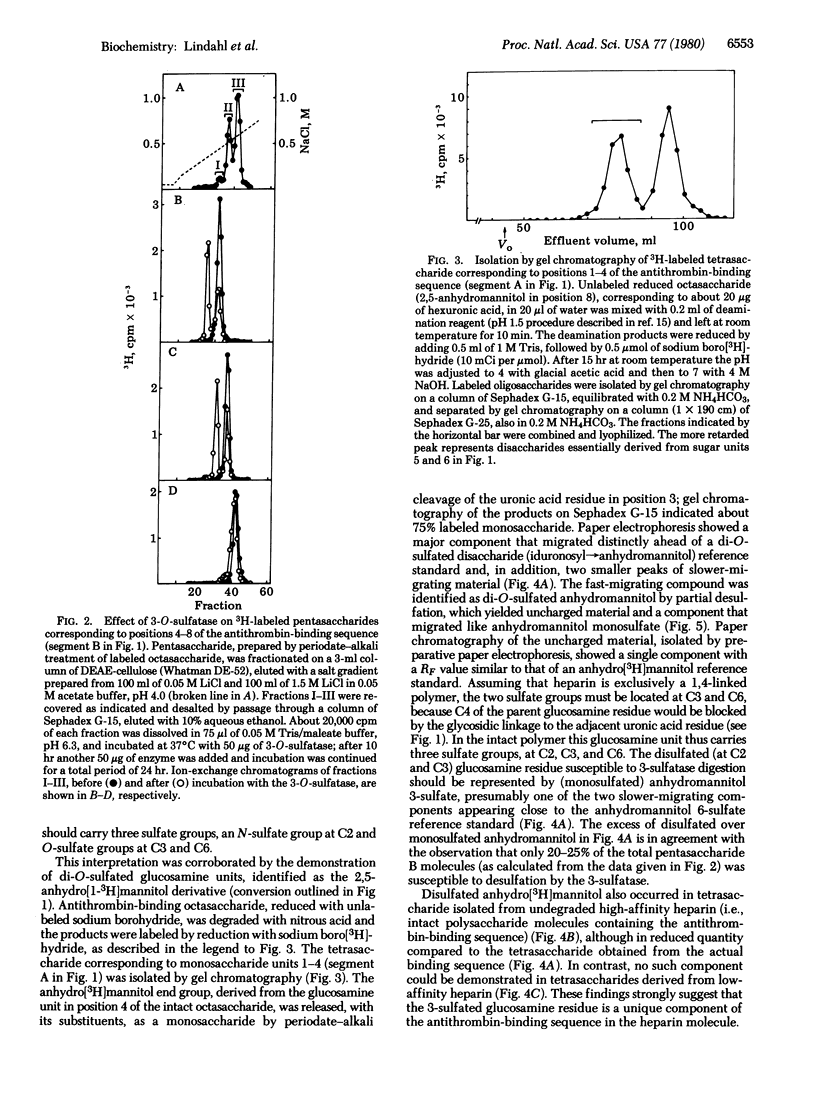
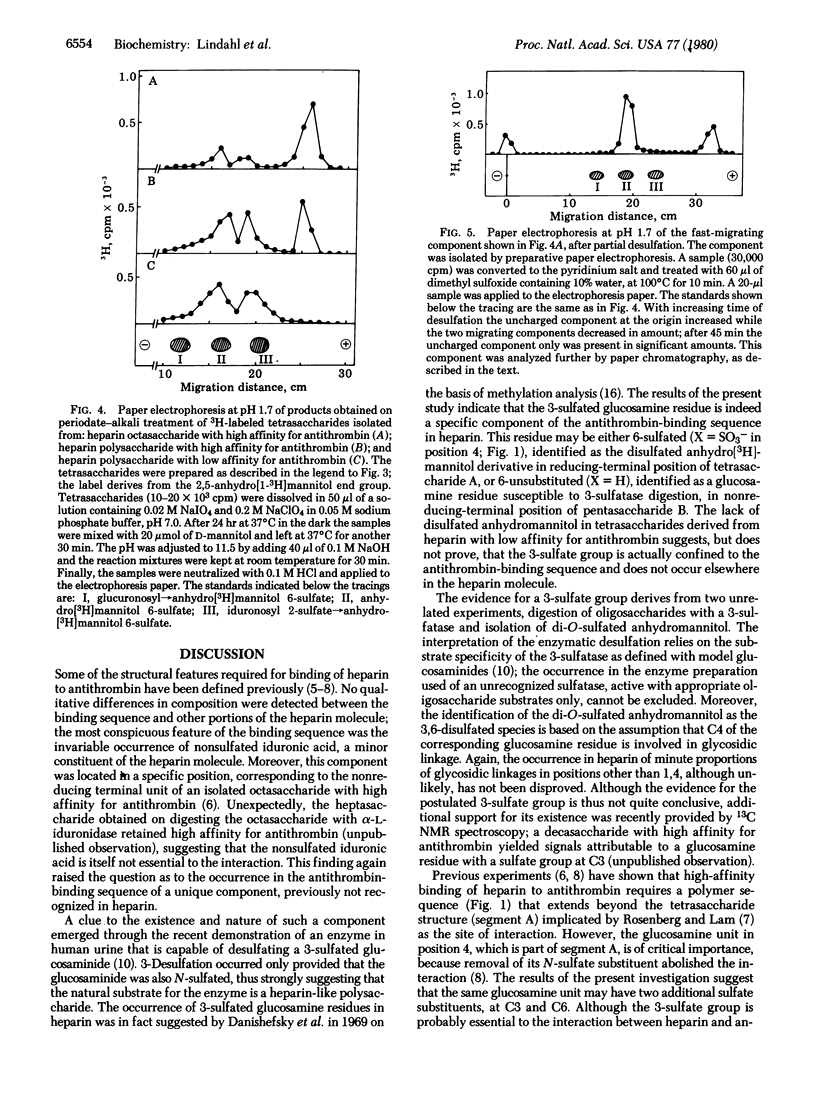
An octasaccharide with high affinity for antithrombin was isolated after partial deaminative cleavage of heparin with nitrous acid. After conversion of the 2,5-anhydro-D-mannose end group to anhydro[1-3H]mannitol, labeled pentasaccharide was released from the octasaccharide by periodate-alkali treatment. Incubation of the pentasaccharide with a recently discovered 3,O-sulfatase from human urine resulted in desulfation, suggesting the occurrence of a 3-sulfate group on the terminal glucosamine residue. The same glucosamine residue was recovered as a 2,5-anhydro[1-3H]mannitol derivative by a procedure involving deamination of the octasaccharide with nitrous acid, reduction of the products with sodium boro[3H]hydride, isolation of 3H-labeled tetrasaccharide by gel chromatography, and release of the labeled end-group by periodate-alkali treatment. Paper electrophoresis indicated disulfated anhydro[3H]mannitol, presumably sulfated at C3 and C6, as a major component, along with smaller amounts of monosulfated (presumably 3-sulfated) anhydro[3H]mannitol. Similar treatment of an analogous tetrasaccharide derived from heparin with low affinity for antithrombin failed to produce any disulfated anhydromannitol. These results suggest that 3-sulfated glucosamine is a unique component of high-affinity heparin, located at a specific position in the antithrombin-binding sequence of the molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Danishefsky I., Steiner H., Bella A., Jr, Friedlander A. Investigations on the chemistry of heparin. VI. Position of the sulfate ester groups. J Biol Chem. 1969 Apr 10;244(7):1741–1745. [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Leder I. G. A novel 3-O sulfatase from human urine acting on methyl-2-deoxy-2-sulfamino-alphs-D-glucopyranoside 3-sulfate. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1183–1189. doi: 10.1016/0006-291x(80)90544-6. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Jacobsson I., Hök M., Backström G., Feingold D. S. Biosynthesis of heparin. Loss of C-5 hydrogen during conversion of D-glucuronic to L-iduronic acid residues. Biochem Biophys Res Commun. 1976 May 17;70(2):492–499. doi: 10.1016/0006-291x(76)91073-1. [DOI] [PubMed] [Google Scholar]
- Nagasawa K., Inoue Y., Kamata T. Solvolytic desulfation of glycosaminoglycuronan sulfates with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1977 Sep;58(1):47–55. doi: 10.1016/s0008-6215(00)83402-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Rosenberg R. D., Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1218–1222. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Grundberg H., Riesenfeld J., Lindahl U. The molecular size of the antithrombin-binding sequence in heparin. FEBS Lett. 1980 Aug 11;117(1):203–206. doi: 10.1016/0014-5793(80)80945-8. [DOI] [PubMed] [Google Scholar]


