Abstract
Although the cerebellar interpositus nuclei are known to be involved in cognitive functions, such as associative motor learning, no anatomical evidence has been available for this issue. Here we used retrograde transneuronal transport of rabies virus to identify neurons in the cerebellar nuclei that project via the thalamus to area 46 of the prefrontal cortex of macaques in comparison with the projections to the primary motor cortex (M1). After rabies injections into area 46, many neurons in the restricted region of the posterior interpositus nucleus (PIN) were labeled disynaptically via the thalamus, whereas no neuron labeling was found in the anterior interpositus nucleus (AIN). The distribution of the labeled neurons was dorsoventrally different from that of PIN neurons labeled from the M1. This defines an anatomical substrate for the contribution of medial cerebellar output to cognitive functions. Like the dentate nucleus, the PIN has dual motor and cognitive channels, whereas the AIN has a motor channel only.
Keywords: acquisition, retention, cerebello-thalamo-cortical pathway
Whereas the cerebellum has long been thought to contribute to motor execution, it has recently been suggested that the cerebellum is involved in cognitive aspects of motor behaviors. With respect to the lateral output of the cerebellum, Leiner and colleagues have reported that the cerebellar dentate nucleus undergoes a marked expansion that parallels the development of the frontal cortex (1). They have proposed cerebellar involvement in human higher-order functions, including language and cognition. Moreover, Middleton and Strick have shown evidence that the ventral aspect of the dentate nucleus projects multisynaptically to the prefrontal cortex (2). In terms of the medial output of the cerebellum, it has been considered that the cerebellar interpositus nuclei play crucial roles in cognitive functions, such as acquisition and retention of classically conditioned behaviors (3–7). Thus, a major question arises as to whether multisynaptic pathways connect the interpositus nuclei to the prefrontal cortex.
To address this issue, we performed transneuronal labeling with rabies virus. Rabies virus is well known to infect axon terminals preferentially and move retrogradely across synapses in a time-dependent manner (8–12). In the present study, rabies injections were made into area 46 of the prefrontal cortex of macaques that is characterized by cognitive functions, such as reward (13), spatial working memory (14), temporal processing (15, 16), evaluation of self-generated decision making (17), categorization (18), and motor learning of classical conditioned action (19). The distribution pattern of disynaptically labeled neurons in the cerebellar interpositus nuclei was analyzed in comparison with that of the labeled neurons from the primary motor cortex (M1).
Results
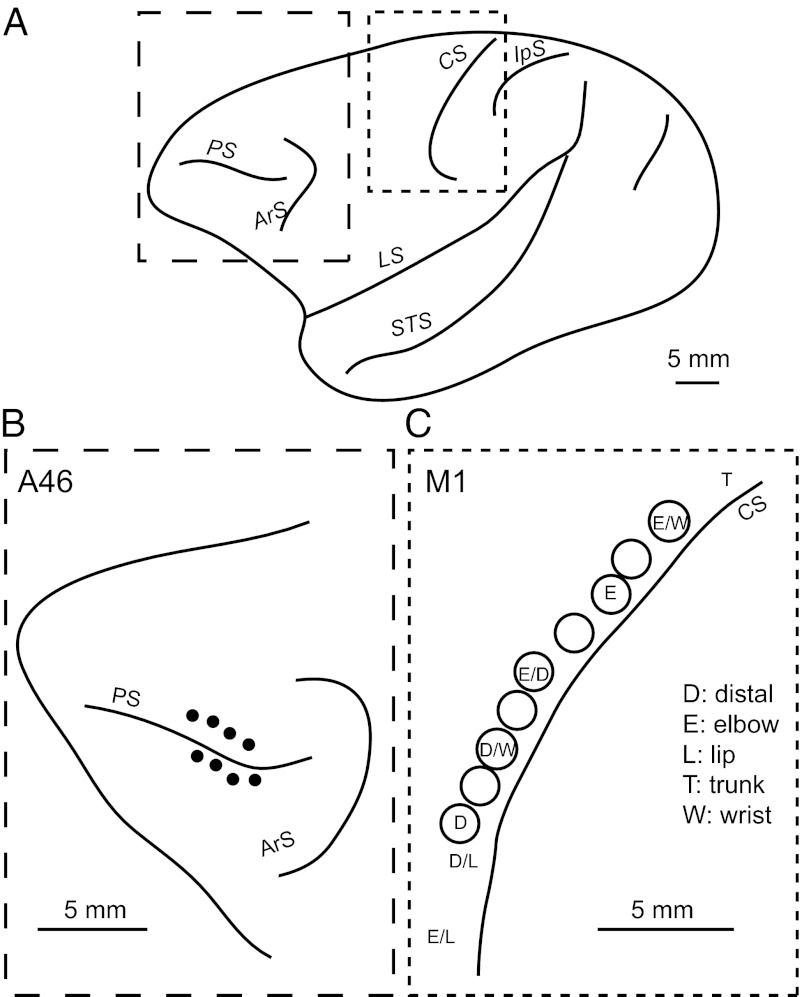
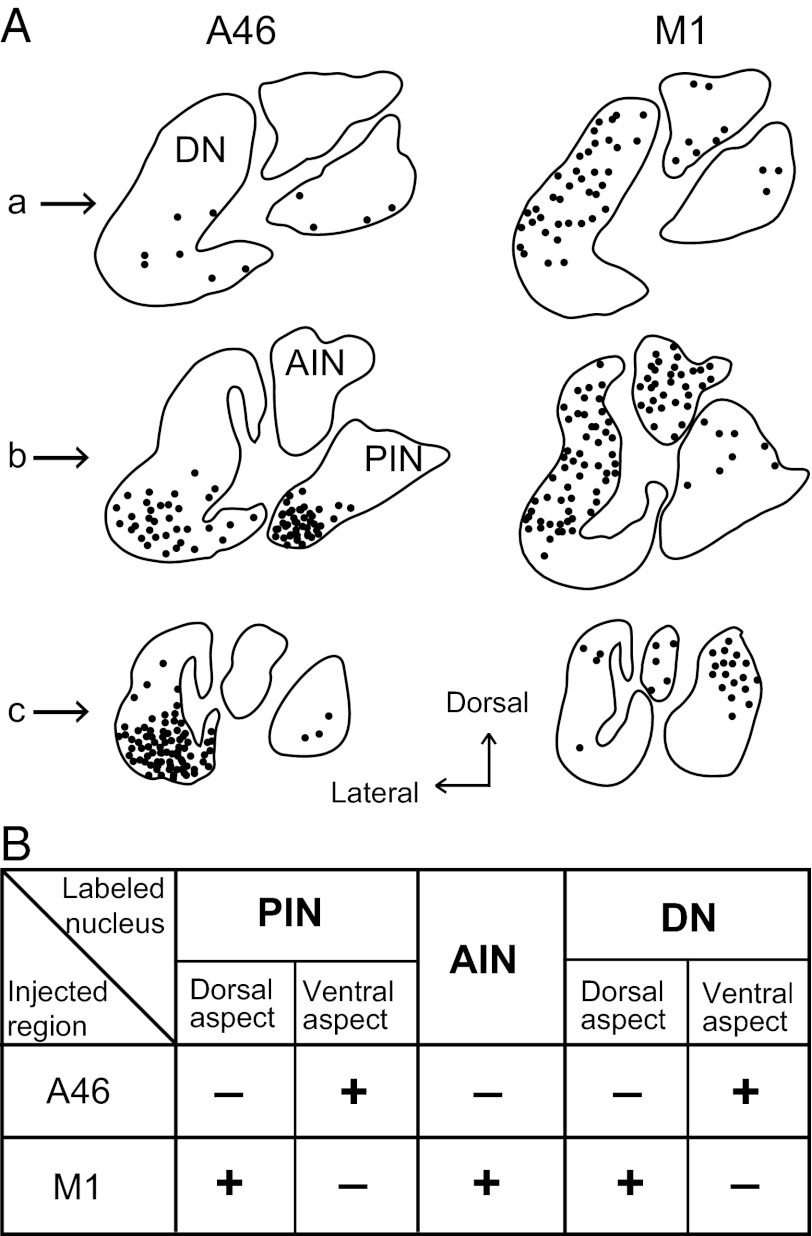
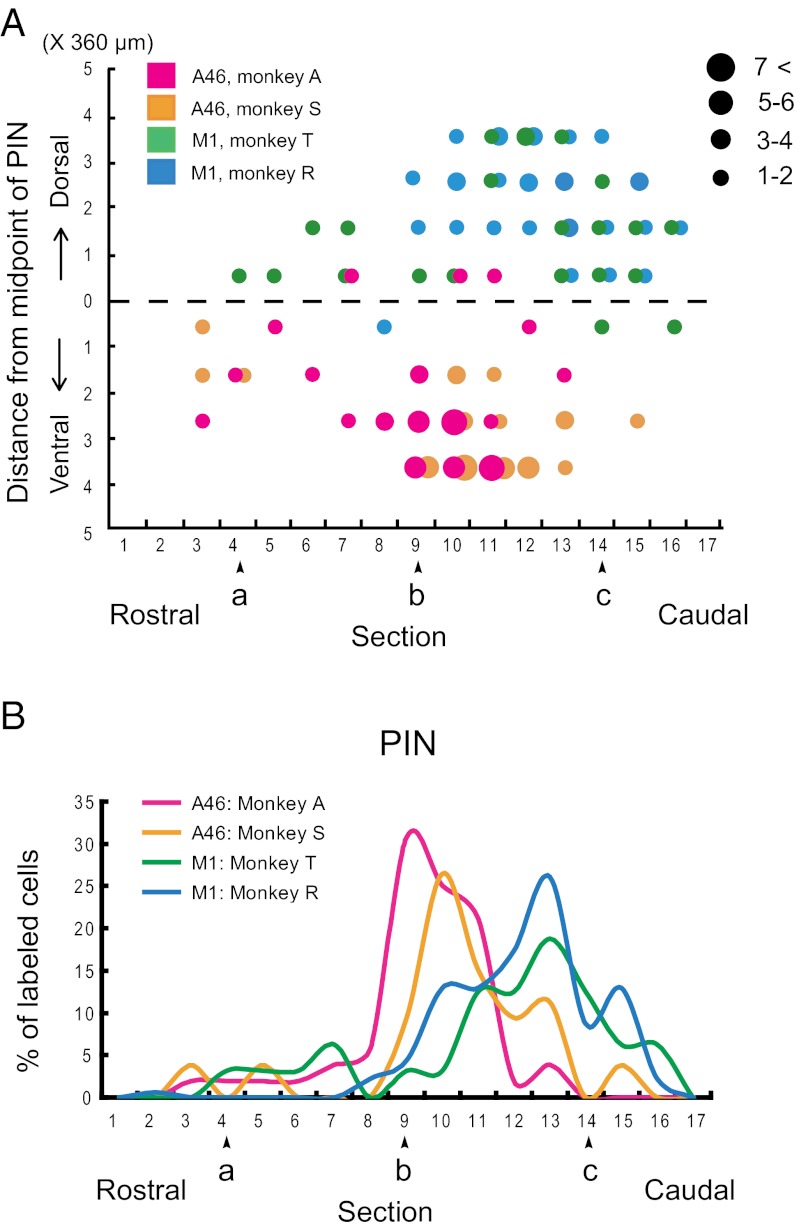
Rabies injections were made into area 46 (n = 2) and forelimb presentation of the M1 (n = 2). The injections into area 46 were placed in the dorsal and ventral banks of the principal sulcus, at least 3 mm rostral to the caudal end of the sulcus (Fig. 1 A and B). The forelimb region of the M1 was identified by means of intracortical microstimulation (Fig. 1 A and C). With the 3-d postinjection period, these cortical injections retrogradely labeled many neurons in the cerebellar nuclei as the second-order neuron labeling via the thalamus (Fig. 2). Most of the labeled neurons were found on the side contralateral to the injection sites. In the interpositus nuclei, neuronal labeling from area 46 was located in the posterior interpositus nucleus (PIN), but not in the anterior interpositus nucleus (AIN). The labeled neurons in the PIN (mean = 52.5, range = 52–53) were confined to the ventral aspect (Figs. 3 and 4A). By contrast, neuronal labeling from the M1 was observed not only in the PIN (mean = 37.5, range = 29–46), but also in the AIN (mean = 50.5, range = 43–58) (Figs. 3 and 4A). Within the PIN, the neurons labeled from the M1 were restricted to the dorsal aspect, unlike the labeling from area 46. Moreover, the rostrocaudal distributions of labeled neurons from area 46 and the M1 were somewhat different. Neuronal labeling from area 46 was localized at the rostrocaudal middle level of the PIN, whereas the peak of PIN neuron labeling from the M1 shifted caudally (Fig. 4B). In addition, a number of labeled neurons were distributed in the ventral or dorsal aspect of the dentate nucleus after the rabies injections into area 46 or the M1, respectively (Fig. 3A), which was consistent with the previous results (2, 20).
Fig. 1.
Viral injection sites along the principle sulcus and central sulcus in macaques. (A) Lateral view of the primate brain. (B) Enlargement of the area enclosed by the larger dashed line in A. Solid circles represent needle entry of the viral injections. (C) Enlargement of the area enclosed by the smaller dashed line in A. Each letter indicates a body part of which movement was evoked by intracortical microstimulation of the corresponding site. ArS, superior limb of the arcuate sulcus; CS, central sulcus; D, dorsal; IpS, intraparietal sulcus; LS, lateral sulcus; PS, principal sulcus; STS, superior temporal sulcus.
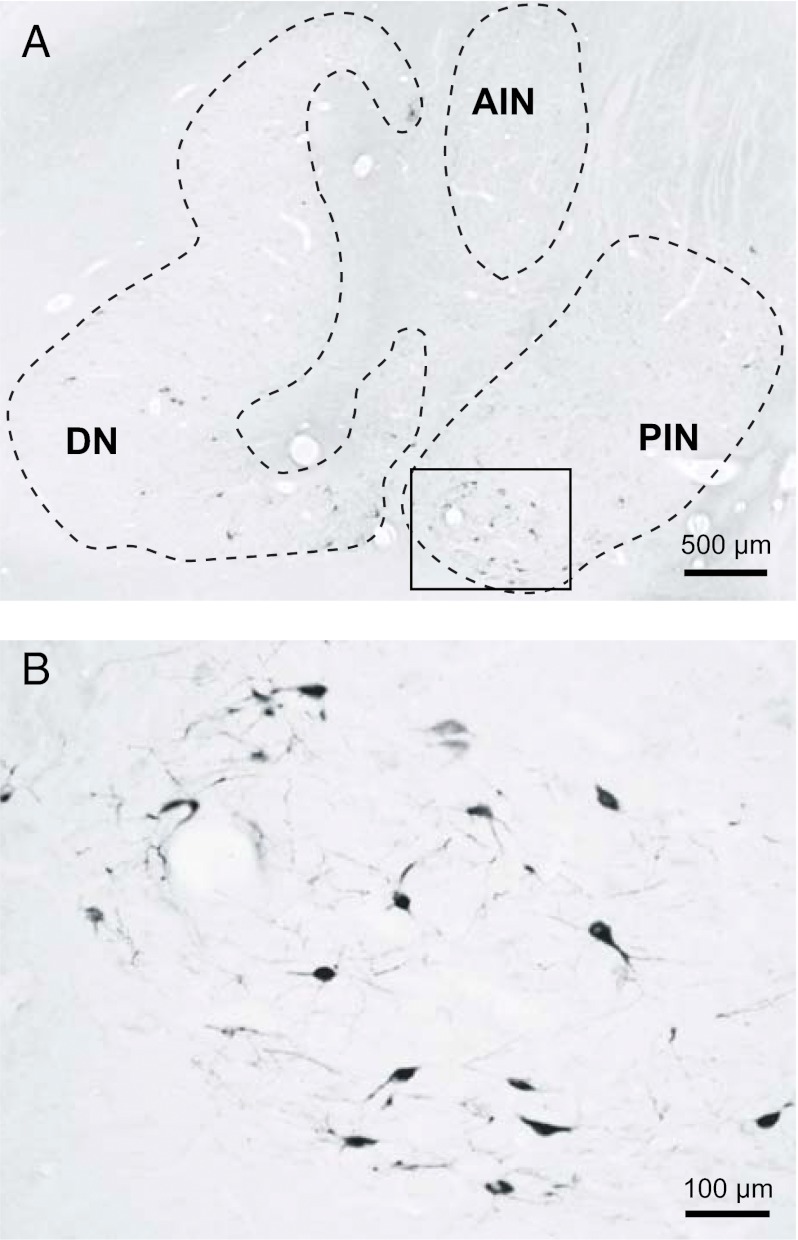
Fig. 2.
Rabies-labeled neurons in the cerebellar nuclei 3 d after the injection into the area 46 region of the prefrontal cortex. (A) Coronal section showing labeled neurons in the cerebellar nuclei. (B) Enlargement of the area enclosed by the rectangle area in A. AIN, anterior interpositus nucleus; DN, dentate nucleus; PIN, posterior interpositus nucleus.
Fig. 3.
Distribution patterns of second-order neuron labeling in the deep cerebellar nuclei 3 d after the rabies injections into area 46 and the M1. (A) Three representative coronal sections in each case are arranged rostrocaudally in a–c. Rostrocaudal level of each section is indicated as the section number (specified by a–c) on the abscissa of the graphs shown in Fig. 4A. (B) Summary of distribution of second-order neuron labeled in the different region of cerebellar nuclei. Abbreviations are as in Fig. 2.
Fig. 4.
Distribution patterns of the PIN neurons labeled from area 46 versus the M1. (A) Dorsoventral extent of labeled neurons in the PIN. Purple and orange dots denote area 46 labeling in monkeys A and S, respectively. Blue and green dots denote the M1 labeling in monkeys T and R, respectively. Other conventions are as in Fig. 3. (B) Graphs showing the rostrocaudal extent of labeled neurons in the PIN. Purple and orange lines denote area 46 labeling in monkeys A and S, respectively. Blue and green lines denote the M1 labeling in monkeys T and R, respectively. Other conventions are as in Fig. 3.
With regard to the thalamic labeling, the major sites of this first-order neuron labeling were the parvocellular division of the ventroanterior nucleus (VApc) and the mediodorsal nucleus (MD) in the area 46 injection case and the oral division of the ventrolateral nucleus and the oral division of the ventroposterolateral nucleus in the M1 injection case. Virtually no labeling of Purkinje cells was seen in the cerebellar cortex in either of the injection cases. The basal ganglia subserved as controls. After the area 46 injections, a number of labeled neurons were observed in the internal segment of the globus pallidus (GPi), especially in its dorsomedial part, whereas the striatum was devoid of neuronal labeling. Following the M1 injections, on the other hand, neuronal labeling was found in the ventral part of the GPi. Thus, distinct output channels to the prefrontal cortex and M1 could be present in the GPi (20).
Overall, the distribution pattern of neurons in the interpositus nuclei that were labeled disynaptically from area 46 was totally distinct from that of neurons labeled from the M1 (Fig. 3B). This indicated that separate cerebellar output channels to the prefrontal cortex and M1 might exist in the interpositus nuclei as well as in the dentate nucleus.
Discussion
Prefrontal Cortex Is a Target of a Cerebello–Thalamo–Cortical Pathway from the Interpositus Nuclei.
In the present study, most of the neurons in the cerebellar interpositus nuclei that were labeled disynaptically from area 46 of the prefrontal cortex via the thalamus were located in the ventral aspect of the PIN, rather than in the AIN. Previous studies have shown that several thalamic nuclei innervate area 46. These nuclei include the VApc, MD, and the caudal division of the ventrolateral nucleus (21–24), which is in agreement with the present findings. In turn, these thalamic nuclei receive inputs from the cerebellar interpositus nuclei (25–29). Additionally, it has been reported that the distribution area of thalamic projections from the PIN overlaps that from the dentate nucleus (29). Together with these data, our results provide evidence that area 46 of the prefrontal cortex is a target of a cerebello–thalamo–cortical pathway arising from the ventral aspect of the PIN. In comparison, the origin of a cerebello–thalamo–cortical pathway to the M1 is distinct from that of the pathway to area 46, with strong output from the dorsal aspect of the PIN and also from the AIN.
It has been demonstrated that area 46 receives disynaptic input from a major output station of the basal ganglia, the GPi, particularly its dorsomedial part (20). Similar results were obtained in the present study. In addition, no neuronal labeling occurred in either the cerebellar cortex or the striatum in our experiments. These findings strongly indicate that rabies labeling of neurons in cerebellar interpositus nuclei corresponds to the second-order labeling.
The previous work using herpes simplex virus failed to detect a disynaptic projection from the PIN to the prefrontal cortex (2). Two reasons for this discrepancy are likely. First, there would be a potential difference in the sensitivity between the two viral tracers used in the present and previous studies; rabies virus is more sensitive than herpes simplex virus. Second, the lack of retrograde labeling with herpes simplex virus in the PIN could be explained by a possible greater collateralization of the PIN efferents compared with the dentate efferents (30, 31).
Anatomical Substrate for the Involvement of Medial Cerebellar Output in Cognitive Functions.
Our data demonstrate the existence of a disynaptic pathway connecting the PIN to area 46 of the prefrontal cortex. This suggests that the medial cerebellar output from the PIN may be involved in behavioral functions.
Previous studies have shown that the cerebellar interpositus nuclei, although not necessarily restricted to the PIN, play crucial roles in the associative motor learning in nonprimate mammals such as rodents, rabbits, and cats. For example, McCormick and Thompson reported that lesions of the interpositus nuclei abolished the overlearned eyeblink response; recordings from these nuclei have revealed neuronal activity in relation to response learning (32). Our findings may be more relevant to some studies in human subjects. Acquisition of trace eyeblink conditioning was impaired in patients with lesions in the interpositus nuclei (33). Imaging studies also identified that the cerebellum is involved in eyeblink conditioning (34–36). Moreover, Molchan and colleagues found that the changes in positron emission tomography and regional cerebral blood flow in several areas (19), including the cerebellum and the prefrontal cortex, were correlated with associative motor learning. Given such diverse behavioral functions that both the cerebellar interpositus nuclei and the prefrontal cortex participate in, the existence of a pathway between these two regions may not be surprising. However, no data have so far been available on the anatomical relationship between the interpositus nuclei and the prefrontal cortex.
Differential Functional Organization of Medial Cerebellar Output from the Interpositus Nuclei.
It has been thought that the dorsal aspect of the PIN is involved specifically in the performance of overlearned eyeblink responses and saccadic eye movements rather than in their new learning (37, 38). If this is the case, then impairment in the acquisition of trace eyeblink conditioning by PIN lesions can be due to functional blockade of the ventral rather than the dorsal aspect of the PIN. On the other hand, many studies using lesioning (39–43), inactivation (44, 45), electron microscopy (46), and functional magnetic resonance imaging (47) have shown that the AIN is the site for memory in eyeblink conditioning rather than learning. Furthermore, Park and colleagues reported that the expression of representative motor memory formation genes was increased selectively in the AIN, but not in the PIN (5). This supports the notion that the AIN is critical for long-term memory of associative motor learning. These results strongly indicate the differential functional organization of the medial cerebellar output from the ventral PIN versus the dorsal PIN/AIN.
Interestingly, it has been shown that the ventral or ventrolateral portion of the PIN projects disynaptically via the thalamus to the frontal eye field and the lateral and medial intraparietal areas (28, 48, 49). The sites of origin of these projections, which are considered to contribute to oculomotor control and visuospatial adaptation, correspond closely with the PIN region giving rise to the projection to area 46. Moreover, it is generally accepted that there is the zonal organization of corticonuclear projections to the AIN and PIN; in both the anterior lobe and lobules of the posterior cerebellum, the so-called C1 and C3 Purkinje cell zones project to the AIN, whereas the intercalated C2 zone projects to the PIN (28, 29). According to the results of the recent study (49), transneuronal retrograde labeling of Purkinje cells from the medial intraparietal area is distributed in the anterior/posterior C2 zone. Together with these results, our present data indicate that a common C2/PIN output system probably exerts cerebellar influences on the prefrontal and parietal cortical areas. Thus, the zonal organization could allow a cooperative action of two different channels, i.e., C1/C3/AIN and C2/PIN output systems, on long-term associative motor learning.
In conclusion, our results have revealed that the outflow from the ventral PIN is directed toward area 46 of the prefrontal cortex by way of the thalamus. This disynaptic pathway provides an anatomical substrate for the involvement of medial cerebellar output in cognitive functions. Overall, it is most likely that there are separate cerebellar output channels to the prefrontal cortex and M1 in the interpositus nuclei. Like the dentate nucleus, the PIN has dual motor and cognitive channels that are dorsoventrally segregated, whereas the AIN has a motor channel only.
Materials and Methods
Subjects and Materials.
This report was based on observations from four macaque monkeys of either sex weighing 3.7–11 kg. Injections of the challenge virus strain 11 of rabies virus were made into area 46 of the prefrontal cortex (n = 2) and the forelimb region of the M1 (n = 2). According to previous studies (8, 11, 12), a 3-d survival period after the rabies injections into area 46/M1 is optimal to analyze second-order neuron labeling in the deep cerebellar nuclei. In the present study, the same time course was adopted to examine the distribution patterns of cerebellar nuclear neurons. The experimental protocol was approved by the Animal Care and Use Committee of the Tokyo Metropolitan Institute for Neuroscience, and all experiments were conducted in accordance with the Guidelines for the Care and Use of Animals (Tokyo Metropolitan Institute for Neuroscience, 2000).
Surgery and Primary Motor Cortex Mapping.
The procedures of surgical operation and electrophysiological mapping of the M1 are described in detail elsewhere (11, 12). Under general anesthesia, a head holder was fixed onto the monkey’s head. After a recovery period of several days, the monkeys prepared for the M1 injections were anesthetized with an intramuscular injection of ketamine hydrochloride and positioned in a stereotaxic frame attached to a primate chair. A portion of the skull over the precentral gyrus was removed and the M1 was mapped by intracortical microstimulation in awake conditions. A glass-coated Elgiloy-alloy microelectrode (0.5–1.5 MΩ at 1 kHz) was inserted perpendicular to the dural surface. When trains of 12 cathodal pulses (200-μs duration at 333 Hz, currents of less than 30 μA) were delivered through a constant-current stimulator, evoked movements of different body parts were carefully monitored. At the end of the mapping, two to three reference points were tattooed on the dura mater so that we were able to target the mapped sites by stereotaxic measurement of the distances between the reference points and the mapped sites when we injected rabies virus into the M1. A rectangular chamber was then fixed onto the skull to preserve the exposed dural surface.
Viral Injections.
A few days after the M1 mapping, monkeys received injections of challenge virus strain 11 into the electrophysiologically mapped representations of the forelimb region of the M1. For the prefrontal cortex (area 46) cases, animals were anesthetized with ketamine hydrochloride (5 mg/kg, i.m.) and sodium pentobarbital (20 mg/kg, i.v.), and a portion of the skull over the principal sulcus (PS) was removed. Dura over the PS was cut open to confirm the locations of the PS and the caudal end of the PS. The injections were placed at least 3 mm rostral to the caudal end of the PS and no more than 2 mm away from the center of the PS. The virus was derived from the Center for Disease Control and Prevention (Atlanta, GA) and donated by Satoshi Inoue (National Institute of Infectious Diseases, Tokyo, Japan). When injections were made along the anterior bank of the central sulcus or along the PS, viral deposits (0.5 μL each) were placed at one or two different levels. For the M1 cases, viral deposits were made 3–5 mm below the surface of the dura, and for the area 46 cases, viral deposits were made 3–5 mm below the surface of the brain. The titer of a stock viral suspension was 1.4 × 108 focus-forming units per milliliter. Under anesthesia with ketamine hydrochloride (5–10 mg/kg, i.m.) and xylazine hydrochloride (0.5–1 mg/kg, i.m.), the viral suspension was injected into multiple sites (0.5 μL per penetration) through a 10-μL Hamilton microsyringe as shown in Fig. 1.
Histology.
At the end of a survival period of 3 d, the monkeys were deeply anesthetized with an overdose of sodium pentobarbital (50 mg/kg, i.v.) and killed by perfusion fixation with a mixture of 8% (vol/vol) formalin and 15% (vol/vol) saturated picric acid in 0.1 M phosphate buffer (pH 7.4). The brains were removed from the skull, postfixed in the same fresh fixative overnight at 4 °C, and saturated with 30% (wt/vol) sucrose at 4 °C. Coronal sections were cut serially at 60 μm thickness on a freezing microtome. Every sixth section was processed for immunohistochemical staining for rabies virus by means of the standard avidin-biotinperoxidase complex method. Following immersion with 1% (wt/vol) skim milk, the sections were incubated overnight with rabbit antirabies virus antibody (diluted at 1:10,000) in 0.1 M PBS (pH 7.4) containing 0.1% Triton X-100 and 1% (vol/vol) normal goat serum (50). The sections were then placed in the same fresh incubation medium containing biotinylated goat antirabbit IgG antibody (diluted at 1:200; Vector Laboratories), followed by the avidin–biotin–peroxidase complex kit (ABC Elite; Vector Laboratories). For visualization of the antigen, the sections were reacted in 0.05 M Tris⋅HCl buffer (pH 7.6) containing 0.04% diaminobenzidine, 0.04% nickel chloride, and 0.002% hydrogen peroxide. A series of the adjacent sections were Nissl stained with 1% (wt/vol) Neutral red or Cresyl violet. Other technical details were as described elsewhere (11). Neuronal labeling was plotted on tracings of equidistant coronal sections (360 μm apart) through the deep cerebellar nuclei.
Analysis of Distribution of Neurons in the Cerebellar Nuclei Labeled Disynaptically from Area 46 Versus the M1.
To display the overall distribution of labeled neurons in the cerebellar nuclei, coronal sections at three levels through the nuclei are shown in Fig. 3. Each level of the section shows the approximate position of neurons labeled by retrograde transneuronal transport observed in five sections spaced 360 μm apart. There are a total of 17 sections. Fifteen of these are included in this analysis because there were no labeled neurons observed in the first or last section. Furthermore, localization of the labeled neurons in the cerebellar interpositus nuclei is analyzed in further detail (Fig. 4). At the middle of the dorsoventral level of each coronal section, a midline is set with equal linear distances from the midline to the ends of the dorsal and ventral edges on photomicrographs of all coronal sections (Fig. 4A). Bins of 360 μm (coronal sections 360 μm apart at rostrocaudal level) × 360 μm (distance from the midline) are set along this dorsoventral axis and the number of labeled neurons in each bin is counted (Fig. 4A). Finally, numbers of the neurons are represented on the sagital-view reconstructions by dots of different sizes (Fig. 4A). For the rostrocaudal extent of labeled neurons, percentages of labeled neurons observed in each coronal section 360 μm apart, are plotted along the rostrocaudal axis (Fig. 4B).
Safety Issues.
All investigators received immunization beforehand and wore protective clothing during the experimental sessions to avoid accidental infection with the virus. The experiments were performed in a special primate laboratory (biosafety level 2) designated for in vivo virus experiments. Throughout the experiments, the monkeys were kept in individual cages that were installed inside a special safety cabinet. Equipment was disinfected with 70% (vol/vol) ethanol after each experimental session and waste was autoclaved before disposal.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (18500247 and 20500293) to X.L. and for Scientific Research on Priority Areas “Integrative Brain Research” (17021050) to M.T. from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16(11):444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- 2.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark RE, Zhang AA, Lavond DG. Reversible lesions of the cerebellar interpositus nucleus during acquisition and retention of a classically conditioned behavior. Behav Neurosci. 1992;106(6):879–888. doi: 10.1037//0735-7044.106.6.879. [DOI] [PubMed] [Google Scholar]
- 4.Krupa DJ, Thompson RF. Reversible inactivation of the cerebellar interpositus nucleus completely prevents acquisition of the classically conditioned eye-blink response. Learn Mem. 1997;3(6):545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Onodera T, Nishimura S, Thompson RF, Itohara S. Molecular evidence for two-stage learning and partial laterality in eyeblink conditioning of mice. Proc Natl Acad Sci USA. 2006;103(14):5549–5554. doi: 10.1073/pnas.0601150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalmbach BE, Ohyama T, Kreider JC, Riusech F, Mauk MD. Interactions between prefrontal cortex and cerebellum revealed by trace eyelid conditioning. Learn Mem. 2009;16(1):86–95. doi: 10.1101/lm.1178309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pakaprot N, Kim S, Thompson RF. The role of the cerebellar interpositus nucleus in short and long term memory for trace eyeblink conditioning. Behav Neurosci. 2009;123(1):54–61. doi: 10.1037/a0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly RM, Strick PL. Rabies as a transneuronal tracer of circuits in the central nervous system. J Neurosci Methods. 2000;103(1):63–71. doi: 10.1016/s0165-0270(00)00296-x. [DOI] [PubMed] [Google Scholar]
- 9.Graf W, Gerrits N, Yatim-Dhiba N, Ugolini G. Mapping the oculomotor system: The power of transneuronal labelling with rabies virus. Eur J Neurosci. 2002;15(9):1557–1562. doi: 10.1046/j.1460-9568.2002.01994.x. [DOI] [PubMed] [Google Scholar]
- 10.Moschovakis AK, et al. Oculomotor areas of the primate frontal lobes: A transneuronal transfer of rabies virus and [14C]-2-deoxyglucose functional imaging study. J Neurosci. 2004;24(25):5726–5740. doi: 10.1523/JNEUROSCI.1223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyachi S, et al. Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J Neurosci. 2005;25(10):2547–2556. doi: 10.1523/JNEUROSCI.4186-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu X, Miyachi S, Ito Y, Nambu A, Takada M. Topographic distribution of output neurons in cerebellar nuclei and cortex to somatotopic map of primary motor cortex. Eur J Neurosci. 2007;25(8):2374–2382. doi: 10.1111/j.1460-9568.2007.05482.x. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M. Reward expectancy in primate prefrontal neurons. Nature. 1996;382(6592):629–632. doi: 10.1038/382629a0. [DOI] [PubMed] [Google Scholar]
- 14.Takeda K, Funahashi S. Population vector analysis of primate prefrontal activity during spatial working memory. Cereb Cortex. 2004;14(12):1328–1339. doi: 10.1093/cercor/bhh093. [DOI] [PubMed] [Google Scholar]
- 15.Niki H. Prefrontal unit activity during delayed alternation in the monkey. II. Relation to absolute versus relative direction of response. Brain Res. 1974;68(2):197–204. doi: 10.1016/0006-8993(74)90389-8. [DOI] [PubMed] [Google Scholar]
- 16.Genovesio A, Tsujimoto S, Wise SP. Neuronal activity related to elapsed time in prefrontal cortex. J Neurophysiol. 2006;95(5):3281–3285. doi: 10.1152/jn.01011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujimoto S, Genovesio A, Wise SP. Evaluating self-generated decisions in frontal pole cortex of monkeys. Nat Neurosci. 2010;13(1):120–126. doi: 10.1038/nn.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman DJ, Riesenhuber M, Poggio T, Miller EK. Categorical representation of visual stimuli in the primate prefrontal cortex. Science. 2001;291(5502):312–316. doi: 10.1126/science.291.5502.312. [DOI] [PubMed] [Google Scholar]
- 19.Molchan SE, Sunderland T, McIntosh AR, Herscovitch P, Schreurs BG. A functional anatomical study of associative learning in humans. Proc Natl Acad Sci USA. 1994;91(17):8122–8126. doi: 10.1073/pnas.91.17.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31(2-3):236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 21.Kievit J, Kuypers HG. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977;29(3-4):299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- 22.Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242(4):535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- 23.Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: Evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8(11):4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313(1):65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- 25.Asanuma C, Thach TW, Jones EG. Cytoarchitectonic delineation of the ventral lateral thalamic region in the monkey. Brain Res Brain Res Rev. 1983;5:919–235. doi: 10.1016/0165-0173(83)90014-0. [DOI] [PubMed] [Google Scholar]
- 26.Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983;286(3):237–265. doi: 10.1016/0165-0173(83)90015-2. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Yoshida K, Yoshikawa H, Kishimoto Y, Oka H. The medial dorsal nucleus is one of the thalamic relays of the cerebellocerebral responses to the frontal association cortex in the monkey: Horseradish peroxidase and fluorescent dye double staining study. Brain Res. 1992;579(2):315–320. doi: 10.1016/0006-8993(92)90067-j. [DOI] [PubMed] [Google Scholar]
- 28.Glickstein M, Sultan F, Voogd J. Functional localization in the cerebellum. Cortex. 2011;47(1):59–80. doi: 10.1016/j.cortex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Voogd J, Ruigrok TJH. Cerebellum and precerebellar nuclei. In: Mai JK, Paxinos G, editors. The Human Nervous System. 3rd Ed. Amsterdam: Elsevier; 2012. pp. 472–545. [Google Scholar]
- 30.Bentivoglio M, Kuypers HGJM. Divergent axon collaterals from rat cerebellar nuclei to diencephalon, mesencephalon, medulla oblongata and cervical cord. A fluorescent double retrograde labeling study. Exp Brain Res. 1982;46(3):339–356. doi: 10.1007/BF00238629. [DOI] [PubMed] [Google Scholar]
- 31.Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ. Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res. 2000;124:141–172. doi: 10.1016/S0079-6123(00)24014-4. [DOI] [PubMed] [Google Scholar]
- 32.McCormick DA, Thompson RF. Cerebellum: Essential involvement in the classically conditioned eyelid response. Science. 1984;223(4633):296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- 33.Gerwig M, et al. Trace eyeblink conditioning in human subjects with cerebellar lesions. Exp Brain Res. 2006;170(1):7–21. doi: 10.1007/s00221-005-0171-2. [DOI] [PubMed] [Google Scholar]
- 34.Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA. 1995;92(16):7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaxton TA, et al. Functional mapping of human learning: A positron emission tomography activation study of eyeblink conditioning. J Neurosci. 1996;16(12):4032–4040. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schreurs BG, et al. Lateralization and behavioral correlation of changes in regional cerebral blood flow with classical conditioning of the human eyeblink response. J Neurophysiol. 1997;77(4):2153–2163. doi: 10.1152/jn.1997.77.4.2153. [DOI] [PubMed] [Google Scholar]
- 37.Lu X, Hikosaka O, Miyachi S. Role of monkey cerebellar nuclei in skill for sequential movement. J Neurophysiol. 1998;79(5):2245–2254. doi: 10.1152/jn.1998.79.5.2245. [DOI] [PubMed] [Google Scholar]
- 38.Gruart A, Guillazo-Blanch G, Fernández-Mas R, Jiménez-Díaz L, Delgado-García JM. Cerebellar posterior interpositus nucleus as an enhancer of classically conditioned eyelid responses in alert cats. J Neurophysiol. 2000;84(5):2680–2690. doi: 10.1152/jn.2000.84.5.2680. [DOI] [PubMed] [Google Scholar]
- 39.Lavond DG, Hembree TL, Thompson RF. Effect of kainic acid lesions of the cerebellar interpositus nucleus on eyelid conditioning in the rabbit. Brain Res. 1985;326(1):179–182. doi: 10.1016/0006-8993(85)91400-3. [DOI] [PubMed] [Google Scholar]
- 40.Skelton RW. Bilateral cerebellar lesions disrupt conditioned eyelid responses in unrestrained rats. Behav Neurosci. 1988;102(4):586–590. doi: 10.1037//0735-7044.102.4.586. [DOI] [PubMed] [Google Scholar]
- 41.Steinmetz JE, Lavond DG, Ivkovich D, Logan CG, Thompson RF. Disruption of classical eyelid conditioning after cerebellar lesions: Damage to a memory trace system or a simple performance deficit? J Neurosci. 1992;12(11):4403–4426. doi: 10.1523/JNEUROSCI.12-11-04403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodruff-Pak DS, Lavond DG, Logan CG, Steinmetz JE, Thompson RF. Cerebellar cortical lesions and reacquisition in classical conditioning of the nictitating membrane response in rabbits. Brain Res. 1993;608(1):67–77. doi: 10.1016/0006-8993(93)90775-i. [DOI] [PubMed] [Google Scholar]
- 43.Swain RA, Shinkman PG, Thompson JK, Grethe JS, Thompson RF. Essential neuronal pathways for reflex and conditioned response initiation in an intracerebellar stimulation paradigm and the impact of unconditioned stimulus preexposure on learning rate. Neurobiol Learn Mem. 1999;71(2):167–193. doi: 10.1006/nlme.1998.3872. [DOI] [PubMed] [Google Scholar]
- 44.Chapman PF, Steinmetz JE, Sears LL, Thompson RF. Effects of lidocaine injection in the interpositus nucleus and red nucleus on conditioned behavioral and neuronal responses. Brain Res. 1990;537(1-2):149–156. doi: 10.1016/0006-8993(90)90351-b. [DOI] [PubMed] [Google Scholar]
- 45.Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci USA. 2002;99(3):1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kleim JA, et al. Synapse formation is associated with memory storage in the cerebellum. Proc Natl Acad Sci USA. 2002;99(20):13228–13231. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller MJ, et al. fMRI of the conscious rabbit during unilateral classical eyeblink conditioning reveals bilateral cerebellar activation. J Neurosci. 2003;23(37):11753–11758. doi: 10.1523/JNEUROSCI.23-37-11753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch JC, Hoover JE, Strick PL. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res. 1994;100(1):181–186. doi: 10.1007/BF00227293. [DOI] [PubMed] [Google Scholar]
- 49.Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: Functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20(1):214–228. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoue S, et al. Cross-reactive antigenicity of nucleoproteins of lyssaviruses recognized by a monospecific antirabies virus nucleoprotein antiserum on paraffin sections of formalin-fixed tissues. Pathol Int. 2003;53(8):525–533. doi: 10.1046/j.1440-1827.2003.01511.x. [DOI] [PubMed] [Google Scholar]