Abstract
Since its discovery, the tumor suppressor phosphatase and tensin homolog (PTEN) has become a molecule with a wide spectrum of functions, which is typically meditated through its lipid phosphatase activity; however, PTEN also functions in a phosphatase-independent manner. It is well established that PTEN regulates several signaling pathways, such as phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), janus kinase (JAK)/signal transducers and activators of transcription (STAT), focal adhesion kinase (FAK), and more recent, extracellular signal-regulated kinase (ERK)1/2, where activation of these pathways typically leads to cancer development and progression. In regard to most of these pathways, the underlining molecular mechanism of PTEN-mediated regulation is well established, but not so much for the ERK1/2 pathway. Indeed, accumulating evidence has shown an inverse correlation between PTEN expression and ERK1/2 in several malignancies. However, the detailed mechanism by which PTEN regulates ERK1/2 is poorly understood. In this review, we discuss the role of PTEN in regulating ERK1/2 by directly targeting shc/Raf/MEK and PI3K/AKT cascades, and a putative cross-talk between the two.
Keywords: ERK1/2, PTEN, PI3K/AKT, Raf, Ras, cancer
Introduction
Tumorigenesis is the result of genetic alterations and/or epigenetic modifications to oncogenes and tumor suppressors. Aberrations in oncogenes results in an increase in mitogenic functions, such as cell proliferation and survival signaling; alterations in tumor suppressor genes are associated with unrestrained cell proliferation. Thus, tumor suppressor proteins generally inhibit cancer development and progression by regulating a spectrum of cellular survival functions, including cell cycle, apoptosis, DNA repair, signal transduction, and cell adhesion. One frequently inactivated tumor suppressor, found in a variety of human cancers, is the phosphatase and tensin homolog deleted on chromosome 10q23 (PTEN) gene. In fact, phosphatase and tensin homolog (PTEN) is the second most mutated tumor suppressor, only to p53, in several malignancies such as prostate, breast, lung, and brain (1–4).
Loss of PTEN in cancer cells is typically associated with increased activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) pathway, thereby facilitating cancer malignant transformation and progression. PTEN negatively regulates AKT through its lipid phosphatase activity; PTEN dephosphorylates phosphatidylinositol 3,4,5-triphosphate (PIP3), a secondary messenger for AKT, to PIP2, thereby preventing the activation of AKT and subsequent downstream survival pathways (5). Other pathways that are under regulation by PTEN are (1): signal transducers and activators of transcription (STAT); (2) Jun N-terminal kinase (JNK); and focal adhesion kinase (FAK) (6–8). Emerging studies have suggested that PTEN may also negatively regulate the extracellular signal-regulated kinase (ERK)1/2 pathway. To date, several studies have established an inverse correlation between PTEN expression and ERK1/2 inhibition, but have not fully investigated the underlining mechanism(s) by which PTEN inhibits ERK1/2 activity. In fact, we have demonstrated that PTEN reconstitution in PC3 prostate cancer cells inhibited ERK1/2 phosphorylation (2). Herein, we will briefly focus on the tumor-suppressive role of PTEN, as well as an emerging target of PTEN regulation, ERK1/2 kinase.
Regulation of PTEN
In 1997, Li et al. (9) and Steck et al. (10) first identified a high frequency of PTEN mutations and deletions in cancers of the brain, bladder, breast, and prostate, implicating PTEN as a novel tumor suppressor. Likewise, Yu et al. demonstrated that inactivation of PTEN was the result of point mutations, epigenetic silencing, and deletions. Apart from its tumor-suppressive role, PTEN aberrations also associate with an array of diverse diseases, such as Cowden disease, autism, Lhermitte Duclos disease, and Bannayan–Zonana syndrome (11).
PTEN is a member of type-I protein tyrosine phosphatase (PTP) family and consists of five domains: (1) N-terminus phosphoinositol 4, 5 bisphosphate (PIP2) binding domain; (2) the phosphatase domain; (3) lipid binding C2 domain; (4) PDZ domain (post synaptic density protein [PSD95], Drosophila disc large tumor suppressor [Dlg A], and zonula occludens-1 protein [zo-1] motif; and (5) a C-terminal tail containing a PEST motif (12). The N-terminus phosphatase domain has dual-specificity activity, which dephosphorylates proteins and phosphoinositides substrates (13). The peptide phosphatase activity is targeted against tyrosine, serine, and threonine residues on proteins, while the lipid phosphatase activity targets PIP3. This dual phosphatase function indicates that PTEN targets a wide range of molecules, and indirectly, molecules that are downstream of these targets, thereby regulating tumorigenic functions, such as apoptosis, cell cycle, cell adhesion, and cell migration (14,15).
PTEN is a potent tumor suppressor; therefore, it is expected that PTEN expression and function would be well regulated to maintain cellular homeostasis. For instance, PTEN expression and enzymatic activity are regulated through transcriptional regulation, microRNA (miRNA) targeting, and post-translational regulation. Transcriptionally, PTEN expression is mediated by growth regulated transcription factor 1 (EGR1), peroxisome proliferator activated receptor γ (PPARγ), and p53, through direct binding to the PTEN promoter region, leading to its gene transcription (16–18). Conversely, PTEN transcriptional silencing is enhanced by NF-κB and Jun in several cancer models; while promoter hypermethylation, another form of repressing gene expression, was identified in lung, thyroid, breast, and ovarian cancers (19–22). Moreover, miRNA targeting, specifically miRNA21 (miR-21), miR-22 and miR-25a, reduced PTEN expression (23,24). As with most proteins, PTEN is also regulated through post-translational modifications, commonly phosphorylation, acetylation, ubiquitylation, and active site oxidation. For instance, Torres et al. (25) demonstrated that phosphorylation of the C-terminus end of PTEN by casein kinase 2 (CK2) rendered PTEN resistant to proteasomal degradation, ultimately, enhancing stability. PTEN degradation is enhanced by the E3 ligase NEDD4-1 through ubiquitin-mediated proteasomal degradation (26). Finally, PTEN activity was regulated by reactive oxygen species (ROS), where accumulation of ROS has been shown to oxidize PTEN within the catalytic domain by forming disulfide bridges, rendering PTEN inactive (27). An interruption in PTEN expression and activity will increase, and enhance expression and catalytic activity of growth-promoting kinases, such as AKT, and consequently, encourage phenotypic behaviors that allow tumor cells to survive and become mobile (28).
Tumor-suppressive functions of PTEN
PTEN is classically associated with inhibiting the PI3K/AKT pathway and ultimately cell survival, proliferation, and migration (5). Accumulating studies have indicated that PTEN exerts its tumor-suppressive functions through its phosphatase activity as well as protein interactions. For instance, PTEN promoted cell cycle G1 phase arrest by downregulating cyclin D1 through its protein phosphatase activity, while upregulating p27 through its lipid phosphatase activity in breast cancer cells. This study suggested that the tumor-suppressive function of PTEN does not depend on the lipid phosphatase function or the protein phosphatase function independently, but coordinating activities of both phosphatases (29). Despite this conclusion, current evidence supports the notion that both phosphatase activities can suppress tumor development independent of each other. Through the peptide phosphatase activity, PTEN targeted the tyrosine residue on FAK, which disrupted cell adhesion and migration (30). As an example of protein phosphatase function, Freeman et al. (31) demonstrated that PTEN regulated p53 stabilization and transcriptional activity by competing with MDM2 for direct binding with p53.
The continued investigation of PTEN has revealed its multi-faceted role in regulating cell proliferation, gene expression, metabolism, migration, and survival, by acting on targets involved in these processes, explaining its potent tumor-suppressive role (32,33). PTEN utilizes its phosphatase domain, as well as its protein–protein domain, to regulate cell signaling and the function of cognate molecules. The ERK1/2 pathway has become a novel target of PTEN regulation (2). Weng et al. (34) reported one of the initial studies to support a cross-talk between PTEN and ERK1/2, where reconstitution of PTEN in breast cancer cells inhibited ERK1/2-mediated phosphorylation of ETS-2, thereby preventing cancer progression. Since then, more studies have provided evidence that ERK1/2 is a target of PTEN, but not enough to fully elucidate the molecular mechanism.
ERK1/2 signaling pathway
The ERK1/2 signaling pathway plays a critical role in regulating cell proliferation, differentiation, survival, and apoptosis of various cancer types such as bone, prostate, breast, and lung. In fact, it has been estimated that ERK1/2 targets more than 180 different molecules that are responsible for cell growth, survival, and differentiation, thus aberrant regulation greatly affects cell growth (35). ERK1/2 kinase couples the signals from cell surface receptors to molecules that transmit cell proliferative signals. Following stimulation of appropriate receptors, the Src homology 2 domain-containing protein (shc), becomes associated with the activated receptor (36). The coupling of shc and a stimulated receptor recruits the growth factor receptor-bound protein 2 (Grb2), and son of sevenless (sos) to form a complex. This complex then exchanges GDP with GTP onto membrane-bound Ras, thereby activating Ras function. Subsequently, Ras-GTP recruits Raf serine/threonine-specific kinase to the membrane, which then becomes activated. Raf then mediates activation of ERK kinase 1 (MEK1) through phosphorylation on two of its serine residues within the activating loop (36). Finally, MEK1 phosphorylates ERK1/2, rendering it active, which then targets a variety of substrates through its kinase activity. ERK1/2 kinase activity then leads to the upregulation of various transcription factors such as Ets-1, c-Jun, c-Myc, and HIF1α (37,38). Each of these transcription factors regulate genes that promote cancer cell survival in some way-from metabolism, to proliferation, to metastasis, and distal colonization (39). Independent of direct activation by ERK1/2 kinase, constitutive activation of molecular events upstream of ERK1/2 can also involve the kinase in cancer progression. For instance, the continuous activation of epidermal growth factor receptor (EGFR) resulted in constitutive activation of downstream target ERK1/2. Likewise, a mutation in the proto-oncogene B-Raf rendered it constitutively active, and as a result, downstream targets like the ERK1/2 pathway also became constitutively active and tumorigenic (40).
PTEN regulation of the ERK1/2 signaling pathway
In cancer cells, an assortment of signaling pathways leads to neoplasia, and eventually, tumor development and progression of the tumor to a malignant disease. Tumor development primarily depends on the homeostatic imbalance of activated oncogenes, and repressed tumor suppressors. It is well established that the tumor suppressor PTEN negatively regulates the oncogenic signaling pathway PI3K/AKT; however, literature suggests that PTEN may also regulate the ERK1/2 pathway to combat neoplastic development. Bouali et al. (41) and Chetram et al. (2) independently demonstrated that reconstitution of PTEN in null prostate cancer cells reduced the phosphorylation of both AKT and ERK1/2, resulting in reduced kinase activity. Inversely, ROS-mediated catalytic inactivation of PTEN resulted in increased phosphorylation of both AKT and ERK1/2 (28), rendering them active. A putative mechanism by which PTEN regulates ERK1/2 may be due to an interaction between PI3K and ERK1/2. An early link between the two kinases was observed in Xenopus laevis oocytes, which demonstrated that an increase in the catalytic subunit (p110α) of PI3K stimulated ERK1/2 activity (42). Likewise, a subsequent investigation in Simia aethiops kidney cells (COS-7) revealed that overexpression of the p110γ subunit of PI3K also facilitated the activation of ERK1/2 (43). In support of these studies, Liu et al. (44) demonstrated that pharmacological inhibition of PI3K suppressed ERK1/2 activity in hepatocellular carcinoma, breast, and prostate cancer cells. It remains unclear, however, whether stimulation of ERK1/2 activity depends on direct interaction with PI3K, or indirectly through a molecule that interacts with both the kinases.
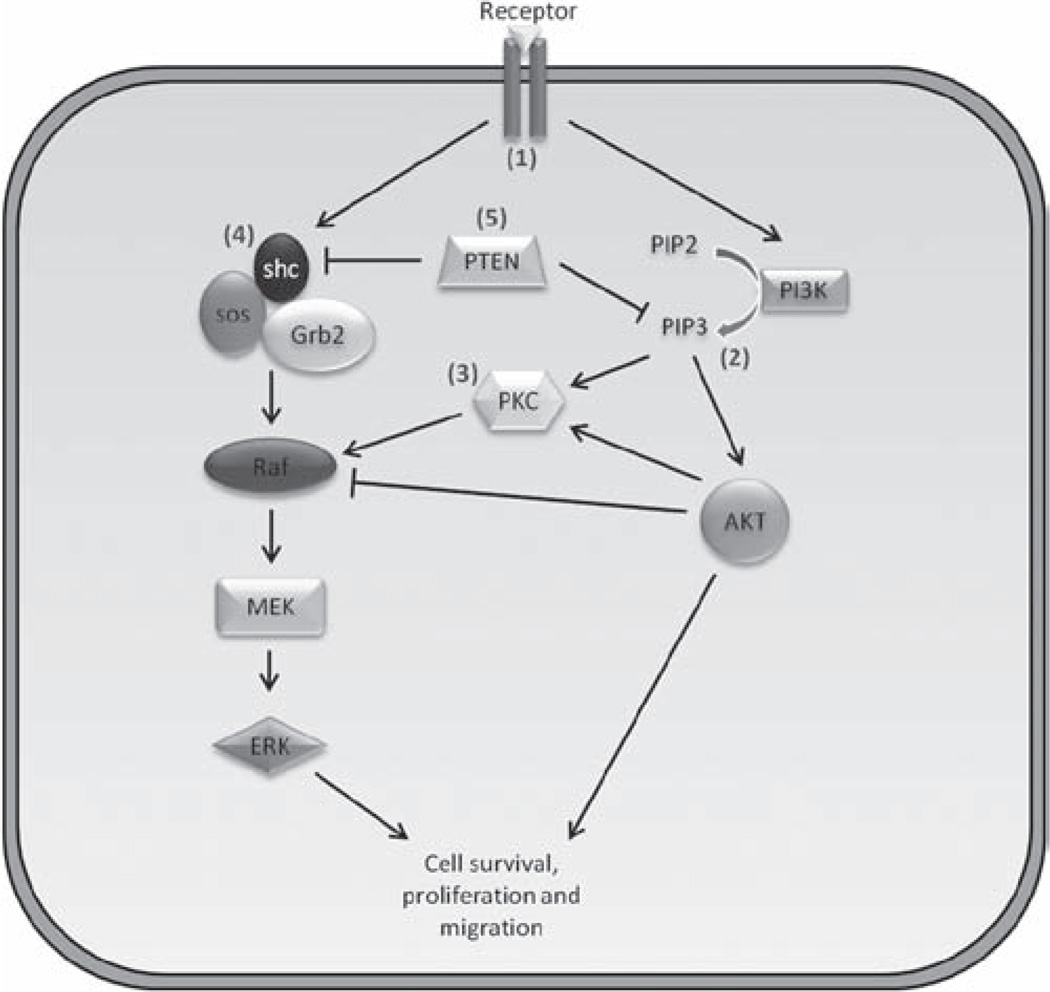
A putative indirect mechanism by which PI3K regulates ERK1/2 is through protein kinase C (PKC) (44). Studies have shown that PKC isoforms, such as PKCε, PKCδ and PKCη, were directly activated by PIP3 or through AKT activation (45,46), which in turn led to the phosphorylation of Raf, and ultimately, lead to ERK1/2 activation (47,48). These observations further suggested that the conversion of PIP3 to PIP2 by PTEN would negatively regulate ERK1/2 by preventing the activation of the AKT/PKC/Raf signaling axis (Figure 1), which indirectly connected PTEN and ERK1/2.
Figure 1.
A schematic of PTEN-mediated regulation of ERK1/2 in cancer cells. (1) The stimulation of a cell surface receptor results in the activation of PI3K (44) and shc complex (51). (2) An increase in PI3K function leads the accumulation of PIP3, which subsequently activates PKC directly (45) or indirectly through AKT (46) activation. (3) Increased PKC activity results in ERK1/2 activation and subsequent downstream functions (47). (4) Furthermore, the stimulation of shc results in the activation of Ras, Raf and ultimately, ERK1/2 (51). (5) PTEN regulates ERK1/2 by dephosphorylating shc and by reducing PIP3-mediated activation of PKC and AKT. PI3K, phosphoinositide 3-kinase; PIP3, phosphatidylinositol 3,4,5-triphosphate; AKT, protein kinase B; PKC, protein kinase C, shc, Src homology 2 domain-containing protein.
Lee et al. (49) demonstrated that AKT kinase directly associated with Raf and caused its inactivation by phosphorylating serine-259 in prostate cancer cells. A similar observation was made in breast cancer cells, where a high dose of insulin-like growth factor I (IGF-I) stimulated AKT and inhibited Raf through serine-259 phosphorylation, while a low dose of IGF-I abrogated these effects (50). In the same study, phorbol 12-myristate 13-acetate (PMA), a differentiation-inducing agent, activated the Raf/ERK pathway, but mildly activated the PI3K/AKT pathway, which did not trigger any cross-talk with Raf (50). It is possible that the differential regulatory role of AKT on the Raf/ERK1/2 pathway may be ligand and/or concentration-dependent.
Another mechanism by which PTEN may regulate ERK1/2 is through the Ras superfamily of small GTPases, which activates Raf. The coupling and phosphorylation of shc adapter protein to a ligand-stimulated receptor recruits the Grb–SOS complex to the plasma membrane, which activates Ras, then Raf, and ultimately, ERK1/2 (51). Gu et al. (52) demonstrated that reconstitution of PTEN in glioblastoma cells dephosphorylated shc adaptor protein, which inhibited activation of ERK1/2. Complementary studies in glioblastoma models confirmed these observations and even reported a correlation between PTEN-mediated shc dephosphorylation and cell migration (53). Further, a study by Nogueira et al. (54) suggested that loss of PTEN is correlated with Ras activation and consequently resulted in melanoma development. The latter studies did not further define the involvement of ERK1/2 in cell migration and melanoma development; however, it is plausible to suggest that increased Ras activation lead to downstream ERK1/2 activity. Therefore, these results support that PTEN regulates ERK1/2 signaling, although indirectly by targeting upstream kinases.
Conclusion
Current knowledge of PTEN-mediated regulation favors its effects on the PI3K/AKT pathway. Emerging studies support a new paradigm that PTEN may also target the ERK1/2 signaling pathway; however, very little information definitely describes how this mechanism may occur. The diverse regulatory molecules of the ERK1/2 signaling pathway suggests its importance in cellular survival that can encourage tumor development; and for this reason, the involvement of PTEN would be integral in regulating these functions. PTEN-mediated regulation appears to be indirect through several molecules, such as PI3K/AKT, shc, PKC, Ras, and Raf, all of which cross-talk and dictate the activation status of the ERK1/2 (Figure 1). For instance, PI3K/AKT may activate ERK1/2 through PKC, while the phosphorylation of Raf by AKT may inhibit ERK1/2 function. The activity status of signaling molecules connected to ERK1/2 may differ among tissue types; hence, the role of PTEN in regulating ERK1/2 pathway may differ among tumor types. Considering that PTEN may modulate both AKT and ERK pathways, it is crucial to evaluate both the pathways in future research. Therefore, assessing the status of PTEN expression in tumors may improve treatment options; current treatments for PTEN-null tumors focus on antagonizing the PI3K/AKT pathway, but should now consider antagonizing ERK1/2 in a combinatory manner. To substantiate our observations, however, further studies are warranted to understand the detailed mechanisms by which PTEN regulates the ERK1/2 pathway.
Acknowledgments
Limitations of space preclude extensive citation of literature; we apologize to those whose work is not mentioned herein. Research in this laboratory is supported, in part, by National Institutes of Health grants RCMI22622A (MAC), F31CA153908 (MAC), 2G12RR003062-22 (CVH) and 1P20MD002285 (CVH).
Footnotes
Declaration of Interest
None.
References
- 1.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 2.Chetram MA, Odero-Marah V, Hinton CV. Loss of PTEN permits CXCR4-mediated tumorigenesis through ERK1/2 in prostate cancer cells. Mol Cancer Res. 2011;9:90–102. doi: 10.1158/1541-7786.MCR-10-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954–2963. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira AM, Ross JS, Fletcher JA. Tumor suppressor genes in breast cancer: the gatekeepers and the caretakers. Am J Clin Pathol. 2005;124(Suppl):S16–S28. doi: 10.1309/5XW3L8LU445QWGQR. [DOI] [PubMed] [Google Scholar]
- 5.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 6.Wu R, Sun S, Steinberg BM. Requirement of STAT3 activation for differentiation of mucosal stratified squamous epithelium. Mol Med. 2003;9:77–84. doi: 10.2119/2003-00001.wu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanco I, Palaskas N, Tran C, Finn SP, Getz G, Kennedy NJ, Jiao J, Rose J, Xie W, Loda M, Golub T, Mellinghoff IK, Davis RJ, Wu H, Sawyers CL. Identification of the JNK signaling pathway as a functional target of the tumor suppressor PTEN. Cancer Cell. 2007;11:555–569. doi: 10.1016/j.ccr.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 10.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 11.Nelen MR, Padberg GW, Peeters EA, Lin AY, van den Helm B, Frants RR, Coulon V, Goldstein AM, van Reen MM, Easton DF, Eeles RA, Hodgsen S, Mulvihill JJ, Murday VA, Tucker MA, Mariman EC, Starink TM, Ponder BA, Ropers HH, Kremer H, Longy M, Eng C. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13:114–116. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, Jiang X. PTEN: a default gate-keeping tumor suppressor with a versatile tail. Cell Res. 2008;18:807–816. doi: 10.1038/cr.2008.83. [DOI] [PubMed] [Google Scholar]
- 13.Myers MP, Stolarov JP, Eng C, Li J, Wang SI, Wigler MH, Parsons R, Tonks NK. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–9057. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deevi R, Fatehullah A, Jagan I, Nagaraju M, Bingham V, Campbell FC. PTEN regulates colorectal epithelial apoptosis through Cdc42 signalling. Br J Cancer. 2011;105:1313–1321. doi: 10.1038/bjc.2011.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen KA, Doan HQ, Zhou BP, Wang Q, Zhou Y, Rychahou PG, Evers BM. PTEN loss induces epithelial–mesenchymal transition in human colon cancer cells. Anticancer Res. 2009;29:4439–4449. [PMC free article] [PubMed] [Google Scholar]
- 16.Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, de Belle I. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3:1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 17.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 18.Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11:764–768. doi: 10.1016/s0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez-Nuñez F, Bussaglia E, Mauricio D, Ybarra J, Vilar M, Lerma E, de Leiva A, Matias-Guiu X Thyroid Neoplasia Study Group. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006;16:17–23. doi: 10.1089/thy.2006.16.17. [DOI] [PubMed] [Google Scholar]
- 20.Soria JC, Lee HY, Lee JI, Wang L, Issa JP, Kemp BL, Liu DD, Kurie JM, Mao L, Khuri FR. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8:1178–1184. [PubMed] [Google Scholar]
- 21.García JM, Silva J, Peña C, Garcia V, Rodríguez R, Cruz MA, Cantos B, Provencio M, España P, Bonilla F. Promoter methylation of the PTEN gene is a common molecular change in breast cancer. Genes Chromosomes Cancer. 2004;41:117–124. doi: 10.1002/gcc.20062. [DOI] [PubMed] [Google Scholar]
- 22.Ho CM, Lin MC, Huang SH, Huang CJ, Lai HC, Chien TY, Chang SF. PTEN promoter methylation and LOH of 10q22-23 locus in PTEN expression of ovarian clear cell adenocarcinomas. Gynecol Oncol. 2009;112:307–313. doi: 10.1016/j.ygyno.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 23.Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T, Holland EC. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH. MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC) Clin Chim Acta. 2010;411:846–852. doi: 10.1016/j.cca.2010.02.074. [DOI] [PubMed] [Google Scholar]
- 25.Torres J, Pulido R. The tumor suppressor PTEN is phosphorylated by the protein kinase CK2 at its C terminus. Implications for PTEN stability to proteasome-mediated degradation. J Biol Chem. 2001;276:993–998. doi: 10.1074/jbc.M009134200. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leslie NR, Bennett D, Lindsay YE, Stewart H, Gray A, Downes CP. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22:5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chetram MA, Don-Salu-Hewage AS, Hinton CV. ROS enhances CXCR4-mediated functions through inactivation of PTEN in prostate cancer cells. Biochem Biophys Res Commun. 2011;410:195–200. doi: 10.1016/j.bbrc.2011.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10:599–604. doi: 10.1093/hmg/10.6.599. [DOI] [PubMed] [Google Scholar]
- 30.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–20703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 31.Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 32.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heeg S, Hirt N, Queisser A, Schmieg H, Thaler M, Kunert H, Quante M, Goessel G, von Werder A, Harder J, Beijersbergen R, Blum HE, Nakagawa H, Opitz OG. EGFR overexpression induces activation of telomerase via PI3K/AKT-mediated phosphorylation and transcriptional regulation through Hif1-alpha in a cellular model of oral-esophageal carcinogenesis. Cancer Sci. 2011;102:351–360. doi: 10.1111/j.1349-7006.2010.01796.x. [DOI] [PubMed] [Google Scholar]
- 34.Weng LP, Brown JL, Baker KM, Ostrowski MC, Eng C. PTEN blocks insulin-mediated ETS-2 phosphorylation through MAP kinase, independently of the phosphoinositide 3-kinase pathway. Hum Mol Genet. 2002;11:1687–1696. doi: 10.1093/hmg/11.15.1687. [DOI] [PubMed] [Google Scholar]
- 35.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 36.Kolch W. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem J. 2000;351(Pt 2):289–305. [PMC free article] [PubMed] [Google Scholar]
- 37.Steelman LS, Franklin RA, Abrams SL, Chappell W, Kempf CR, Bäsecke J, Stivala F, Donia M, Fagone P, Nicoletti F, Libra M, Ruvolo P, Ruvolo V, Evangelisti C, Martelli AM, McCubrey JA. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25:1080–1094. doi: 10.1038/leu.2011.66. [DOI] [PubMed] [Google Scholar]
- 38.Richard DE, Berra E, Gothié E, Roux D, Pouysségur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 39.Nicholson B, Schaefer G, Theodorescu D. Angiogenesis in prostate cancer: biology and therapeutic opportunities. Cancer Metastasis Rev. 2001;20:297–319. doi: 10.1023/a:1015543713485. [DOI] [PubMed] [Google Scholar]
- 40.Andreadi C, Noble C, Patel B, Jin H, Aguilar Hernandez MM, Balmanno K, Cook SJ, Pritchard C. Regulation of MEK/ERK pathway output by subcellular localization of B-Raf. Biochem Soc Trans. 2012;40:67–72. doi: 10.1042/BST20110621. [DOI] [PubMed] [Google Scholar]
- 41.Bouali S, Chrétien AS, Ramacci C, Rouyer M, Becuwe P, Merlin JL. PTEN expression controls cellular response to cetuximab by mediating PI3K/AKT and RAS/RAF/MAPK downstream signaling in KRAS wild-type, hormone refractory prostate cancer cells. Oncol Rep. 2009;21:731–735. [PubMed] [Google Scholar]
- 42.Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, Xie Y, Lou L. PI3K is required for insulin-stimulated but not EGF-stimulated ERK1/2 activation. Eur J Cell Biol. 2006;85:367–374. doi: 10.1016/j.ejcb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Toker A, Meyer M, Reddy KK, Falck JR, Aneja R, Aneja S, Parra A, Burns DJ, Ballas LM, Cantley LC. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 46.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 47.Schönwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naranatt PP, Akula SM, Zien CA, Krishnan HH, Chandran B. Kaposi’s sarcoma-associated herpesvirus induces the phosphatidylinositol 3-kinase-PKC-zeta-MEK-ERK signaling pathway in target cells early during infection: implications for infectivity. J Virol. 2003;77:1524–1539. doi: 10.1128/JVI.77.2.1524-1539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee JT, Steelman LS, Chappell WH, McCubrey JA. Akt inactivates ERK causing decreased response to chemotherapeutic drugs in advanced CaP cells. Cell Cycle. 2008;7:631–636. doi: 10.4161/cc.7.5.5416. [DOI] [PubMed] [Google Scholar]
- 50.Moelling K, Schad K, Bosse M, Zimmermann S, Schweneker M. Regulation of Raf-Akt Cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 51.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, Batzer A, Thomas S, Brugge J, Pelicci PG. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 52.Gu J, Tamura M, Pankov R, Danen EH, Takino T, Matsumoto K, Yamada KM. Shc and FAK differentially regulate cell motility and directionality modulated by PTEN. J Cell Biol. 1999;146:389–403. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas SL, Alam R, Lemke N, Schultz LR, Gutiérrez JA, Rempel SA. PTEN augments SPARC suppression of proliferation and inhibits SPARC-induced migration by suppressing SHC-RAF-ERK and AKT signaling. Neuro-oncology. 2010;12:941–955. doi: 10.1093/neuonc/noq048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nogueira C, Kim KH, Sung H, Paraiso KH, Dannenberg JH, Bosenberg M, Chin L, Kim M. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–6232. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]