Abstract
Increases in plasma lipids, tissue triglycerides and decreases in mitochondrial function have been linked to insulin resistance and aging. In animals, peroxisome proliferator-activated receptor-α (PPARα) agonists decrease plasma lipids, intramyocellular fat (IMCL) and liver fat (LFAT) and improve mitochondrial β- oxidative function and insulin sensitivity, but the effects in elderly were not known. Insulin sensitivity was assessed with a 2-hour oral glucose tolerance test, magnetic resonance spectroscopy was used to asses IMCL, LFAT and plasma lipids were measured before and after 6, 11 and 61 days of PPAR-α agonist (fenofibrate) administration in 19 elderly (age 70±1 years) volunteers. Volunteers were stratified into healthy (N=7) and insulin resistant (N=12) groups. The baseline insulin sensitivity index (8.1±1.2 vs. 3.8±0.5, healthy vs. insulin resistant; P<0.001) was significantly higher in the healthy group. Fenofibrate treatment induced significant reductions in plasma triglycerides (P<0.001) and total cholesterol (P<0.001) in both groups. Nonetheless, neither fasted free fatty acids, glucose, insulin, nor insulin sensitivity improved in either group (Day 1 vs. day 61, 8.1±1.2 vs. 8.1±0.9, healthy; and 3.8±0.5 vs. 4.2±0.05, insulin resistant). Furthermore, there was no change in IMCL or LFAT. These results indicate that whereas fenofibrate significantly lowers plasma lipids it does not affect insulin sensitivity nor intracellular lipids in elderly.
Keywords: Intramuscular triglyceride, liver fat, aging, insulin resistance, plasma lipids
1.1 Background
An increase in the prevalence of insulin resistance in aging has been well documented (Petersen KF, Befroy D et al. 2003; Cree, Newcomer et al. 2004). The insulin resistance of aging may be related to the rate of delivery and breakdown of lipids within the muscle and the liver (Levadoux, Morio et al. 2001). An imbalance between delivery and oxidation can lead to increased intracellular triglycerides (Petersen KF, Befroy D et al. 2003; Shulman 2004). Whereas intra-cellular triglycerides per se may not cause insulin resistance, they may be associated with increases in triglyceride metabolites such as diacylglycerol (DAG) and long chain fatty acyl CoA that decrease insulin signaling (Shulman 2004). The degradation of fats occurs by β-oxidation within the mitochondria, and recent studies in elderly individuals have found that mitochondrial dysfunction occurs in conjunction with increased intracellular lipids in both the muscle (IMCL) and liver (LFAT) (Petersen KF, Befroy D et al. 2003; Cree, Newcomer et al. 2004). Some attribute this mitochondrial dysfunction to cumulative mitochondrial DNA damage (Wang, Michikawa et al. 2001). Others have proposed that the mitochondrial dysfunction is simply a manifestation of reduced physical activity levels that can be reversed with regular physical activity (Rimbert, Boirie et al. 2004). The accumulation of tissue lipids may not only be due to a decrease in the oxidation rate, but also to an increase of delivery through plasma triglycerides (TG) and free fatty acids (FFA).
Peroxisome proliferator-activated receptor-α (PPAR) are nuclear receptors that, when stimulated by endogenous lipids, activate specific genes involved in fat metabolism. PPAR-α receptors are present primarily in the liver and muscle tissue(Su, Simmons et al. 1998). In animals, PPAR-α activators such as WY14643, a potent PPAR-α agonist, have been shown to affect multiple aspects of fat and glucose metabolism (Guerre-Millo M, Gervois P et al. 2000; Boden G, Lebed B et al. 2001). Of interest is the apparent ability of PPAR-α agonists to lower plasma TG, increase fat oxidation and improve insulin sensitivity. Significant decreases in fasting plasma glucose, insulin and TG, with a concomitant decrease in IMCL and LFAT have been reported in lipoatrophic mice, mice given high fat diets and diabetic Zucker rats after treatment with a PPAR-α agonists (Guerre-Millo M, Gervois P et al. 2000; Chou CJ, Haluzik M et al. 2002). PPAR-α agonist treatment in human myocytes increased β-oxidation of oleate and decreased oleate incorporation into TG. More recently we have found that PPAR-α agonist treatment in patients with burn trauma significantly improved insulin stimulated glucose uptake through improving whole body β- oxidation of palmitate and the proportion of mitochondrial respiration associated with ADP use (Cree, Qian et al. 2006; Cree, Newcomer et al. 2007). Thus in certain models the PPAR-α agonists affect glucose and fat metabolism by increasing mitochondrial oxidative capacity.
Despite the encouraging results in animals, in-vitro and trauma patients, results of PPAR-α agonist treatment in young and middle aged humans have not been as clear-cut. Some studies have found that PPAR-α agonists lower triglycerides and fasting concentrations of insulin, and thus improve insulin sensitivity as assessed by the homeostasis model, or improve insulin area under the curve following an oral glucose tolerance test (OGTT) (Yong, Thavintharan et al. 1999; Idzior-Walus, Sieradzki et al. 2000; Tan, Chew et al. 2001). Other studies have also found a decrease in fasting plasma glucose concentrations, and HbA1C following treatment (Kobayashi, Shigeta et al. 1988; Damci, Tatliagac et al. 2003). In contrast, some studies failed to identify an effect of PPAR-α agonists on insulin sensitivity in varied populations (Forcheron, Cachefo et al. 2002; Vega, Cater et al. 2003). Thus the role of PPAR-α in improving insulin sensitivity in humans is not well established.
Thus far, all studies to date have been performed in younger to middle aged populations with plasma hyper-lipidemia and/or type 2 diabetes. While the theory of mitochondrial dysfunction leading to increased lipid accumulation has also been presented for type 2 diabetes, this theory is based on the premise of inherited genetic causes of mitochondrial dysfunction (Shulman 2004). Several studies in offspring of diabetics who appear otherwise healthy have demonstrated numerous metabolic abnormalities. These included increases in IMCL and LFAT, decreases in mitochondrial number, size, and fatty acid oxidation rates (Kelley and Simoneau 1994; He, Watkins et al. 2001).
The cause of the mitochondrial dysfunction in elderly is unknown, but is theorized to be acquired with age, in part because of its widespread occurrence (Shulman 2004) and may not be the same genetically acquired mitochondrial dysfunction. For this reason, we investigated if the PPAR-α agonist fenofibrate could improve insulin sensitivity, and decrease intracellular lipids, presumably through lowering plasma lipids and increasing mitochondrial fatty acid oxidation in elderly individuals. We found that whereas PPAR-α agonist treatment in elderly does decrease plasma TG’s, it has no effect on IMCL, LFAT or insulin sensitivity. This may indicate that there is not an associated between the plasma and tissue lipids in this patient population, as previously thought.
1.2 Methods
Nineteen volunteers, 12 female and 7 male, ages 65-72 (average 70±1 years) were enrolled in the study. All subjects read and signed an informed consent. The project was approved by the Institutional Review Board at the University of Texas Medical Branch, Galveston, Texas. All volunteers were generally healthy by history and physical exam, and none were participating in regular aerobic or resistance training routines. Exclusion criteria included a total cholesterol <300 mg/dL (6.5 mmol/L), abnormal thyroid stimulating hormone, palpable liver enlargement, positive Hepatitis B, C or HIV tests, anemia or elevation of more than one of the following: alkaline phosphatase >122 U/l, ALT > 51U/I, AST >40 U/I. Subjects did not use lipid lowering medications, diabetes medications, anticoagulants, illicit drugs or consume alcohol in excess (>1 drink/day or 6/wk). Following the baseline oral glucose tolerance test (OGTT), subjects were stratified into healthy (HE; N=7) or insulin resistant (IR; N=12) groups, according to the 2005 American Diabetes Association OGTT criteria (Position-Statement 2005). The mean baseline clinical data are shown in Table 1.
Table 1.
Volunteer baseline measurements, taken from blood drawn during the screening examination are shown, with the exception of glucose and insulin. Body Composition measurements taken during the study day are shown. Percent Body Fat, Trunk fat and extremity fat are calculated from a DEXA whole body scan. Data are Mean ± SEM. P values are calculated from a 2 tailed Students t-test.
| HE | IR | |
|---|---|---|
|
|
||
| Number (M/F) | 7(2/5) | 12(5/7) |
| Age -years | 70±1 | 70±1 |
| Family History of Diabetes | 3 | 5 |
| Caucasian | 7 | 9 |
| Black/Hispanic | 0/0 | 2/1 |
| Glucose Metabolism | ||
| Fasting Glucose mg/dL (mmol/L) | 90± 2.2 (4.95±0.12) | 101± 2.3* (5.55±0.12) |
| Fasting Insulin μU/mL (pmol/L) | 6.5± 1.9 (45±13) | 9.1± 1.3* (63±9) |
| HbA1C | 5.6±0.1 | 5.8±0.1 |
| ISI | 8.6±0.9 | 4.1±0.6 |
| Cholesterol | ||
| Total Cholesterol mg/dL (mmol/L) | 205 ± 14 (5.3±0.4) | 210 ± 11 (5.4±0.3) |
| HDL mg/dL (mmol/L) | 64 ± 4 (1.66±0.1) | 51 ± 4 (13. ±0.1)* |
| LDL mg/dL (mmol/L) | 116 ± 10 (3.0±0.3) | 134 ± 10 (3.5±0.3) |
| Ratio | 3.2 ± 0.2 | 4.3 ± .02 * |
| TG’s mg/dL (mmol/L) | 125 ± 33 (1.4±0.4) | 126 ± 10 (15±0.1) |
| Liver Function | ||
| ALK Phosphotase (U/L) | 67±5 | 85±9 |
| ALT (u/L) | 25±2 | 27±2 |
| AST (U/L) | 23±2 | 26±3 |
| Body Composition | ||
| Height (m) | 171±11 | 160±6 |
| Weight (kg) | 75±5 | 77±5 |
| Percent Total Body Fat | 34±3 | 34±3 |
| Percent Trunk Fat | 32±3 | 34±2 |
| Percent Extremity Fat | 37±4 | 36±4 |
indicates P<0.05 between HE and IR
The subjects were studied on five occasions –three inpatient and two outpatient. On days 1, 11 and 61, volunteers were admitted to the General Clinical Research Center (GCRC) at the University of Texas Medical Branch. Following a 12 hour overnight fast, IMCL and LFAT were measured with magnetic resonance spectroscopy (MRS) and body composition with a dual x-ray absorptiometry (DEXA) scan. Then a 20 gauge IV catheter was inserted in the antecubital vein for blood sampling. After 2 baseline samples, a 2-hour OGTT with 75 mg dextrose was performed. The volunteers were fasted for the outpatient visits, which occurred on day 6 (OGTT) and day 35 (cholesterol and hepatic panel tests), respectively.
Fenofibrate treatment (160mg micronized tablets once daily) was started on day 1, following the completion of the baseline study. Subjects were given the full 60 days dose in a special pill pack, with each day and date clearly labeled. Subjects were required to bring the pill pack with them to each visit in order to assess compliance. 15 subjects took all pills, 2 subjects missed one dose, 1 missed 2 doses and one missed 3 doses. None of the missed doses were in the first 11 days, and none were within 5 days of any study measurements.
Minor side effects such as headache, flatulence and diarrhea were reported by 5 subjects. 2 subjects had elevated liver enzymes 3 times normal at day 35 and were subsequently withdrawn from the study at day 40. An additional subject withdrew from the study for personal reasons after day 35. Thus, results presented for days 1-35 represent 19 subjects, whereas the data from day 61 represents 16 subjects. 2 of the dropouts were in the IR group, and one in the HE group.
1.3 Sample Analysis
1.3.1 MRS soleus
Samples were analyzed as previously described (Cree, Newcomer et al. 2004). Briefly, IMCL was measured from the soleus with a 1H knee coil on a GE Advantage 1.5 Tesla whole body imager (General Electric, Milwaukee, WI). A plastic container of 20 % Intralipid© (IV high fat total parenteral feeding solution – Baxter Healthcare Deerfield Park, IL) was placed inside the knee coil to obtain a standard external reference to normalize IMCL concentrations (Cree, Newcomer et al. 2004). After a preliminary localization image, 4 voxels (approximately 4 mm x 4 mm x 10 mm each) were chosen in soleus muscle free from fascia, gross fat marbling and vessels and the Intralipid© external reference. An optimized PRESS sequence with repetition time (TR) of 2000 ms and echo time (TE) of 35 ms was run. Peak positions and areas of interest (extramuscular (CH2)n, intramuscular CH2)n, extramuscular (CH3), intramuscular CH3, total creatine (tCr), and tri-methylamines (TMA) were determined by time domain fitting using jMRUi (van den Boogaart A, Van Hecke A et al. 1996; Rico-Sanz J, Thomas EL et al. 1999). The prior knowledge information used for the AMARES fits have been previously published by Rico-Sanz (Rico-Sanz J, Thomas EL et al. 1999). This process was repeated for the Intralipid© phantom. The tissue TG levels were computed as a relative unit-less ratio of the tissue TG to the Intralipid© standard using the following formula:
where PM is the tissue lipid methylene peak area, VM is the total measured tissue voxel volume, PI is the Intralipid© peak area, and VI is the Intralipid© voxel volume.
1.3.2 MRS liver
The LFAT was measured with a GE 1.5 T 1H whole body coil. Hepatic measurements were performed in the middle right lobe. A tube of Intralipid© was again used for reference. After a preliminary localization scan, a voxel (approximately 30 mm x 30 mm x 20 mm) was chosen at a location free from large vessels. An optimized PRESS sequence with repetition time (TR) of 5000 ms and echo time (TE) of 40 ms was performed. The spectra represent an average LFAT measurement over the mid right lobe as respiratory gating was not conducted. Spectra were manually phased and final analysis was performed with jMRUI software, as previously described for muscle.
1.3.3 Glucose analysis
Plasma glucose concentrations were measured with an YSI 2300 Stat glucose/lactate analyzer (YSI, Inc. Yellow Springs, OH).
1.3.4 Insulin Analysis
Plasma insulin concentrations were measured using Radioactive Immuno-Assay (Diagnostic Laboratories, Los Angeles, CA). The coefficient of variation for these measurements in our lab is <10%.
1.3.5 Free Fatty Acid Analysis
Total free fatty acids were measured using a chromatographic kit (Wako Diagnostics, Osaka, Japan)
1.3.6 Dual X-ray Energy absorptiometry (DEXA)
All DEXA scans were performed on a Hologic QDR 4500A system by the same technician.
1.4 Calculations
We used the Defronzo/Matsuda ISI model following a 75 g oral glucose challenge to assess insulin sensitivity.
| (2) |
Where FPG is fasting plasma glucose and FPI is fasting plasma insulin. The value derived from this equation is an M value of glucose uptake in mg/kg/min which is approximated to results that would likely have been obtained if a more invasive hyperinsulinemic-euglycemic clamp had been performed (Matsuda M and RA DeFronzo 1999; Position-Statement 2005). The calculated values range from 0-12, with 12 being the highest level of insulin sensitivity, and 0 the lowest level of insulin sensitivity.
1. 5 Statistics
All results were reported as mean± standard error of the mean. Baseline comparison were performed with a 2 tailed unpaired students T-test. Differences between the groups and across time were analyzed using a 2 way ANOVA, with a Tukeys test to delineate any detected differences.
1.6 Results
1.6.1 Baseline Parameters
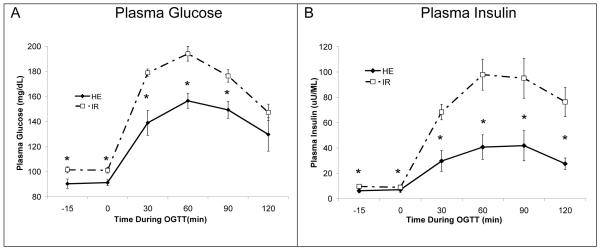
The two groups were significantly different according to their baseline OGTT. OGTT plasma glucose was significantly (P<0.01) higher at all points except for 120 min in the IR group (Figure 1A). Further, the 30, 60 and 120 min timepoint glucose concentrations were in the insulin resistant range (Position-Statement 2005). The post glucose plasma insulin response to the glucose load was also significantly (P<0.01) higher at all time points in the IR group, reaching a peak of 97±11.4 μU/mL compared to a peak of 41±3.8μU/mL in the normal group (Figure 1B). There were few differences between the groups other than the glucose and insulin (Table 1). HDL was higher in HE, and the ratio of LDL to HDL was higher in IR. While the BMI was higher in IR, DEXA results showed that the two groups were comparable in terms of total, trunk and extremity percent body fat percentages.
Figure 1.
A. Plasma glucose during the baseline OGTT in HE and IR subjects. Plasma glucose was significantly elevated in IR groups at 30, 60, and 90 min post-drink. B. Plasma insulin during baseline OGTT. IR plasma insulin was significantly elevated vs HE at all time points following the glucose drink. * indicates P<0.05 between HE and IR. P–values are calculated with ANOVA.
1.6.2 Changes over treatment duration
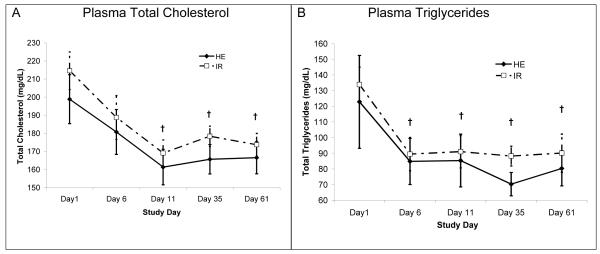
Fenofibrate treatment significantly (P<0.001) decreased total plasma cholesterol (Figure 2A). The lowest values occurred at day 11. Plasma triglycerides followed a similar pattern with a significant (P<0.001) drop seen by day 6, and no difference between the groups (Figure 2B). Basal plasma FFA concentrations were similar between groups, and FFA concentrations were suppressed similarly during the OGTT. Plasma FFA concentrations at 120 min of the OGTT were approximately 9% of the fasted values, and neither the fasted concentrations, nor the level of suppression changed with treatment (data not shown).
Figure 2.
Both groups experienced a significant drop from baseline in both total cholesterol and triglycerides. However the groups were not different from each other. N=6 in HE and N=10 in IR at day 61 due to dropout. † indicates that P<0.05 compared to baseline, as calculated with ANOVA.
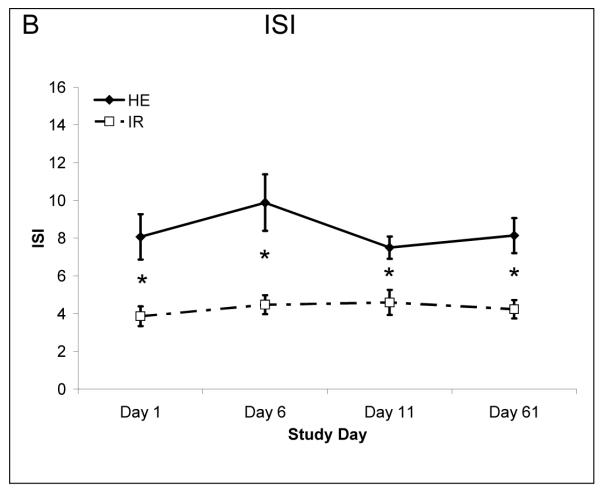
Treatment did not affect and glucose metabolism parameters, including fasting glucose (HE 90±2.2:91±2.4 mg/dL; Day 1:Day 61 and IR 101±2.3:96±1.5 Day 1: Day 61) fasting insulin concentrations (HE 6.5±1.9:5.7±1.2 μmol/mL Day 1:Day 61 and IR 9.1±1.3:8.6±1.0 Day1: Day 61) or the ISI (Figure 3).
Figure 3.
Insulin sensitivity as assessed by the ISI model. There was no change with treatment in insulin sensitivity. N=6 in HE and N=10 in IR at day 61 due to dropout.
Whereas the fenofibrate treatment reduced certain plasma lipids there was no change in the intracellular fat measurements (Table 2). The HE trended to have lower IMCL and LFAT values at baseline than IR, but these were not significant. There was no change in fasted FFA in either group, and the level of plasma FFA suppression seen during the OGTT remained the same across fenofibrate treatment (Table 3). The suppression of plasma FFA’s from 0 to 120 min of the OGTT was significant in both groups, during all 4 OGTT’s.
Table 2.
LFAT and IMCL measurements for both groups are shown above. Neither group was significantly different from each other, and there was no effect of treatment in either group. N=6 in HE and N=10 in IR at day 61 due to dropout.
| Day 1 | Day 11 | Day 61 | ||
|---|---|---|---|---|
|
|
||||
| LFAT | ||||
| HE | 0.22±0.04 | 0.26±0.04 | 0.23±0.06 | |
| IR | 0.38±0.09 | 0.36±0.07 | 0.46±0.06 | |
| IMCL | ||||
| HE | 0.09±0.01 | 0.11±0.01 | 0.09±0.01 | |
| IR | 0.10±0.01 | 0.11±0.01 | 0.13±0.02 | |
Table 3.
Plasma Free Fatty Acid levels drawn at 0, 60 and 120 min of the OGTT’s on days 0, 5, 10 and 60 are shown above. Both groups experienced a significant decrease in plasma FFA following the ingestion of glucose, and this response was not different between groups, and was not changed with fenofibrate treatment. N=6 in HE and N=10 in IR at day 61 due to dropout.
| Study Day |
|||||
|---|---|---|---|---|---|
| OGTT Time | Day 1 | Day 6 | Day 11 | Day 61 | |
|
|
|||||
| HE | |||||
| 0 | 613±60 | 709±39 | 781±77 | 608±55 | |
| 60 | 171±36* | 154±13* | 155±27* | 165±34* | |
| 120 | 84±16* | 60±7* | 69±13* | 53±13* | |
| IR | |||||
| 0 | 687±57 | 619±47 | 665±49 | 651±91 | |
| 60 | 164±18* | 191±10* | 171±28* | 189±35* | |
| 120 | 63±7* | 60±8* | 87±26* | 52±6* | |
indicates P<0.05 compared to time 0.
1.7 Discussion
Consistent with its clinical application, fenofibrate treatment lowered plasma triglycerides significantly within 6 days of treatment and total cholesterol within 11 days of treatment. Thus, a therapeutic dose of fenofibrate was given to all subjects. However, despite good compliance and a significant decrease in plasma lipids, neither intracellular lipids, plasma FFA’s nor insulin sensitivity were affected by fenofibrate treatment.
In concurrence with some previous research, we found no change in the OGTT ISI index either the IR or HE groups, despite the fact that insulin sensitivity was significantly reduced by more than 50% in the IR group compared to HE, and was similar to that seen in patients with type 2 diabetes (Matsuda M and RA DeFronzo 1999). Elevated fasting blood glucose concentrations generally provide the first clinical indication of diabetes. However, an OGTT provides a better representation of the post-prandial period, and has a higher sensitivity than the fasting value (Matsuda M and RA DeFronzo 1999). Other methods such as an intravenous glucose tolerance test, or a hyperinsulinemic-euglycemic clamp are more invasive and do not activate all of the physiologic responses accompanying a gastric caloric load, although they are better for assessing muscle and hepatic specific insulin sensitivity. Further, the ISI model with which we analyzed the OGTT correlates well with the clamp technique (Matsuda M and RA DeFronzo 1999). The ISI uses measurements of both plasma glucose and insulin concentrations following the glucose drink, and neither were affected by fenofibrate treatment (data not shown). Thus, the lack of findings in terms of insulin sensitivity is not likely to be due to use of the OGTT’s for assessment.
There was no effect of treatment on either IMCL or LFAT in either group. The baseline values obtained are comparable to those from a previous study from this group, and the DEXA values, a more accurate measure than BMI, are similar between groups (Cree, Newcomer et al. 2004). The relationship between IMCL, LFAT and insulin resistance has been well documented in cross sectional studies in recent years, including our own previous study (Marchesini G, Brizi M et al. 1999; Petersen KF, Befroy D et al. 2003; Cree, Newcomer et al. 2004). Further, this relationship is maintained in experimental conditions, such as following gastric bypass surgery or high fat infusion (Boden G, Lebed B et al. 2001; Greco AV, Mingrone G et al. 2002). It is therefore not surprising that fenofibrate treatment failed to affect insulin sensitivity in light of the lack of change in intracellular lipid stores. On the other hand, we have recently reported that in severely burned patients fenofibrate treatment counteracted insulin resistance but did not affect either IMCL or LFAT (Cree, Qian et al. 2006). Thus, whereas ample data suggest a relation between intracellular lipids and insulin resistance, the relationship may not always apply.
There are several reports of an inverse relationship between plasma TG’s and insulin sensitivity (Taniguchi, Fukushima et al. 2000; Kanaya, Fyr et al. 2005). Additionally, dyslipedmia and insulin resistance are the basis for the definition of the metabolic syndrome (Klein and Romijn 2003). Thus, since fenofibrate decreased plasma TG’s, it is surprising that insulin sensitivity was not affected. A study similar to ours examined the effects of PPAR-α and PPARγ agonists in middle-aged type II diabetics. These authors found that the PPARγ improved insulin sensitivity, decreased intracellular lipids and decreased plasma FFA’s, while their results with the PPAR-α were similar to ours (Bajaj, DeFronzo et al. 2004) . It may be that an improvement in insulin sensitivity is dependant on a reduction in plasma free fatty acids and not plasma TG’s or that the plasma TG’s were not adequately elevated at the outset of the study.
Several studies have found an association between plasma FFA’s and insulin sensitivity (Paolisso, Tataranni et al. 1995; Charles, Eschwege et al. 1997). Furthermore, infusions of a lipid emulsion and/or heparin to increase plasma FFA concentrations have been shown to rapidly induce insulin resistance in several populations, including healthy, obese, healthy diabetic relatives and diabetics (Boden, Chen et al. 1994; Griffin, Marcucci et al. 1999). The acute increase in insulin resistance following intravenous FFA administration has been associated with increases in IMCL in some cases, but often this parameter has not been measured (Boden, Lebed et al. 2001). The use of plasma FFA lowering drugs such as Acipimox improve in insulin sensitivity within three days (Paolisso, Tagliamonte et al. 1998). The increase in insulin stimulated glucose uptake was associated with an increase in glucose oxidation. Thus, there seems to be a clear relationship between plasma FFA’s and insulin mediated glucose uptake (Boden, Chen et al. 1994).
Despite the reduction in plasma TG’s, there was no change in the either the fasted or the OGTT FFA levels in our subjects. Damci et al (Damci, Tatliagac et al. 2003) saw a change of insulin sensitivity with fenofibrate and reported a decrease in plasma FFA’s following treatment, whereas Vega et al (Vega, Cater et al. 2003) found no change in either fasted plasma FFA’s, FFA turnover rates or insulin sensitivity. Serum FFA’s were not measured in the other studies that saw either a positive or negative response of insulin sensitivity to fenofibrate (Yong, Thavintharan et al. 1999; Idzior-Walus, Sieradzki et al. 2000; Tan, Chew et al. 2001; Forcheron, Cachefo et al. 2002).
Several studies have assessed the effect of PPARα agonists on plasma triglycerides and indices of insulin sensitivity, but not IMCL or LFAT, in middle aged patient populations with conflicting results. Patients with hyper-lipidemia and metabolic syndrome experienced no improvement in any glucose or insulin measurements following 8 weeks of fenofibrate treatment (Vega, Cater et al. 2003). Similarly, there was no improvement in insulin sensitivity in hyperlipidemic diabetics following 4 months of fenofibrate treatment (Forcheron, Cachefo et al. 2002). However, fenofibrate may not work in these populations if the mitochondrial defects in diabetes and metabolic syndrome are genetic and consequently less likely to be reversed by pharmacologic treatment. Young healthy patients treated with 1,200 mg of gemfibrozil daily experienced no improvement in any glucose or insulin parameters, and no change in plasma FFA’s (Sane, Knudsen et al. 1995). In these volunteers the inability of fenofibrate to improve insulin sensitivity may be due to an intrinsically small margin for improvement. Middle aged men with hyper-lipidemia had a decrease in fasting insulin and the insulin response to a glucose load at 60 and 180 min after either 3 or 6 months of treatment (Yong, Thavintharan et al. 1999; Idzior-Walus, Sieradzki et al. 2000). 31 patients with metabolic syndrome experienced an improvement in insulin sensitivity index following fenofibrate administration and patients with long standing (7.7±5.9 years) diabetes had lower fasted and post-prandial glucose levels, lower HbA1C, and decreased fasted insulin levels (Tan, Chew et al. 2001; Wysocki, Belowski et al. 2004). The true efficacy of the fenofibrate in middle aged population is thus hard to interpret.
While we did affect a change in plasma triglycerides, it may be that a larger dose is needed to cause a measurable change in insulin sensitivity or intracellular fat. In the five positive human studies that used fenofibrate, three gave 200mg micronized fenofibrate (Lipanthyl©), and in the other two, 250 and 300 mg were given(Yong, Thavintharan et al. 1999; Idzior-Walus, Sieradzki et al. 2000; Tan, Chew et al. 2001; Damci, Tatliagac et al. 2003; Wysocki, Belowski et al. 2004). These doses are all greater than the 160 mg “supra-available” co-micronized (Tricor©) dose available in the United States (Najib 2002). In our trauma study, we used a dose of 5mg/kg (Cree, Qian et al. 2006). The co-micronized formulation is coated to increase the absorption yield, so that a lower dose can be given with presumed effectiveness. 200 mg micronized and 160 mg co-micronized are thought to be equivalent in terms of efficacy on plasma lipids, but this may not be the case with insulin sensitivity (Najib 2002; Ramjattan, Callaghan et al. 2002). In animal studies showing positive effects large doses were given, either as fenofibrate or through experimental compounds such as WY14,643 or GW7647 which are more potent than fenofibrate (Chou CJ, Haluzik M et al. 2002) Thus, a potential reason for the conflicting data on the effects of PPAR-α on insulin sensitivity in both animals and humans may be related to dose.
1.8 Conclusions
In conclusion, treatment for 60 days with 160 mg co-micronised fenofibrate failed to affect a change in insulin sensitivity, IMCL of LFAT levels in either healthy or insulin resistant elderly, despite dramatic decreases in plasma cholesterol and triglyceride.
Acknowledgments
The investigators would like to thank the volunteers who made this study possible. Also the metabolism nurses & technicians, Pepper center recruiters, the GCRC staff, and the Dept. of Radiology MRI Technicians.
Funding for this project was provided by Shriners Hospital Grant 8940 (R.R. Wolfe), and the NIH Claude D. Pepper Older Americans Independence Center Grant P60 AG17231-01 (James Goodwin, MD Dept of Internal Medicine, UTMB).
This study was conducted at the General Clinical Research Center, University of Texas Medical Branch (Galveston, TX) funded by grant M01-RR-00073 from the National Center for Research Resources, NIH, USPHS.
List of Abbreviations
- DAG
Diacylglycerol
- DEXA
Dual X-ray absorptiometry
- FFA
free fatty acids
- GCRC
General Clinical Research Center
- HDL
High density lipoprotein cholesterol
- HE
Healthy
- IMCL
Intramyocellular fat
- IR
Insulin resistant
- ISI
Insulin sensitivity index
- LDL
Low density lipoprotein cholesterol
- LFAT
Liver fat
- MRS
Magnetic Resonance
- OGTT
Oral glucose tolerance test
- PPAR-α
Peroxisome proliferating agonist receptor
- TE
Echo time
- TG
Triglycerides
- TR
Repetition time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests There are no financial conflicts of interest between the authors and any private funding entity. This work was in no way sponsored by the maker of the drug used, fenofibrate (Abbott Corporation)
References
- Bajaj M, DeFronzo R, et al. Effects of PPAR-a/g Therapy on Glucose/Lipid Metabolism, Hepatic fat and Adiponectin in Type 2 Diabets (T2DM) Diabetes. 2004;53(S2):A32. [Google Scholar]
- Boden G, Lebed B, et al. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–7. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- Boden G, Chen X, et al. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93(6):2438–46. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Lebed B, et al. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50(7):1612–7. doi: 10.2337/diabetes.50.7.1612. [DOI] [PubMed] [Google Scholar]
- Charles MA, Eschwege E, et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia. 1997;40(9):1101–6. doi: 10.1007/s001250050793. [DOI] [PubMed] [Google Scholar]
- Chou CJ, Haluzik M, et al. WY14,643, a peroxisome proliferator-activated receptor alpha (PPARalpha ) agonist, improves hepatic and muscle steatosis and reverses insulin resistance in lipoatrophic A-ZIP/F-1 mice. J Biol Chem. 2002;277(27):24484–9. doi: 10.1074/jbc.M202449200. [DOI] [PubMed] [Google Scholar]
- Cree MG, Newcomer BR, et al. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab (Lond) 2007;4:9. doi: 10.1186/1743-7075-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cree MG, Newcomer BR, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864–71. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- Cree MG, Qian T, et al. Fenofibrate improves insulin sensitivity and mitochondrial function in children with burn injury. Annals of Surgery. 2007 Feb;245(2):214–21. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damci T, Tatliagac S, et al. Fenofibrate treatment is associated with better glycemic control and lower serum leptin and insulin levels in type 2 diabetic patients with hypertriglyceridemia. Eur J Intern Med. 2003;14(6):357–360. doi: 10.1016/s0953-6205(03)90001-x. [DOI] [PubMed] [Google Scholar]
- Forcheron F, Cachefo A, et al. Mechanisms of the triglyceride- and cholesterol-lowering effect of fenofibrate in hyperlipidemic type 2 diabetic patients. Diabetes. 2002;51(12):3486–91. doi: 10.2337/diabetes.51.12.3486. [DOI] [PubMed] [Google Scholar]
- Greco AV, Mingrone G, et al. Insulin Resistance in Morbid Obesity: Reverasl with intramyocellular fat depletion. Diabetes. 2002;51:144–151. doi: 10.2337/diabetes.51.1.144. [DOI] [PubMed] [Google Scholar]
- Griffin ME, Marcucci MJ, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–4. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Gervois P, et al. Peroxisome proliferator-activated receptor alpha activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275(22):16638–42. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- He J, Watkins S, et al. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50(4):817–23. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- Idzior-Walus B, Sieradzki J, et al. Effects of comicronised fenofibrate on lipid and insulin sensitivity in patients with polymetabolic syndrome X. Eur J Clin Invest. 2000;30(10):871–8. doi: 10.1046/j.1365-2362.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Fyr CL, et al. Predicting the Development of Diabetes in Older Adults: The derivation and validation of a prediction rule. Diabetes Care. 2005;28(2):404–8. doi: 10.2337/diacare.28.2.404. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(6):2349–56. doi: 10.1172/JCI117600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Romijn J. Obesity. In: PR Larsen HK, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. Saunders; Philadelphia, PA: 2003. [Google Scholar]
- Kobayashi M, Shigeta Y, et al. Improvement of glucose tolerance in NIDDM by clofibrate. Randomized double-blind study. Diabetes Care. 1988;11(6):495–9. doi: 10.2337/diacare.11.6.495. [DOI] [PubMed] [Google Scholar]
- Levadoux E, Morio B, et al. Reduced whole-body fat oxidation in women and in the elderly. Int J Obes Relat Metab Disord. 2001;25(1):39–44. doi: 10.1038/sj.ijo.0801530. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, et al. Assocation of Nonalcoholic Fatty Liver Disease with Insulin resistance. Am J. Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA. Insulin Sensitivity Indices Obtained From Oral Glucose Tolerance Testing. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- Najib J. Fenofibrate in the treatment of dyslipidemia: a review of the data as they relate to the new suprabioavailable tablet formulation. Clin Ther. 2002;24(12):2022–50. doi: 10.1016/s0149-2918(02)80095-9. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Tagliamonte MR, et al. Lowering fatty acids potentiates acute insulin response in first degree relatives of people with type II diabetes. Diabetologia. 1998;41(10):1127–32. doi: 10.1007/s001250051041. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Tataranni PA, et al. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia. 1995;38(10):1213–7. doi: 10.1007/BF00422371. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Position-Statement Standards of Medical Care in Diabetes. Diabetes Care. 2005;28(suppl_1):S4–S36. [PubMed] [Google Scholar]
- Ramjattan BR, Callaghan DJ, et al. Efficacy and tolerability of a “suprabioavailable” formulation of fenofibrate in patients with dyslipidemia: a pooled analysis of two open-label trials. Clin Ther. 2002;24(7):1105–16. doi: 10.1016/s0149-2918(02)80022-4. [DOI] [PubMed] [Google Scholar]
- Rico-Sanz J, Thomas EL, et al. Diversity in levels of intracellular total creatine and triglycerides in human skeletal muscles observed by 1H-MRS. J. Appl. Physiol. 1999;87(6):2068–2072. doi: 10.1152/jappl.1999.87.6.2068. [DOI] [PubMed] [Google Scholar]
- Rimbert V, Boirie Y, et al. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. Faseb J. 2004;18(6):737–9. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- Sane T, Knudsen P, et al. Decreasing triglyceride by gemfibrozil therapy does not affect the glucoregulatory or antilipolytic effect of insulin in nondiabetic subjects with mild hypertriglyceridemia. Metabolism. 1995;44(5):589–96. doi: 10.1016/0026-0495(95)90115-9. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–90. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- Su JL, Simmons CJ, et al. Monitoring of PPAR alpha protein expression in human tissue by the use of PPAR alpha-specific MAbs. Hybridoma. 1998;17(1):47–53. doi: 10.1089/hyb.1998.17.47. [DOI] [PubMed] [Google Scholar]
- Tan CE, Chew LS, et al. Benefits of micronised Fenofibrate in type 2 diabetes mellitus subjects with good glycemic control. Atherosclerosis. 2001;154(2):469–74. doi: 10.1016/s0021-9150(00)00497-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi A, Fukushima M, et al. The role of the body mass index and triglyceride levels in identifying insulin-sensitive and insulin-resistant variants in Japanese non-insulin-dependent diabetic patients. Metabolism. 2000;49(8):1001–5. doi: 10.1053/meta.2000.7735. [DOI] [PubMed] [Google Scholar]
- van den Boogaart A, Van Hecke A, et al. MRUI: a graphical user interface for accurate routine MRS data analysis. Proceedings of the ESMRMB 13th Annual Meeting; Prague. 1996. p. 318. [Google Scholar]
- Vega GL, Cater NB, et al. Free fatty acid metabolism during fenofibrate treatment of the metabolic syndrome. Clin Pharmacol Ther. 2003;74(3):236–44. doi: 10.1016/S0009-9236(03)00170-X. [DOI] [PubMed] [Google Scholar]
- Wang Y, Michikawa Y, et al. Muscle-specific mutations accumulate with aging in critical human mtDNA control sites for replication. Proc Natl Acad Sci U S A. 2001;98(7):4022–7. doi: 10.1073/pnas.061013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki J, Belowski D, et al. Effects of micronized fenofibrate on insulin resistance in patients with metabolic syndrome. Int J Clin Pharmacol Ther. 2004;42(4):212–7. doi: 10.5414/cpp42212. [DOI] [PubMed] [Google Scholar]
- Yong QW, Thavintharan S, et al. The effect of fenofibrate on insulin sensitivity and plasma lipid profile in non-diabetic males with low high density lipoprotein/dyslipidaemic syndrome. Ann Acad Med Singapore. 1999;28(6):778–82. [PubMed] [Google Scholar]