Abstract
In children under 5 years of age, human parainfluenza viruses (HPIVs) as a group are the second most common etiology of acute respiratory illness leading to hospitalization, surpassed only by respiratory syncytial virus but ahead of influenza viruses. Using reverse genetics systems for HPIV serotypes 1, 2 and 3 (HPIV1, 2 and 3), several live-attenuated HPIVs have been generated and evaluated as intranasal vaccines in adults and in children. Two vaccines against HPIV3 were found to be well tolerated, infectious and immunogenic in Phase I trials in HPIV3-seronegative infants and children and should progress to proof-of-concept trials. Vaccines against HPIV1 and HPIV2 are less advanced and have just entered pediatric trials.
Keywords: acute respiratory illness, clinical trial, intranasal, live-attenuated, parainfluenza virus vaccine, pediatric, vaccine
Epidemiology & clinical disease
Acute lower respiratory illness (ALRI) is a major cause of morbidity and mortality. Globally, ALRI remains the most important cause of postneonatal mortality in children under 5 years of age, accounting for approximately 1.6 million deaths every year [1]. In addition to bacterial pathogens such as Streptococcus pneumoniae and Haemophilus influenzae type b, which were estimated to account for approximately half (36 and 16%, respectively) of the global pneumonia mortality in children under 5 years of age in 2000 [2,3], the major viral contributors to childhood ALRI are respiratory syncytial virus (RSV), human parainfluenza viruses (HPIVs), human metapneumovirus (HMPV) and influenza viruses [4-6]. Whereas licensed vaccines against invasive pneumococcal and H. influenzae type b disease are available and increasingly accessible, vaccines against RSV, the HPIVs and HMPV are still in development.
Globally, RSV is the most common cause of childhood ALRI [5] and the HPIVs as a group are the second most common etiology, responsible for more hospitalizations in children under the age of 5 years (1/1000 per year) than influenza [7-9]. A recent population-based burden of hospitalization study conducted by the New Vaccine Surveillance Network estimated that, in the USA, HPIVs accounted for approximately 7% of all hospitalizations for fever, acute respiratory illness (ARI) or both in children under 5 years of age [7]. This estimate translates into 23,000 HPIV-attributable hospitalizations per year in the USA, with HPIV3 responsible for half of that burden and HPIV1 responsible for the majority of the remainder [7].
Of the four HPIV serotypes, types 1, 2 and 3 (HPIV1, 2 and 3) are common causes of respiratory illness in infants and young children [6,10]. HPIV3, like RSV, frequently causes bronchiolitis and pneumonia in young infants. HPIV1 and HPIV2 are responsible for epidemics of croup, with HPIV1 being the most common etiologic agent of that disease [7,11]. Although HPIV1 and HPIV2 disease is most commonly seen in 1-6 year olds, hospitalization rates for all three HPIVs are highest in the first 6 months of life, with bronchiolitis, fever/possible sepsis, upper respiratory illness, pneumonia, croup and apnea as the most frequent discharge diagnoses. In infants and children 6 months of age and older, asthma and croup are the most common discharge diagnoses [7]. The use of corticosteroids and nebulized epinephrine to treat croup requiring urgent medical care has decreased croup-related hospitalization significantly and also explains a reported decrease in the contribution of HPIV1 to overall HPIV-attributable hospitalization [11-13].
In the USA, HPIVs can be isolated throughout the year, but HPIV3 circulation tends to peak in the spring, HPIV2 in autumn and HPIV1 in the autumn of odd-numbered years [14]. Reinfections with the HPIVs are frequent, although usually associated with milder illness and restricted to the upper respiratory tract (URT) [15]. Indeed, most HPIV-associated clinical illness is mild, even in primary infection. Rhinitis, pharyngitis, coryza and fever are common, whereas otitis media, croup, bronchitis, bronchiolitis and pneumonia are only observed in a minority children. Therefore, most HPIV-associated illness is treated on an outpatient basis and is not diagnosed with regard to viral etiology, leading to an underestimation of the HPIV-attributable burden of disease.
In addition to infants and young children, immunocompromised patients and the elderly are also at increased risk for severe HPIV disease. However, our understanding of the burden of disease in the elderly is very limited since most epidemiologic studies in this population focus on RSV and influenza. In a prospective study of healthy elderly individuals and of adults with chronic heart or lung disease, RSV infection was responsible for 11% of hospitalizations for pneumonia, 11% for chronic obstructive pulmonary disease, 5% for congestive heart failure and 7% for asthma [16]. In patients hospitalized with acute cardiopulmonary conditions, mortality was similar in RSV and influenza-infected patients [16]. If one assumes that the HPIVs behave like RSV in rhe aforementioned population and that their relative contribution to the burden of disease is similar to that observed in children, then the impact of HPIVs may be significant. However, data to substantiate this assumption are not available. In the immuno-compromised, especially in hematopoietic cell transplant (HCT) and in lung transplant patients, HPIVs can cause severe morbidity and mortality [17]. HPIVs are known ro be responsible for ALRI outbreaks in HCT units and outpatient clinics, with high transmission rates and high mortality (up to 45%) [18-21]. In HCT patients, HPIVs are as common a cause of viral pneumonia as RSV and high viral loads are found in bronchoalveolar lavage fluid from these patients [22].
Is there a need for HPIV vaccines?
Studies conducted decades ago (reviewed elsewhere [6]), as well as recent epidemiologic studies [7], indicate that the HPIVs as a group cause at least as much ARI in infants and young children as influenza. Whereas universal influenza vaccination of children is recommended, no licensed vaccine against the HPIVs exists. Since cross-protection between HPIV serotypes is very short-lived or insignificant, a decision is needed as to which serotypes should be included in HPIV vaccines. As previously indicated, HPIV3 is responsible for more hospital admissions than HPIV1 and HPIV2 combined. Therefore, a HPIV3 vaccine for infants is desirable and would ideally be given to infants as young as 1 or 2 months of age since much of the severe illness occurs in the first several months of life. As both RSV and HPIV3 can infect very early in life and in the presence of maternally-derived serum antibody, a combined RSV/HPIV3 vaccine that can induce protective immunity in this young infant population is probably the most important goal in pediatric respiratory viral vaccine development. A recent study conducted in rural Kenya indicated that the prevention of RSV-associated severe pneumonia alone might reduce all-cause (i.e., including bacterial) pneumonia hospital admissions of children under 13 years of age by a third [23]. A HPIV1/HPIV2 vaccine could be given later than an RSY/HPIV3 vaccine because clinical disease from these serotypes is less common in the first 6 months of life. Since the relative contribution to the burden of disease in infants and young children is estimated at approximately 4:2:1 for HPIV3, HPIV1 and HPIV2, respectively [6,24], a HPIV2 vaccine is not an attractive target as a stand-alone vaccine. However, if a HPIV1/HPIV2 vaccine could be developed as a single vaccine or be combined with a boost against RSV, it might prove to be a worthy and medically meaningful target.
Viruses
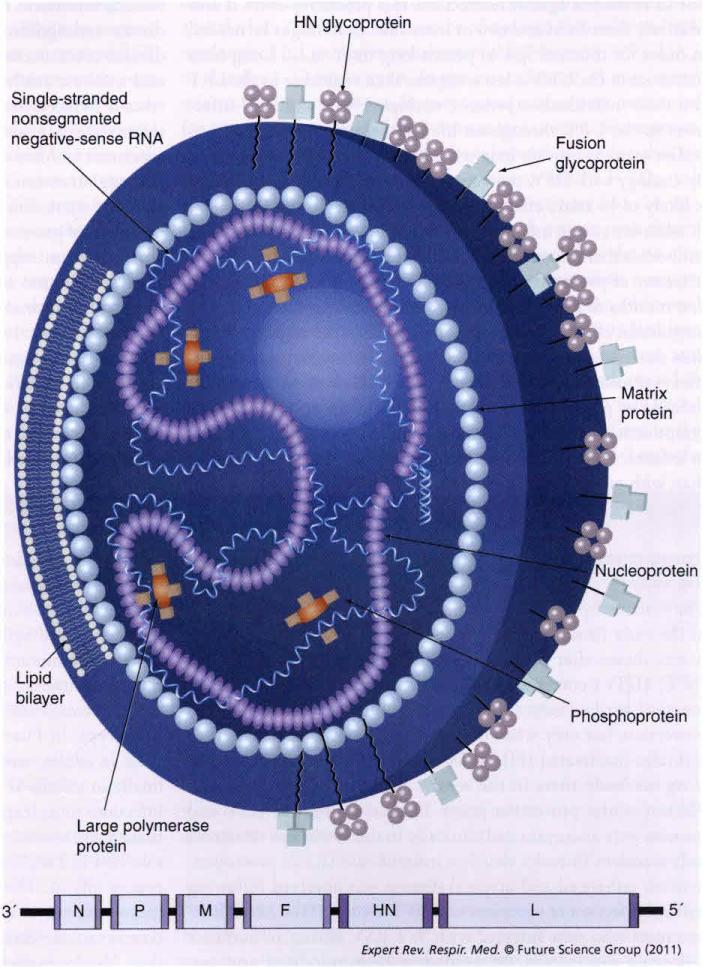
The HPIVs are enveloped, nonsegmented, negative-sense RNA viruses belonging to the Paramyxoviridae family in the order Mononegavirales. HPIV1 and HPIV3 are members of genus Respirovirus, while HPIV2 is a member of genus Rubulavirus. The genomes of all three HPIVs are approximately 15,500 nucleotides in length and encode six common proteins in the invariant order N-P-M-F-HN-L (Figure 1). Infection of a host cell by HPIV is initiated by binding of the hemagglutinin-neuraminidase (HN) glycoprotein on the virion envelope to sialic acid on cellular membrane proteins [25,26]. The fusion (F) protein then mediates the fusion between the viral envelope and the host-cell plasma membrane. This releases the viral nucleocapsid, which consists of the viral genome tightly bound by the nucleoprotein (N) and associated with the phosphoprotein (P) and the large RNA-dependent RNA polymerase L protein. The nucleocapsid-bound polymerase directs copying of the viral genes into separate mRNA transcripts and also directs replication of the RNA genome [27]. Progeny nucleocapsids assemble and are packaged into virions that bud from the plasma membrane. The matrix (M) protein coats the inner surface of the envelope and the spike-like glycopcoteins F and HN project from the outer surface of the envelope [6,28].
Figure 1. Human parainfluenxa virus virion and genome organization.
Parainfluenza virus are enveloped viruses in the paramyxovirus family. Their single-strand negative-sense RNA genomes are approximately 15,500 nucleotides in length and encode six common proteins in the invariant order N-P-M-F-HN-L. The N, P and L proteins form the viral nucleocapsid. The M protein is involved in virion morphogenesis whereas the HN and F proteins mediate adhesion to and fusion with the host cell membrane, respectively. Each gene encodes a single major protein with the exception of the P gene, which, in addition to the P protein, encodes one or more accessory proteins, as indicated.
F: Fusion; HN: Hemagglutinin–neuraminidase; L: Large; M: Matirx; N: Nucleo; P: Phospho.
In addition to these six common proteins, each of the HPIVs encodes at least one additional nonessential protein from alternate open reading frames in the P gene. HPIV1 and HPIV3 encode short C proteins, while HPIV2 encodes a V protein that is distinguished by having a C-terminal domain that contains a conserved cysteine-rich motif. HPIV3 also encodes a D protein and may express small amounts of a V protein. The V and C proteins primarily act as inhibitors of the host innate immune response, whereas the function of D is unknown [29-32]. Although the C and V proteins are unrelated in either sequence or mechanism of action, they have a common function: to suppress the antiviral activity of type I interferons (IFNs) by blocking both their induction and their ability to signal amiviral responses by host cells.
Tropism
Replication of HPIV1, 2 and 3 occurs in the superficial epithelial cells lining the respiratory tract. Infection typically starts in the mucous membranes of the nose and throat but an infectious virus may also be transmitted directly to the lower respiratory tract (LRT). Within the LRT, the ciliated and alveolar cells but not the basal cells of the bronchial epithelium are infected [26]. Whether disease progresses from the URT to the LRT depends on several factors, including previous exposure to that serotype, virus titer in the URT and genetic susceptibility to severe disease [27,33,34). HPIV infection, unlike influenza, does not cause extensive cytopathic effect or tissue destruction in an in vitro model of the respiratory airway epithelium [26,32,35]. This suggests that the host immune response may contribute significantly to pathogenesis. HPIV infection is generally limited to the respiratory tract and does not spread systemically unless the infected individual is severely immunocompromised. Newly formed virions are primarily released from the apical surface of ciliated respiratory cells into the lumen of the respiratory tract [26,32,35]. This directional budding is thought to play a role in limiting HPIVs and RSV to the respiratory tract. However, RSV RNA can be detected in the blood of 10% of severely immunocompromised patients with RSV pneumonia, suggesting that, in addition to directional budding, some degree of host immunity might be needed to prevent systemic spread [22].
Immune response & correlates of protection
Human parainfluenza virus infections can induce potent humoral and cellular immune responses in the infected host. Innate immune responses, local and systemic IgG and IgA responses and CDS+ and CD4+ T-cell responses are known to be induced [36,37]. Although cellular responses are important in restricting virus replication and clearing primary HPIV infection, neutralizing antibodies targeting the HN and F glycoproteins of HPIV play a major role in conferring long-term protection from HPIV disease [6,38]. Both serum and mucosal neutralizing antibodies can provide lasting protection against disease. The presence of nasal antibody was found to be a good correlate of protection whereas serum antibody titers needed to be high in order to confer protection [43,44]. Thus, the induction of neutralizing antibodies is thought to be essential for a successful HPIV vaccine. As discussed earlier, virus-encoded IFN antagonists permit HPIVs to replicate efficiently in vivo; however, IFN production and activity are usually not completely blocked and probably contribute to host defense. Many patients with primary HPIV infections develop a detectable IFN response during the acute stage of illness [39]. The induction of T-cell responses also contributes to viral clearance [40,41]. Cellular immunity confers some resistance to reinfection but this effect wanes over a period of weeks to months [42].
Mucosal antibodies that neutralize virus infectivity appear to be the best correlates of protection against HPIV disease in adults [43] and may also correlate with protection in children, though measurement of mucosal immunity in children has been difficult. IgA has the advantage of being specifically transported through the epithelium to the lumenal surface and IgA is also able ro neutralize the virus within infecred epithelial cells. Although local IgA plays a key role in resistance against reinfection, this protective effect is also relatively short-lived and two or more infections might be needed in order for mucosal IgA to persist long term [43,44]. Long-term resistance in the URT is less complete than resistance in the LRT and most individuals experience multiple HPIV and RSV infections in the URT throughout life [45]. Serum neutralizing antibodies seem to provide long-term resistance to virus replication. By analogy with RSV, protection conferred by serum antibodies is likely to be more effective in the LRT than in the URT [46]. In addition, young infants possess maternally-derived serum IgG antibodies that are transferred across the placenta during the last trimester of pregnancy and provide some protection during the first months of life. Immune responses in infants are reduced in magnitude, effectiveness and durability compared with older children due to immunologic immaturity and the immunosuppressive effects of maternal antibodies, as reponed below [47-49]. Immunity induced by primary infection with HPIV often does not prevent symptomatic reinfection for more than a few months, especially in infants. However, illness upon reinfection is generally milder than with primary infection, with restricted virus replication and infrequent progression co ALRI [15].
Approaches to vaccine development & obstacles along the way
The first attempts at developing a HPIV vaccine were undertaken in the early 1960s, not long after the discovery of these viruses. It was shown that protection against challenge with wild-type (WT) HPIV1 correlated with the presence of neutralizing nasal mucosal antibodies [50]. Serum antibodies also contributed to protection, but only when the serum antibody titer was high. A formalin-inactivated (FI) HPIV1 vaccine that induced neutralizing antibody titers in the serum, but not the nasal mucosa, did not confer protection [43,50]. FI vaccines against RSV and measles were also evaluated clinically in the 1960s and conferred only transiem (measles virus) or insignificant (RSV) protection; instead, enhanced and atypical disease was observed following natural infection of vaccinees with WT virus. Of the 23 FI-RSV vaccinees who were infected with WT RSV during an outbreak following vaccination, 18 needed to be hospitalized and two infants died of pneumonia (reviewed elsewhere [6]). As a result of the failure of these FI vaccines, new attempts at developing HPIV and RSV vaccines for use in children have focused, for many years, on live-attenuated approaches. This is because preclinical and clinical data demonstrate that disease enhancement is not associated with live vaccines [51]. Only very recently has the interest in developing protein-based nonlive vaccines re-emerged and several vaccine developers have invested in the development of nonlive RSV vaccines.
At first consideration, HPIVs appear to be an easy target for vaccine development because these viruses cause acute, self-limiting disease and do not establish persistent infection. However, vaccine development has proved to be far from trivial and several obstacles need to be overcome. First, immunity induced by a single infection with WT HPIV does not prevent symptomatic reinfection. Most children and adults experience multiple symptomatic infections. However, reinfections generally induce milder disease and significant LRI is infrequent. Second, severe HPIV3 disease often occurs in young infants under 6 months of age and a robust antibody response to the viral surface glycoproteins is induced less frequently in this age group than in older infants [52,53]. In addition, young infants have a less diverse B-cell repertoire and less efficient antibody affinity maturation [54-56]. Although they can mount a protective immune response, as indicated by restriction of a second dose of live vaccine, measurable correlates of protection are not well defined [47]. Third, maternal antibodies can suppress the immunogenicity of both parenterally-administered nonlive and mucosally-delivered live vaccines [49,57,58]. In summary, one would want an ideal HPIV vaccine to be immunogenic in young infants in the presence of maternal IgG, to protect against LRI during the first infection with WT virus and to be well-tolerated and safe. A live-attenuated intranasally-administered HPIV or RSV vaccine would probably need to be given in twO or three doses, perhaps at 2, 4 and 6 months of age to fit the pediatric vaccination schedule.
Individual vaccines in clinical development HPIV3 vaccines
HPIV3cp45 was derived from the JS strain of HPIV3 by 45 passages at low temperature (cold-passage [cp]) [59]. During this process, the virus acquired 20 nucleotide substitutions, 15 of which were considered significant because they occurred in or near RNA signals (five mutations) or caused amino acid substitutions (ten mutations) (Figure 2) [60,61]. Six of the amino acid substitutions were found to contribute independently to the HPIV3cp45 attenuation phenotype. In Phase I trials, HPIV3cp45 was evaluated sequentially in adults, seropositive children, seronegative children and finally in infants at 1-2 months of age (Table 1). At a dose of 104 infectious units (expressed as plaque-forming units [PFU] or 50% tissue culture infectious doses [TCID50]), HPIV3cp45 was well-tolerated and highly infectious (>90%) in 1–3 month-old seronegative infants. The vaccine virus was shed for 2–3 weeks. with a mean peak titer of 103.3 PFU/ml of nasal wash fluid [47,62]. A second dose of vaccine was administered either 1 or 3 months after the first dose. Vaccinees who received dose two at 3 months after dose one were more likely to shed the vaccine virus and to shed higher titers of the vaccine virus than vaccinees who received dose two 1 month after dose one, indicating that protective immunity induced by the first dose had partially waned by 3 months. No reliable serological correlate of the observed protection could be identified in this youngest cohorr of vacinees. HPIV3-specific IgG responses were infrequent, potentially obscured owing to the presence of maternally-derived HPIV3-specific IgG. HPIV3 HN-specific serum IgA responses were detected in the majority of infants but did not correlate well with protection against replication of a second dose of vaccine [47]. In a separate trial involving 380 children aged 6–18 months that included 226 seronegative infants and children, a single dose of 105 PFU ofHPIV3cp45 was found to be well-tolerated, safe and immunogenic [63]. No significant difference in the frequency of adverse events (rhinitis, cough, fever or otitis media) was noted during the first 2 weeks after vaccination and 84% of seronegative vaccinees seroconverted to HPIV3, indicating that the vaccine was safe, appropriately attenuated and immunogenic in this age group [63]. Compatibility between live-attenuated RSV and HPIV3 components of an experimental bivalent vaccine was assessed by simultaneous intranasal vaccination with 105 PFU each of RSV cold-passaged and temperature sensitive [cpts]248/404 and HPIV3cp45 in 6–18 month-old seronegative children. In this trial, 92% of vaccinees were infected with HPIV3cp45 when the vaccine was given as a monovalem vaccine, whereas 76% were infected following co-administration with the RSV component, suggesting that the replication of RSVcpts248/404 might have interfered with that of HPIV3cp45. Nonetheless, antibody responses against HPIV3 were similar in subjects receiving monovalent versus bivalent vaccine [64]. The above mentioned trials were performed with biologically-derived virus. Subsequently, the vaccine virus was rederived from cDNA using a reverse genetics system and recombinant (r)HPIV3cp45 is the drug substance used in current clinical development efforts. Derivation of the vaccine virus from cDNA provides a preparation with a short, well-defined passage history that minimizes the risk of potential biological contamination of the vaccine seed virus. This technology also enables rederivation of the vaccine virus from cDNA at any time. The clinical development of rHPIV3cp45 is conducted in a cooperative research and development agreement between the National Institute of Allergy and Infectious Diseases (NIAID) and MedImmune, LLC. Currently, two Phase I trials are being sponsored by NIAID. The first protocol (ClinicalTrials.gov identifier: NCT00308412) [101] enrolled a total of 45 children 6–36 months of age into two cohorts, both randomized 2:1 to receive two doses of rHPIY3cp45 (105 TCID50) at placebo 4–10 weeks apart. In the first cohort of 24 unscreened infants 6–12 month of age, frequent nasal washes were performed for quantitative virology. In this unscreened cohort, all ten seronegative vaccinees had the vaccine virus detected in nasal washes for approximately 2 weeks, with a mean peak titer of 103.6 TCID50/ml, whereas only two out of five seropositive vaccinees had the vaccine virus detected for a single day each, with a mean peak titer of 100.9 TCID50/ml. Only one of the 15 vaccinees shed the vaccine following dose two, again suggesting that a protective immune response restricted replication of the vaccine virus. An additional 21 seronegative children 6–36 months of age were enrolled into this study to expand the safety and immunogenicity data and the findings from the first cohort were confirmed with regard to safety, infectivity and immunogenicity. rHPIV3cp45 was found to be safe, well-tolerated, immunogenic and bioequivalent to the biologically-derived vaccine virus, but an interval of 1–2 months was determined to be insufficient to allow for reinfection and boosting of the immune response. In order to test whether a longer interval between doses would increase the infectivity of a second dose of vaccine, a second NIAID-sponsored study (ClinicalTrials. gov identifiers: NCT01021397 and NCT01254175) [101] is currently enrolling seronegative children 6–36 months of age to evaluate the safety and immunogenicty of two doses of vaccine given 6 months apart. In addition to the above NIAID-sponsored studies. MedImmune has initiated a Phase I study evaluating the safety and immunogenicity of three doses of rHPIV3cp45 given 2 months apart (ClinicalTrials.gov identifier: NCT01150799) [101,65].
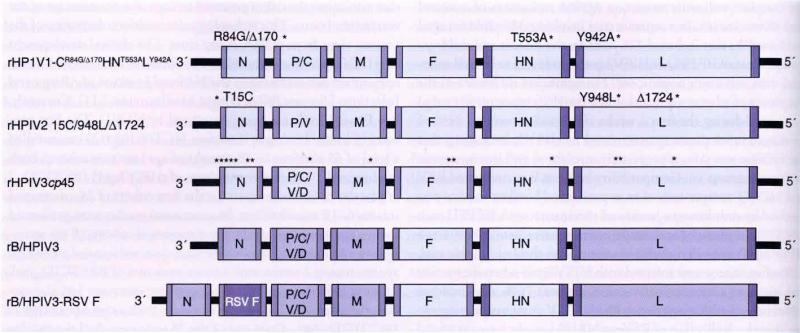
Figure 2. Investigational live-attenuated parainfluenza virus vaccines currently in clinical development.
Mutations that were introduced into the wild-type virus genome are indicated by asterisks. For HPIV1 and HPIV2, the type and positions of amino acid substitutions are indicated. The T15C mutation is a non coding mutation in the leader sequence of HPIV2. For rHPIV3cp45 (also referred to as MEDI-560) only the position of mutations is indicated. For B/HPIV3 and B/HPIV3-RSV F, BPIV3 genes are in light purple and HPIV3 genes are in light blue. In B/HPIV3-RSV F (also referred to as MEDI-534), the HRSV (subgroup A F protein is expressed from an additional open reading frame inserted between BPIV3 N and P).
BPIV3: Bovine parainfluenza viruses; cp: Cold-passaged; HPIV: Human parainfluenza virus; HRSV: human respiratory syncytial virus; MEDI: MedImmune. LLC; N: Nucleo; P: Phospho.
Table 1.
Selected published human parainfluenza virus 3 vaccine trials.
| Vaccine | Age range | Number and HPIV3 serostatus of subjects enrolled | Type of study and main outcome | Ref. |
|---|---|---|---|---|
| HPIV3cp45 | 4–48 months | 80 seronegative | Transmission study. No child fulfilled criteria for vaccine virus transmission | [101] |
| HPIV3cp45 ± RSV cpts-248/404 | 6–18 months | 54 seronegative for both HPIV3 and RSV | Phase I study. Safe and immunogenic. Simultaneous RSV/PIV3 vaccination is feasible | [64] |
| HPIV3cp45 | 6–18 months | 380 total 226 seronegative |
Phase II study. Safe and immunogenic | [63] |
| HPIV3cp45 | 18–50 years 6–59 months 1–2 months |
20 adults 24 seropositive children 52 seronegative children 49 unscreened infants |
Phase I study. Two doses in the youngest cohort. Safe and immunogenic | [47] |
| HPIV3cp45 | 6 months–10 years | 56 seropositive and 58 seronegative | Phase I study. Safe and immunogenic in seronegative children | [62] |
| BPIV3 | 2-<6 months 6–36 months |
19 unscreened infants 11 seronegative children |
Phase I study. Safe and immunogenic. Lower antibody titers against HPIV3 than against BPIV3 | [67] |
| BPIV3 | 2 months | 192 unscreened infants | Phase II study of four doses of vaccine or placebo. Safe and immunogenic. Lower antibody titers against HPIV3 than against BPIV3 | [68] |
| rB/HPIV3-RSV F (MEDI-534) | 1–9 years | 120 seropositive | Phase I study. Minimal shedding and immunogenicity, as expected for seropositive children | [75] |
BPIV: Bovine parainfluenza virus serotype 3; HPIV3cp45: Cold-passaged; cpts: Cold-passaged and temperature sensitive; HPIV3: Human parainfluenza virus serotype 3; MEDI: Medlmmune, LLC; RSV: Respiratory syncytial virus.
Bovine PIV3 & bovine/human HPIV3
Bovine PIV3 (BPIV3) and HPIV3 are closely related viruses that have evolved separately in their respective hosts. The two viruses are approximately 25% related antigenically, as determined by reciprocal cross-neutralization [66]. Compared with HPIV3, BPIV3 replication is restricted 100- to 1000-fold in the respiratory tract in rhesus monkeys [66]. BPIV3 was evaluated as a live-attenuated vaccine against HPIV3, a strategy similar to the use of cowpox as a vaccine against smallpox. In Phase I trials in seronegative infants and children, BPIV3 was found to be highly infectious, safe and immunogenic [67]. However, owing to antigenic differences between the BPIV3 and HPIV3 glycoproteins, the geometric mean hemagglutination-inhibiting (HAI) antibody titer in BPIV3 vaccinees was lower against HPIV3 than against BPIV3 and the seroconversion fate against HPIV3 was only 62% [67]. A Phase II trial of BPIV3 was conducted in 1922-month-old infants with four doses of 105 TCID50 or 106 TCID50 administered at 2, 4, 6 and 12–15 months of age [68]. With the exception of fever following dose two of the vaccine, the frequency of adverse events was equivalent in vaccinees and in placebo recipients. As in the Phase I study, seroconversion rates were satisfactory against BPIV3 but only modest against HPIV3 [68]. To improve immunogenicity against HPIV3, two independent but similar versions of a cDNA-derived chimeric bovine/human PIV3 virus (rB/HPIV3) were constructed by reverse genetics, one by NIAID and one by MedImmune. These chimeras were engineered to contain the WTHPIV3 HN and F genes in place of the respective BPIV3 genes [69,70]. In rhesus monkeys, rB/HPIV3 was as attenuated as BPIV3 but was more immunogenic against HPIV3 [69]. As expected for a live-attenuated vaccine, rB/HPIV3 was poorly infectious and highly restricted in replication in adults and in seropositive children. Single-dose studies in 6–36 month-old seronegative children are currently ongoing (ClinicalTrials.gov identifier: NCT00366782) [101] and preliminary data suggest that rB/HPIV3 is as attenuated and as infectious as rHPIV3cp45. Analysis of safety and immunogenicity data is pending.
Combined HPIV3 & RSV vaccines
The aforementioned chimeric rB/HPIV3 viruses were modified to express either the RSV F protein alone [71,72] or both the G and F proteins [73,74] from additional genes inserted into the B/HPIV3 genome. Each of these constructs is a bivalent vaccine virus expressing the major protective antigens of both RSV and HPIV3. These constructs were developed by MedImmune and NIAID, respectively, The MedImmune construct expressing the RSV F protein (Table 2), referred to as MedImmune, LLC (MEDI)-534, was well-tolerated in 1–9 year old seropositive children [75]. In 6–23 month-old children seronegative for both RSV and HPIV3, 67 and 100% of subjects who received 106 TCID50 ofMEDI-534 seroconvetted to RSV and HPIV3, respectively [76]. The NIAID construct expressing both RSV F and G has not yet entered clinical trials.
Table 2.
Recent and ongoing clinical trials of human parainfluenza virus vaccines.
| Target | Vaccine | Clincaltrials.gov identifier | Sponsor | Patients (n) | Current serostatus enrolling | Current age group (months) | Doses, n (interval) |
|---|---|---|---|---|---|---|---|
| HPIV3 | rHPIV3cp45 | NCT00308412 | NIAID | 24 | Unscreened | 6–12 | 2 (1–2 months) |
| rHPIV3cp45 | NCT00308412 | NIAID | 21 | Seronegative | 6–36 | 2 (1–2 months) | |
| rHPIV3cp45 |
NCT01021397 NCT01254175 |
NIAID | 30 | Seronegative | 6–36 | 2 (6 months) | |
| MEDI-560 (rHPIV3cp45) | NCT01150799 | MedImmune | 30 | Unscreened | 1–12 | 3 (2 months) | |
| rB/HPIV3 | NCT00366782 | NIAID | 21 | Seronegative | 6–36 | 1 | |
| MEDI-534 (rB/HPIV3-RSVF | NCT00686075 | MedImmune | 80 | Seronegative | 6-<24 | 3 (2 months) | |
| MEDI-534 (rB/HPIV3-RSVF) | NCT00493285 | MedImmune | 80 | Seronegative | 6-<24 | 3 (2 months) | |
| HPIV1 | CR84G/Δ170HNT553ALY942A | NCT00641017 | NIAID | 21 | Seronegative | 6–36 | 1 |
| Sendai virus | NCT00186927 | St Jude | 18 | Seropositive | 12–59 | 1 | |
| HPIV2 | 15C/948L/Δ1724 | NCT01139437 | NIAID | 21 | Seropositive | 15–59 | 1 |
cp: Cold-passage; HPIV: Human parainfluenza virus serotype; MEDI: MedImmune, LLC; NIAID: National Institute of Allergy and Infectious Diseases; rB/HPIV3: Recombinant Bovine/Human PIV3; rHPIV: Recombinant HPIV.
HPIV1 vaccines
The NIAID has explored two approaches to developing a live-attenuated HPIV1 vaccine. Initially, rHPIV3cp45 was used as a vector for the HPIV1 F and HN antigens [77–79]. Replacement of the F and HN glycoproteins of rHPIV3cp45 with those of HPIV1 yielded a virus that was attenuated in hamsters and offered protection against challenge with WT HPIV1 at 1 month post-vaccination [78,79]. However, the chimeric virus was less immunogenic in HPIV3-immune animals. probably owing to restriction of replication mediated by cellular immunity against the internal HPIV3 proteins. Since infants would probably receive an HPIV3 vaccine prior to HPIV1 vaccination, an HPIV3-based HPIV1 vaccine was deemed suboptimal and this approach was abandoned. The current approach relies on a reverse genetics system for HPIV1 [80] that was used to import attenuating mutations from related viruses (HPIV3, BPIV3 and RSV) into homologous sites of the HPIV1 genome [81–84]. Mutations introduced into the P/C and L genes in several combinations yielded attenuated HPIV1 mutants. Single amino acid substitutions in the C proteins, such as the phenylalanine-to-serine substitution at amino acid residue 170 (F170S), were found to restrict HPIV1 replication of the respiratory tract of hamsters and of African green monkeys (AGMs) [82,84,81]. The F170S mutation in the C protein was subsequently stabilized by deletion of codons 169 and 170 (rHPIV1-CΔ170) and this mutant was found to be as attenuated as rHPIV1-CF170S in AGMs [84,85]. Another rHPIVl mutant with one substitution each in C and HN, rHPIVI-CR84GHNT553A, was also attenuated in AGMs. In addition, an attenuating substitution in the L protein, Y942A, was developed using a codon sequence chosen for stability against reversion. The live-attenuated HPIV1 vaccine that is currently being developed in clinical trials contains the three sets of attenuating elements previously mentioned and is called rHPIV1-CR84G/Δ170HNT553ALY942A [83,86]. This vaccine candidate conferred protection against HPIV1 challenge in AGMs and is highly attenuated in adults and seropositive children [86]. A study in seronegative children is in progress (ClinicalTrials.gov identifier: NCT00641017) [101].
Similar to the use of BPIV3 as a vaccine against HPIV3, murine PIV1 or Sendai virus (SeV), is being evaluated as a live-attenuated xenotropic vaccine against HPIV1. Intranasal administration of SeV was well-toletated in a Phase I study in healthy adults [87] and an open-label Phase I dose-escalation study in children 1 to <6 years of age is currently ongoing (ClinicalTrials.gov identifier: NCT00186927) [101].
Human parainfluenza virus serotype 1 has also been evaluated as a vector for RSV and HMPV glycoproteins [88]. Interestingly, immunization of hamsters with an attenuated RSV vaccine virus, followed by a boost with HPIV1 expressing the RSV F protein, was substantially more immunogenic than two doses of the attenuated RSV strain [Peter Collins; Unpublished Data]. This is probably because replication of the HPIV1 vector – and the immunogenicity of its expressed RSV F insert – would nor be restricted by prior immunization against RSV. Thus, a live-attenuated HPIV1 or HPIV2 vaccine in which the components are engineered to express the RSV F and G proteins might provide a more potent boost against RSV than a second dose of a live-attenuated RSV vaccine.
HPIV2 vaccines
The development of a live-attenuated HPIV2 vaccine is based on a cDNA-derived full-length HPIV2 [89,90]. In preclinical studies, the HPIV2 L protein was identified as a major target for mutagenesis to develop attenuated mutant HPIVs [82,91,92]. Several HPIV2 mutants were created by importing known arrenuating mutations from other paramyxoviruses into homologous sites in the L protein of HPIV2 [61,93–99]. This strategy effectively identified mutations at three sites in L – at amino acid positions 460, 948 and 1724 – that generated rHPIV2 mutants that were temperature sensitive in vitro and attenuated in vivo [99,100]. At these three amino acid positions, alternative codons that would require two or three nucleotide changes to revert to the WT amino acid assignment, as well as codon deletions, were employed to increase the genetic stability of these mutations [99]. In addition to the mutations in the L protein, a spontaneous T to C nucleotide substitution within the 3' extragenic leader region of the genome was found to attenuate HPIV2 [100]. An HPIV2 vaccine candidate called rHPIV2–15C/948L/Δ1724 was developed using this 15C leader mutation combined with an amino acid substitution (948L) and deletion (Δ1724) in the L protein. This vitus is highly attenuated in AGMs and provides significant protection against WT HPIV2 challenge [100]. In addition, the vaccine virus was found to be as attenuated in an in vitro model of primary human airway epithelium as in AGMs, suggesting that the in vitro model is useful for the identification of appropriately attenuated vaccines [32]. The vaccine was also highly attenuated in unscreened adult volunteers and is currently being evaluated in seropositive children (ClinicalTrials.gov identifier: NCT01139437) [100]. Additional HPIV2 mutants have been tested preclinically and are available for clinical development should the current investigational vaccine be unsatisfactorily attenuated. Specifically, viruses with mutations in the V protein that abrogate its inhibition of the type I IFN response and therefore induce a strong antiviral IFN response, were highly attenuated in AGMs [Collins P; Unipublished Data]. Some of these mucants might deserve evaluation in clinical trials.
Expert commentary & five-year view
The need for pediatric vaccines that protect against the HPIVs and RSV has long been recognized but progress toward such vaccines has been slow. This was partly owing to the negative consequences of early RSV and HPIV vaccine trials using FI vaccines, resulting in a lack of enthusiasm to test new vaccines, in academia, government and in industry. NIAID and partners in industry have pursued the development of live-attenuated vaccines but struggled to identify vaccine viruses that were immunogenic yet sufficiently attenuated for seronegative infants. Both reactogenicity and immunogenicity correlate with the magnitude of vaccine virus replication in the respiratory tract and the ‘therapeutic window’ for a suitably attenuated yet immunogenic vaccine virus seems quite narrow. However, the availability of reverse genetics systems has allowed a deliberate design of vaccine viruses from cDNA (instead of random mucation during in vitro passage) and yielded several promising live-attenuated vaccines that are now in Phase I/II clinical development. Of the three HPIV serotypes targeted. HPIV3 vaccine development is furthesr along and proof-of-concept trials are planned for two of the investigational vaccines, rHPIV3cp45 (or MEDI-560) and B/HPIV3–RSV–F (or MEDI-534). The target group for HPIV3 and RSV vaccines is ideally 1–2-month-old infants whereas HPIV1 and HPIV2 vaccines could be given at 6 momhs of age or even later. All of the live-attenuated HPIV (and RSV) vaccines will probably need to be given two or three times and sterilizing immunity cannot be expected. Rather, the goal is to protect against ALRI requiring medical anemion and that goal should be within reach. Apart from St Jude Children Research Hospital's HPIV1 vaccine trials, the NIAID/MedImmune program is currently the only acdve program evaluating HPIV vaccines in children. However, the imerest in HPIV and RSV vaccines seems to be on the rise in industry. Several companies are now pursuing RSV vaccine developmem and several invesrigational vaccines are expected to enter clinical trials within the next few years. This field will certainly benefit from new ideas, and new approaches and competition might encourage a commitmem to develop these vaccines through Phase III to marketing approval. The children most in need, many of them in resource-poor countries and/or without access to supportive care. would certainly deserve them. Although PIV and RSV vaccines will not be on the market within the next 5 years, the hope is to have more than one investigational vaccine in Phase III trials.
Key issues.
The human parainfluenza viruses (HPIVs) are a common cause of acute upper and lower respiratory illness in infants, young children, the elderly and the immunocompromised.
HPIV3 is similar to respiratory syncytial virus (RSV) in that it is a common cause of lower respiratory illness, such as bronchiolitiS, in the first year of life.
HPIV1 and HPIV2 tend to infect later than HPIV3 and are a common cause of croup.
HPIV1 and HPIV2 vaccines expressing RSV antigens could be used to boost the immune response against RSV.
Reverse genetics systems (cDNAs) for HPIVs have helped to identify attenuating mutations and to incrementally attenuate HPIVs for use as intranasal vaccines.
HPIV3 vaccines have passed Phase 1 evaluation and need to be tested in proof-of-concept trials.
Attenuated HPIV1 and HPIV2 vaccines have entered pediatric trials in Phase I.
Acknowledgements
Many of the studies reviewed here were supported by the Intramural Research Program of the National Institutes of Allergy and Infectious Diseases (NIAID), National Institutes of Health. NIAID-sponsored clinical trials were conducted as part of research contracts between NIAID and the Johns Hopkins Bloomberg School of Public Health (JHSPH). We would like to thank all of the clinical research teams for their excellent work. Since 2006, NIAID and MedImmune have a collaborative research and development agreement for the development of RSV, HPIV and HMPV vaccines in place. We are grateful to be part of this collaboration. Brian Murphy led the PIV vaccine development program unti1 2010 and we appreciate his leadership and support. Sonja Surman was instrumental in generating vaccine seed viruses and Mario Skiadopoulos oversaw the preclinical research and development of a many PIV vaccine candidates. Finally, we would like to thank the many individuals and families who participated in the investigational vaccine studies reviewed here.
Footnotes
Financial & competing interests disclosure
Alexander C Schmidt, Emmalene J Bartlett and Peter L Collins are inventors on PIV patents and patent applications. NIAID and MedImmune have a cooperative research and development agreement in place for the delvelopment of RSV, PIV and HMPV vaccines. NIAID receives royaltits (not related to P/V vaccints) from MedImmune. Alexander C Schmidt is an Associate Scientist at NIAID and the Project Officer responsible for translational and clinical PIV vaccine development. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter materials discussed in the manuscript apart from thou disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Black RE, Cousens S, Johnson HL, et al. Global, regional and national causes of child monality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Watt JP, Wolfson LJ, O'Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374(9693):903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 4.Mullins JA, Erdman DO, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg. Infect. Dis. 2004;10(4):700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy BR, Prince GA, Collins PL, et al. Current approaches to the development of vaccines effective against parainfluenza and respiratory syncytial viruses. Virus Res. 1988;11(l):1–15. doi: 10.1016/0168-1702(88)90063-9. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J. Pediatr. 2009;154(5):694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Weinberg GA, Iwane MK, et al. The butden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009;360(6):588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poehling KA, Talbot HK, Williams JV, et al. Impact of a school-based influenza immunization program on disease burden: comparison of two Tennessee counties. Vaccine. 2009;27(20):2695–2700. doi: 10.1016/j.vaccine.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forster J, Ihorst G, Rieger CH, et al. Prospective population-based study of viral lower respiratory tract infections in children under 3 years of age (the PRI.DE study). Eur. J. Pediatr. 2004;163(12):709–716. doi: 10.1007/s00431-004-1523-9. [DOI] [PubMed] [Google Scholar]
- 11.Marx A, Torok TJ, Holman RC, Clarke MJ, Anderson LJ. Pediatric hospitalizations for croup (Iaryngotracheobronchitis): biennial increases associated with human parainfluenza virus 1 epidemics. J. Infect. Dis. 1997;176:1423–1427. doi: 10.1086/514137. [DOI] [PubMed] [Google Scholar]
- 12.Bjornson CL, Johnson DW. Croup. Lancet. 2008;371(9609):329–339. doi: 10.1016/S0140-6736(08)60170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosychuk RJ, Klassen TP, Metes D, Voaklander DC, Senthilsdvan A, Rowe BH. Croup presentations to emergency departments in Alberta, Canada: a large population-based study. Pediatr. Pulmonol. 2010;45(1):83–91. doi: 10.1002/ppul.21162. [DOI] [PubMed] [Google Scholar]
- 14.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990-2004. Clin. Infect. Dis. 2006;43(8):1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 15.Glezen WP, Frank AL, Taber LH, Kasel JA. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J. Infect. Dis. 1984;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- 16.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 17.Wendt CH, Weisdorf DJ, Jordan MC, Balfour HH, Jr, Hertz MI. Parainfluenza virus respiratory infection after bone marrow transplantation. N. Engl. J. Med. 1992;326(14):921–926. doi: 10.1056/NEJM199204023261404. [DOI] [PubMed] [Google Scholar]
- 18.Maziarz RT, Sridharan P, Slater S, et al. Control of an outbreak of human parainfluenza virus 3 in hematopoietic stem cell transplant recipients. Biol. Blood Marrow Transpl. 2010;16(2):192–198. doi: 10.1016/j.bbmt.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piralla A, Percivalle E, Di Cesare-Merlone A, Locatelli F, Gerna G. Multicluster nosocomial outbreak of parainfluenza virus type 3 infeerion in a pediatric oncohematology unit: a phylogenetic study. Haematologica. 2009;94(6):833–839. doi: 10.3324/haematol.2008.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols WG, Erdman DD, Han A, Zukerman C, Corey L, Boeckh M. Prolonged outbreak of human parainfluenza virus 3 infection in a stem cell transplant outpatient department: insights from molecular epidemiologic analysis. Biol. Blood Marrow Transpl. 2004;10(1):58–64. doi: 10.1016/j.bbmt.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Jalal H, Bibby DF, Bennett J, et al. Molecular investigations of an outbreak of parainfluenza virus type 3 and respiratory syncytial virus infections in a hematology unit. J. Clin. Microbiol. 2007;45(6):1690–1696. doi: 10.1128/JCM.01912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell AP, Chien JW, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation (HCT): associations between viral load in bronchoalveolar lavage samples, viral RNA detection in serum samples and clinical outcomes of HCT. J. Infect. Dis. 2010;201(9):1404–1413. doi: 10.1086/651662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303(20):2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J. Infect. Dis. 1997;175(4):807–813. doi: 10.1086/513975. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki T, Portner A, Scroggs RA, et al. Receptor specificities of human respiroviruses. J. Virol. 2001;75(10):4604–4613. doi: 10.1128/JVI.75.10.4604-4613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Bukreyev A, Thompson CI, et al. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 2005;79(2):1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karron RA, Collins PL. Parainfluenza viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilbns; PA, USA: 2007. pp. 1497–1526. [Google Scholar]
- 28.Murphy BR. Current approaches to the development of vaccines effective against parainfluenza viruses. Bull. World Health Organ. 1988;66(3):391–397. [PMC free article] [PubMed] [Google Scholar]
- 29.Bartlett EJ, Cruz AM, Boonyaratanakornkit J, et al. A novel human parainfluenza virus type 1 (HPIVl) with separated P and C genes is useful for generating C gene mutants for evaluation as live-attenuated virus vaccine candidates. Vaccine. 2010;28(3):767–779. doi: 10.1016/j.vaccine.2009.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boonyaratanakornkit J, Bartlett E, Schomacker H, et al. The C proteins of human parainfluenza virus type 1 limit double stranded RNA accumulation that would otherwise trigger MDA5 and PKR activation. J. Virol. 2011;85(4):1495–1506. doi: 10.1128/JVI.01297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boonyaratanakornkit JB, Bartlett EJ, Amaro-Carambot E, Collins PL, Murphy BR, Schmidt AC. The C proteins of human parainfluenza virus type 1 (HPIV1) control the transcription of a broad array of cellular genes that would otherwise respond to HPIV1 infection. J. Virol. 2009;83(4):1892–1910. doi: 10.1128/JVI.01373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaap-Nutt A, Scull MA, Schmidt AC, Murphy BR, Pickles RJ. Growth restriction of an experimental live attenuated human parainfluenza virus type 2 vaccine in human ciliated airway epithelium in vitro parallels attenuation in African green monkeys. Vaccine. 2010;28(15):2788–2798. doi: 10.1016/j.vaccine.2010.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson J, Rowlands K, Rockett K, et al. Genetic variation at the IL10 gene locus is associated with severity of respiratory syncytial virus bronchiolitis. J. Infect. Dis. 2005;191(10):1705–1709. doi: 10.1086/429636. [DOI] [PubMed] [Google Scholar]
- 34.Crowe JE, Jr, Williams JV. Immunology of viral respiratory tract infection in infancy. Paediatr. Respir. Rev. 2003;4(2):112–119. doi: 10.1016/s1526-0542(03)00033-2. [DOI] [PubMed] [Google Scholar]
- 35.Bartlett EJ, Hennessey M, Skiadopoulos MH, et al. The role of interferon in the replication of human parainfluenza virus type 1 wild type and mutant viruses in human ciliated airway epithelium. J. Virol. 2008 doi: 10.1128/JVI.02263-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gitlin L, Benoit L, Song C, et al. Melanoma differentiation-associated gene 5 (MDA5) is involved in the innate immune response to Paramyxoviridae infection in vivo. PLoS Pathog. 2010;6(1):e1000734. doi: 10.1371/journal.ppat.1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J. Immunol. 1992;149(4):1319–1325. [PubMed] [Google Scholar]
- 38.Spriggs MK, Murphy BR, Prince GA, Olmsted RA, Collins PL. Expression of the F and HN glycoproteins of human parainfluenza virus type 3 by recombinant vaccinia viruses: contributions of the individual proteins to host immunity. J. Virol. 1987;61(11):3416–3423. doi: 10.1128/jvi.61.11.3416-3423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall CB, Douglas RG, Jr, Simons RL, Geiman JM. Interferon production in children with respiratory syncytial, influenza and parainfluenza virus infections. J. Pediatr. 1978;93(1):28–32. doi: 10.1016/s0022-3476(78)80594-0. [DOI] [PubMed] [Google Scholar]
- 40.Hou S, Doheny PC. Clearance of Sendai virus by CD8+ T cells requires direct targeting to virus-infected epithelium. Eur. J. Immunol. 1995;25(1):111–116. doi: 10.1002/eji.1830250120. [DOI] [PubMed] [Google Scholar]
- 41.Zhong W, Marshall D, Coledough C, Woodland DL. CD4+ T cell priming accelerates the clearance of Sendai virus in mice, but has a negative effect on CD8+ T cell memory. J. Immunol. 2000;164(6):3274–3282. doi: 10.4049/jimmunol.164.6.3274. [DOI] [PubMed] [Google Scholar]
- 42.Takamura S, Roberts AD, Jelley-Gibbs DM, Wiumer ST, Kohlmeier JE, Woodland DL. The route of priming influences the ability of respiratory virus-specific memory CD8+ T cells to be activated by residual antigen. J. Exp. Med. 2010;207(6):1153–1160. doi: 10.1084/jem.20090283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith CB, Purcell RH, Bellanti JA, Chanock RM. Protective effect of antibody to parainfluenza type 1 virus. N. Engl. J. Med. 1966;275(21):1145–1152. doi: 10.1056/NEJM196611242752101. [DOI] [PubMed] [Google Scholar]
- 44.Tremonti LP, Lin JS, Jackson GG. Neutralizing activity in nasal secretions and serum in resistance of volunteers to parainfluenza virus type 2. J. Immunol. 1968;101(3):572–577. [PubMed] [Google Scholar]
- 45.Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska native children. RSV Alaska Study Group. J. lnfect. Dis. 1999;180(1):41–49. doi: 10.1086/314841. [DOI] [PubMed] [Google Scholar]
- 46.Malley R, DeVincenzo J, Ramilo O, et al. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J. Infect. Dis. 1998;178(6):1555–1561. doi: 10.1086/314523. [DOI] [PubMed] [Google Scholar]
- 47.Karran RA, Belshe RB, Wright PF, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatr. Infect. Dis. J. 2003;22(5):394–405. doi: 10.1097/01.inf.0000066244.31769.83. [DOI] [PubMed] [Google Scholar]
- 48.Shinoff JJ, O'Brien KL, Thumar B, et al. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J. Infect. Dis. 2008;198(7):1007–1015. doi: 10.1086/591460. [DOI] [PubMed] [Google Scholar]
- 49.Crowe JE., Jr Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Inftcl. Dis. 2001;33(10):1720–1727. doi: 10.1086/322971. [DOI] [PubMed] [Google Scholar]
- 50.Smith CB, Bellanti JA, Chanock RM. Immunoglobulins in serum and nasal secretions following infection with type 1 parainfluenza virus and injection of inactivated vaccines. J. lmmunol. 1967;99(1):133–141. [PubMed] [Google Scholar]
- 51.Wright PF, Karron RA, Belshe RB, et al. The absence of enhanced disease with wild type respiratory syncytial virus infection occurring after receipt of live, attenuated, respiratory syncytial virus vaccines. Vaccine. 2007;25(42):7372–7378. doi: 10.1016/j.vaccine.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karron RA, Wright PF, Selshe RB, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J. Infect. Dis. 2005;191(7):1093–1104. doi: 10.1086/427813. [DOI] [PubMed] [Google Scholar]
- 53.Wright PF, Karron RA, Belshe RB, et al. Evaluation of a live, cold-passaged, temperature-sensitive, respiratory syncytial virus vaccine candidate in infancy. J. Infect. Dis. 2000;182(5):1331–1342. doi: 10.1086/315859. [DOI] [PubMed] [Google Scholar]
- 54.Zemlin M, Hoersch G, Zemlin C, et al. The postnatal maturation of the immunoglobulin heavy chain IgG repertoire in human preterm neonates is slower than in term neonates. J. Immunol. 2007;178(2):1180–1188. doi: 10.4049/jimmunol.178.2.1180. [DOI] [PubMed] [Google Scholar]
- 55.Delgado MF, Coviello S, Monsalvo AC, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15(1):34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kallewaard NL, McKinney BA, Gu Y, Chen A, Prasad BV, Crowe JE., Jr Functional mawration of the human antibody response to rotavirus. J. Immunol. 2008;180(6):3980–3989. doi: 10.4049/jimmunol.180.6.3980. [DOI] [PubMed] [Google Scholar]
- 57.Murphy BR, Olmsted RA, Collins PL, Chanock RM, Prince GA. Passive transfer of respiratory syncytial virus (RSV) antiserum suppresses the immune response to the RSV fusion (F) and large (G) glycoproteins expressed by recombinant vaccinia viruses. J. Virol. 1988;62(10):3907–3910. doi: 10.1128/jvi.62.10.3907-3910.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Premenko-Lanier M, Hodge G, Rota P, Tamin A, Bellini W, McChesney M. Maternal antibody inhibits both cellular and humoral immunity in response to measles vaccination at birth. Virology. 2006;350(2):429–432. doi: 10.1016/j.virol.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 59.Belshe RB, Hissom FK. Cold adaptation of parainfluenza virus type 3: induction of three phenotypic markers. J. Med. Virol. 1982;10(4):235–242. doi: 10.1002/jmv.1890100403. [DOI] [PubMed] [Google Scholar]
- 60.Durbin AP, Hall SL, Siew JW, Whitehead SS, Collins PL, Murphy BR. Recovery of infectious human parainfluenza virus type 3 from eDNA. Virology. 1997;235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- 61.Skiadopoulos MH, Surman S, Tatem JM, et al. Identification of mutations contributing to the temperature-sensitive, cold-adapted and attenuation phenotypes of the live-attenuated cold-passage 45 (cp45) human parainfluenza virus 3 candidate vaccine. J. Virol. 1999;73(2):1374–1381. doi: 10.1128/jvi.73.2.1374-1381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karron RA, Wright PF, Newman FK, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. J. Infect. Dis. 1995;172(6):1445–1450. doi: 10.1093/infdis/172.6.1445. [DOI] [PubMed] [Google Scholar]
- 63.Belshe RB, Newman FK, Tsai TF, et al. Phase II evaluation of parainfluenza type 3 cold passage mutant 45 live anenuated vaccine in healthy children 6–18 months old. J. Infect. Dis. 2004;189(3):462–470. doi: 10.1086/381184. [DOI] [PubMed] [Google Scholar]
- 64.Belshe RB, Newman FK, Anderson EL, et al. Evaluation of combined live, attenuated respiratory syncytial virus and parainfluenza 3 virus vaccines in infants and young children. J. Infect. Dis. 2004;190(12):2096–2103. doi: 10.1086/425981. [DOI] [PubMed] [Google Scholar]
- 65.Bernstein D, Charenkavanich S, Falloon J. International RSV Symposium. Vol. 147. GCO BV; Rotterdam, The Netherlands: 2010. Safety profile, immunogenicity and viral shedding of rHPIV3cp45, a live, attenuated human parainfluezavirus type 3 (PIV3) vaccine. [Google Scholar]
- 66.van Wyke Coelingh KL, Winter CC, Tierney EL, London WT, Murphy BR. Attenuation of bovine parainfluenza virus type 3 in nonhuman primates and its ability to confer immunity to human parainfluenza virus type 3 challenge. J. Infect. Dis. 1988;157(4):655–662. doi: 10.1093/infdis/157.4.655. [DOI] [PubMed] [Google Scholar]
- 67.Karron RA, Makhene M, Gay K, Wilson MH, Clements ML, Murphy SR. Evaluation of a live attenuated bovine parainfluenza type 3 vaccine in two- to six-month-old infants. Pediatr. Infect. Dis. J. 1996;15(8):650–654. doi: 10.1097/00006454-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Greenberg DP, Walker RE, Lee MS, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. J. Infect. Dis. 2005;191(7):1116–1122. doi: 10.1086/428092. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt AC, McAuliffe JM, Huang A, et al. Bovine parainfluenza virus type 3 (BPIV3) fusion and hemagglutinin-neuraminidase glycoproteins make an important contribution to the restricted replication of BPTV3 in primates. J. Virol. 2000;74(19):8922–8929. doi: 10.1128/jvi.74.19.8922-8929.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haller AA, Miller T, Miriku M, Coelingh K. Expression of the surface glycoproteins of human parainfluenza virus type 3 by bovine parainfluenza virus type 3, a novel attenuated virus vaccine vector. J. Virol. 2000;74(24):11626–11635. doi: 10.1128/jvi.74.24.11626-11635.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang RS, MacPhail M, Schickli JH, et al. Parainfluenza virus type 3 expressing the native or soluble fusion (F) protein of respiratory syncytial virus (RSV) confers protection from RSV infeerion in African green monkeys. J. Virol. 2004;78(20):11198–11207. doi: 10.1128/JVI.78.20.11198-11207.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang RS, Spaete RR, Thompson MW, et al. Development of a PIY-vectored RSV vaccine: preclinical evaluation of safety, toxicity and enhanced disease and initial clinical testing in healthy adults. Vaccine. 2008;26(50):6373–6382. doi: 10.1016/j.vaccine.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Schmidt AC, McAuliffe JM, Murphy BR, Collins PL. Recombinant bovine/human parainfluenza virus type 3 (B/HPIV3) expressing the respiratory syncytial virus (RSV) G and F proteins can be used to achieve simultaneous mucosal immunization against RSV and HPIV3. J. Virol. 2001;75(10):4594–4603. doi: 10.1128/JVI.75.10.4594-4603.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmidt AC, Wenzke DR, McAuliffe JM, et al. Mucosal immunization of rhesus monkeys against respiratory syncytial virus subgroups A and B and human parainfluenza virus type 3 by using a live cDNA-derived vaccine based on a host range-attenuated bovine parainfluenza virus type 3 vector backbone. J. Virol. 2002;76(3):1089–1099. doi: 10.1128/JVI.76.3.1089-1099.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase-I study MEDI-534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza-3 virus in seropositive children. Pediatr. Infect. Dis. J. 2009;28(7):655–658. doi: 10.1097/INF.0b013e318199c3b1. [DOI] [PubMed] [Google Scholar]
- 76.Bernstein D, Malkin E, Charenkavanich S, Dubovsky F. Safety profile, immunogenicity and viral shedding of MEDI-534, a live, attenuated respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccine in RSV/PIV3 seronegative children.. Presented at: International RSV Symposium.; GCO BV, Rotterdam, The Netherlands. 2-5 December 2010. [Google Scholar]
- 77.Tao T, Durbin AP, Whitehead SS, Davoodi F, Collins PL, Murphy BR. Recovery of a fully viable chimeric human parainfluenza virus (PIV) type 3 in which the hemagglutinin-neuraminidase and fusion glycoproteins have been replaced by those of PIV type 1. J Virol. 1998;72(4):2955–2961. doi: 10.1128/jvi.72.4.2955-2961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skiadopoulos MH, Tao T, Surman SR, Collins PL, Murphy BR. Generation of a parainfluenza virus type 1 vaccine candidate by replacing the HN and F glycoproteins of the live-attenuated PIV3 cp45 vaccine virus with their PIV1 counterparts. Vaccine. 1999;18(5-6):503–510. doi: 10.1016/s0264-410x(99)00227-3. [DOI] [PubMed] [Google Scholar]
- 79.Skiadopoulos MH, Tatem JM, Surman SR, et al. The recombinant chimeric human parainfluenza virus type 1 vaccine candidate, rHPIV3–1cp45, is attenuated, immunogenic and protective in African green monkeys. Vaccine. 2002;20(13):1846–1852. doi: 10.1016/s0264-410x(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 80.Newman JT, Surman SR, Riggs JM, et al. Sequence analysis of the Washington/1964 strain of human parainfluenza virus type 1 (HPIV1) and recovery and characterization of wild-type recombinant HPIV1 produced by reverse genetics. Virus Genes. 2002;24(1):77–92. doi: 10.1023/a:1014042221888. [DOI] [PubMed] [Google Scholar]
- 81.McAuliffe JM, Surman SR, Newman JT, et al. Codon substitution mutations at two positions in the L polymerase protein of human parainfluenza virus type 1 yield viruses with a spectrum of attenuation in vivo and increased phenotypic stability in vitro. J. Virol. 2004;78(4):2029–2036. doi: 10.1128/JVI.78.4.2029-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newman JT, Riggs JM, Surman SR, et al. Generation of recombinant human parainfluenza virus type 1 vaccine candidates by importation of temperature-sensitive and attenuating mutations from heterologous paramyxoviruses. J. Virol. 2004;78(4):2017–2028. doi: 10.1128/JVI.78.4.2017-2028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bartlett EJ, Amaro-Carambot E, Surman SR, et al. Human parainfluenza virus type 1 (HPIV1) vaccine candidates designed by reverse genetics are attenuated and efficacious in African green monkeys. Vaccine. 2005;23(38):4631–4646. doi: 10.1016/j.vaccine.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 84.Bartlett EJ, Amaro-Carambot E, Surman SR, Collins PL, Murphy BR, Skiadopoulos MH. Introducing point and deletion mutations into the P/C gene of human parainfluenza virus type 1 (HPIV1) by reverse genetics generates attenuated and efficacious vaccine candidues. Vaccine. 2006;24(14):2674–2684. doi: 10.1016/j.vaccine.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 86.Van Cleve W, Amaro-Carambot E, Surman SR, et al. Attenuating mutations in the P/C gene of human parainfluenza virus type 1 (HPIV1) vaccine candidates abrogate the inhibition of both induction and signaling of type I interferon (IFN) by wild-type HPIV1. Virology. 2006;352(1):61–73. doi: 10.1016/j.virol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Bartlett EJ, Castano A, Surman SR, Collins PL, Skiadopoulos MH, Murphy BR. Attenuation and efficacy of human parainfluenza virus type 1 (HPIV1) vaccine candidates containing stabilized mutations in the P/C and L genes. Virol. J. 2007;4(1):67. doi: 10.1186/1743-422X-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Slobod KS, Shenep JL, Lujan-Zilbermann J, et al. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine. 2004;22(23-24):3182–3186. doi: 10.1016/j.vaccine.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 88.Skiadopoulos MH, Biacchesi S, Buchholz UJ, et al. The two major human metapneumovirus generic lineages are highly related antigenically and the fusion (F) protein is a major concributor to this ancigenic relatedness. J. Virol. 2004;78(13):6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawano M, Kaito M, Kozuka Y, et al. Recovery of infectious human parainfluenza type 2 virus from cDNA clones and properties of the defective virus without V-specific cysteine-rich domain. Virology. 2001;284(1):99–112. doi: 10.1006/viro.2001.0864. [DOI] [PubMed] [Google Scholar]
- 90.Skiadopoulos MH, Vogel L, Riggs JM, Surman SR, Collins PL, Murphy BR. The genome length of human parainfluenza virus type 2 follows the rule of six and recombinant viruses recovered from non-polyhexameric-length antigenornic cDNAs contain a biased distribution of correcting mutations. J. Virol. 2003;77(1):270–279. doi: 10.1128/JVI.77.1.270-279.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collins PL, Murphy BR. Respiratory syncytial virus: reverse genetics and vaccine strategies. Virology. 2002;296(2):204–211. doi: 10.1006/viro.2002.1437. [DOI] [PubMed] [Google Scholar]
- 92.Murphy BR, Collins PL. Live-atcenuared virus vaccines for respiratory syncytial and parainfluenza viruses: applications of reverse genetics. J. Clin. Invest. 2002;110(1):21–27. doi: 10.1172/JCI16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Juhasz K, Murphy BR, Collins PL. The major attenuating mutations of the respiratory syncytial virus vaccine candidate cpts530/l009 specify temperature-sensitive defects in transcription and replication and a non-temperature-sensitive alteration in mRNA termination. J. Virol. 1999;73(6):5176–5180. doi: 10.1128/jvi.73.6.5176-5180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Juhasz K, Whitehead SS, Boulanger CA, Firestone CY, Collins PL, Murphy BR. The two amino acid substitutions in the L protein of cpts530/1009, a live-attenuated respiratory syncytial virus candidate vaccine, are independent temperature-sensitive and attenuation mutations. Vaccine. 1999;17(11-12):1416–1424. doi: 10.1016/s0264-410x(98)00381-8. [DOI] [PubMed] [Google Scholar]
- 95.Whitehead SS, Firestone CY, Karron RA, et al. Addition of a missense mutation present in the L gene of respiratory syncytial virus (RSV) cpts530/1030 to RSV vaccine candidate cpts248/404 increases its attenuation and temperature sensitivity. J. Virol. 1999;73(2):871–877. doi: 10.1128/jvi.73.2.871-877.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Skiadopoulos MH, Schmidt AC, Riggs JM, et al. Determinants of the host range restriction of replication of bovine parainfluenza virus type 3 in rhesus monkeys are polygenic. J. Virol. 2003;77(2):1141–1148. doi: 10.1128/JVI.77.2.1141-1148.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Skiadopoulos MH, Durbin AP, Tatem JM, et al. Three amino acid substitutions in the L protein of the human parainfluenza virus type 3 cp45 live attenuated vaccine candidate contribute to its temperature-sensitive and attenuation phenotypes. J. Virol. 1998;72(3):1762–1768. doi: 10.1128/jvi.72.3.1762-1768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feller JA, Smallwood S, Skiadopoulos MH, Murphy BR, Moyer SA. Comparison of identical temperarure-sensitive mutations in the L polymerase proteins of sendai and parainfluenza3 viruses. Virology. 2000;276(1):190–201. doi: 10.1006/viro.2000.0535. [DOI] [PubMed] [Google Scholar]
- 99.Nolan SM, Surman SR, Amaro-Carambot E, Collins PL, Murphy BR, Skiadopoulos MH. Live-attenuated intranasal parainfluenza virus type 2 vaccine candidates developed by reverse genetics containing L polymerase protein mutations imported from heterologous paramyxoviruses. Vaccine. 2005;39(23):4765–4774. doi: 10.1016/j.vaccine.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 100.Nolan SM, Skiadopoulos MH, Bradley K, et al. Recombinant human parainfluenza virus type 2 vaccine candidates containing a 3′ genomic promoter mutation and L polymerase mutations are attenuated and protective in non-human primates. Vaccine. 2007;25(34):6409–6422. doi: 10.1016/j.vaccine.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Madhi SA, Cutland C, Zhu Y, et al. Transmissibility, infectivity and immunogenicity of a live human parainfluenza type 3 virus vaccine (HPIV3cp45) among susceptible infants and toddlers. Vaccine. 2006;24(13):2432–2439. doi: 10.1016/j.vaccine.2005.12.002. [DOI] [PubMed] [Google Scholar]