Abstract
Determining the signalling pathways that direct tissue expansion is a principal goal of regenerative biology. Vigorous pancreatic β-cell replication in juvenile mice and humans declines with age, and elucidating the basis for this decay may reveal strategies for inducing β-cell expansion, a long-sought goal for diabetes therapy. Here we show that platelet-derived growth factor receptor (Pdgfr) signalling controls age-dependent β-cell proliferation in mouse and human pancreatic islets. With age, declining β-cell Pdgfr levels were accompanied by reductions in β-cell enhancer of zeste homologue 2 (Ezh2) levels and β-cell replication. Conditional inactivation of the Pdgfra gene in β-cells accelerated these changes, preventing mouse neonatal β-cell expansion and adult β-cell regeneration. Targeted human PDGFR-α activation in mouse β-cells stimulated Erk1/2 phosphorylation, leading to Ezh2-dependent expansion of adult β-cells. Adult human islets lack PDGF signalling competence, but exposure of juvenile human islets to PDGF-AA stimulated β-cell proliferation. The discovery of a conserved pathway controlling age-dependent β-cell proliferation indicates new strategies for β-cell expansion.
Acquired tissue insufficiency underlies the pathogenesis of diverse human diseases; for example, absolute or relative deficits in pancreatic β-cells underlie diabetes mellitus. To expand insulin-secreting β-cells, investigators have modulated intracellular factors, including β-cell cycle regulators, or activated intrinsic regulatory pathways with extrinsic mitogenic factors1,2. In mice or other experimental animals, β-cell expansion from such efforts has been transient or limited, and often complicated by uncontrolled β-cell growth, loss of defining β-cell features and impaired metabolic control3,4. Likewise, induction of human islet β-cell proliferation remains an unattained goal, with studies reporting inconsistent mitogenic responses5,6. Thus, we sought to identify native signalling pathways governing physiological β-cell proliferation and regeneration.
During physiological growth in humans, mice and other species, juvenile β-cells expand by self-renewal7,8 while maintaining their hallmark functions. Pancreatic β-cell proliferation decreases rapidly in juvenile mice and humans, then declines more slowly in adults9,10. Studies indicate that age-dependent increases of p16INK4a, a cyclin-dependent kinase inhibitor encoded by Cdkn2a, restrict proliferation of mouse and human β-cells and other tissues with ageing11,12. We showed that the polycomb group protein Ezh2 represses Cdkn2a and promotes β-cell proliferation in juvenile mouse islets13. Ezh2 is a histone methyltransferase crucial for trimethylation of histone H3 on Lys 27 (H3K27me3), a modification mediating transcriptional repression14,15. Ezh2 expression in mouse and human β-cells declines rapidly in juveniles, then slowly in adults13, similar to the tempo of age-dependent decline in β-cell proliferation9,10. Conditional Ezh2 inactivation in β-cells accelerated loss of H3K27me3 repression at Cdkn2a, resulting in premature p16INK4a expression, impaired β-cell proliferation, reduced β-cell mass and inadequate β-cell regenerative recovery after β-cell ablation13. We proposed that conserved regulatory pathways governing Ezh2 expression in ageing islets could be targeted to stimulate adult β-cell proliferation.
Pdgfr signalling promotes proliferation, survival and migration in diverse cell types16. Pdgf signalling activation stimulates DNA synthesis in cultured islets17,18, but the roles of Pdgf signalling in β-cell proliferation and expansion were unknown. Pdgf-induced actin polymerization in fibroblasts requires Ezh2 (ref. 19), suggesting that Pdgf signalling might regulate Ezh2 expression and age-dependent β-cell proliferation in islets. Here we demonstrate that a pathway regulated by Pdgfr-α, governs the expression and activity of intrinsic β-cell factors—including the retinoblastoma protein (Rb), E2f transcription factors and Ezh2—tocontrol β-cell proliferation. Modulation of β-cell Pdgf signalling attenuates age-dependent proliferative failure and Ezh2 loss, permitting in vivo expansion of β-cells with regulated function. Cardinal features of this pathway are conserved in human islets, permitting conditional induction of human β-cell replication.
Age-dependent β-cell Pdgfr-α loss
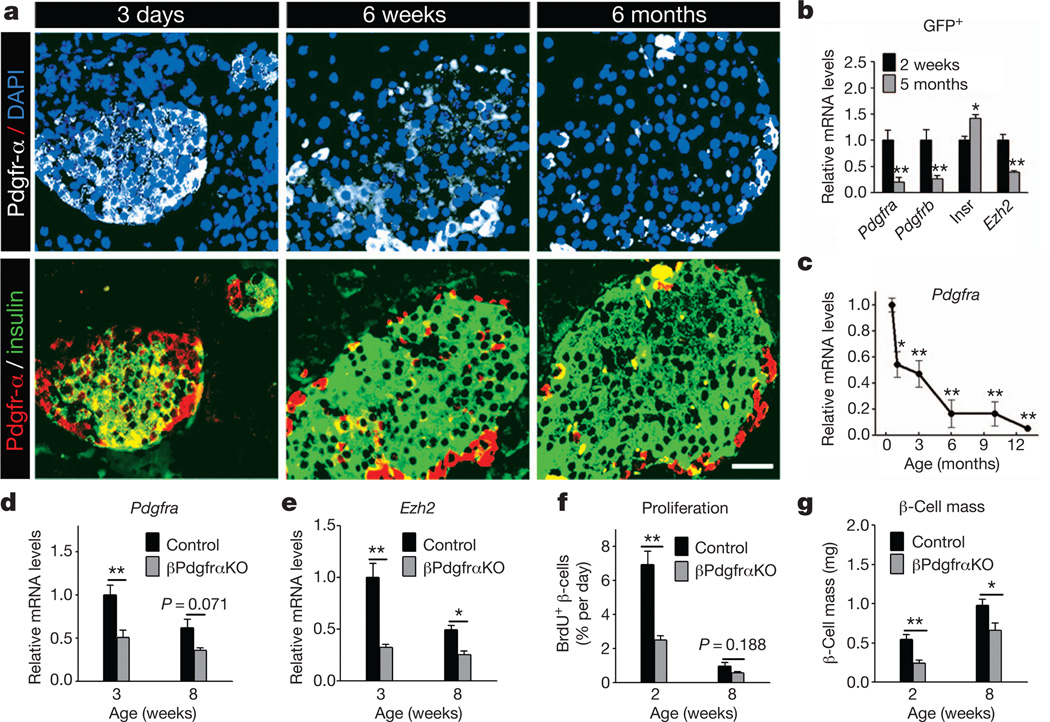
To investigate whether Pdgf signalling might regulate islet β-cell proliferation and Ezh2 expression, we assessed Pdgf receptor and ligand expression in islets and β-cells. Immunohistology revealed that Pdgfr-α and Pdgfr-β levels were markedly reduced in islet β-cells at 6 weeks and 6 months of age, compared to neonatal islets (Fig. 1a and Supplementary Fig. 1a). Real-time polymerase chain reaction with reverse transcription (RT–PCR) studies of β-cells purified by fluorescence-activated cell sorting (FACS) confirmed that Pdgfra, Pdgfrb and Ezh2 messenger RNA levels were reduced in adult β-cells at 5 months compared to 2-week-old β-cells (Fig. 1b). Further RT–PCR analyses of isolated islets from wild-type mice ranging from 2 weeks to 13 months revealed an age-dependent decline of islet mRNAs encoding Pdgfra, Pdgfrb and Pdgf ligands (Fig. 1c and Supplementary Fig. 1b–e). In contrast, islet mRNAs encoding the insulin receptor, prolactin receptor, glucocorticoid receptor and intrinsic regulators of β-cell proliferation like Pdx1, Foxo1, Foxo3, Rb1 (hereafter referred to as Rb), p130 (also known as Rbl2) and E2f1, did not decline in mice with advancing age (Supplementary Fig. 2). Thus, reduced β-cell levels of Pdgf receptors and ligands corresponded with Ezh2 loss and age-dependent reduction of β-cell replication13.
Figure 1. Age-dependent attenuation of Pdgfr-α limits β-cell Ezh2 expression and proliferation in neonatal and juvenile mice.
a, Pdgfr-α and insulin immunostaining on wild-type mouse pancreas sections at indicated ages. DAPI, 4′,6-diamidino-2-phenylindole. Scale bar, 25 µm. b, c, mRNA levels of indicated genes in FACS-purified β-cells (b) or wild-type islets (c) at specified ages. n = 3–5 experiments per group or time point. *P < 0.05, **P < 0.01 compared to samples from 2 weeks (b) or 15 days (c). Insr. refers to mRNA encoding insulin receptor. d–g, Relative mRNA levels of Pdgfra (d) and Ezh2 (e) in isolated islets, and percentage β-cell BrdU incorporation (f) and pancreatic β-cell mass (g) of βPdgfαKO and control mice at specified ages. n = 3–5 independent islet preparations or mice per group. *P < 0.05, **P < 0.01 for the comparison as indicated. Error bars denote s.e.m.
To assess whether premature Pdgfr loss might compromise Ezh2-dependent neonatal β-cell proliferation, we generated RIP-Cre; Pdgfraf/f mice (abbreviated to βPdgfrαKO) that permit conditional Pdgfra inactivation in islet β-cells and loss of detectable Pdgfra mRNA and protein expression (Fig. 1d and Supplementary Fig. 3a). Compared to controls, β-cell Ezh2 protein and islet Ezh2 mRNA levels were reduced whereas p16INK4a mRNA levels were increased in βPdgfrαKO islets (Fig. 1e and Supplementary Fig. 3a, b); in contrast, Pdgfrb and Ezh1 mRNA levels were unchanged in βPdgfrαKO islets (Supplementary Fig. 3c, d). In 2–3-week-old βPdgfαKO mice, we detected a threefold reduction in the β-cell proliferation rate and a 50% reduction in β-cell mass (Fig. 1f, g). βPdgfαKO mice at 3 weeks had mild hyperglycaemia during ad libitum feeding and impaired glucose tolerance in a glucose challenge test (Supplementary Fig. 3e, f). Thus, premature loss of Pdgfra impairs β-cell proliferation and expansion and glucose control in young mice.
Pdgfr-α controls adult β-cell regeneration
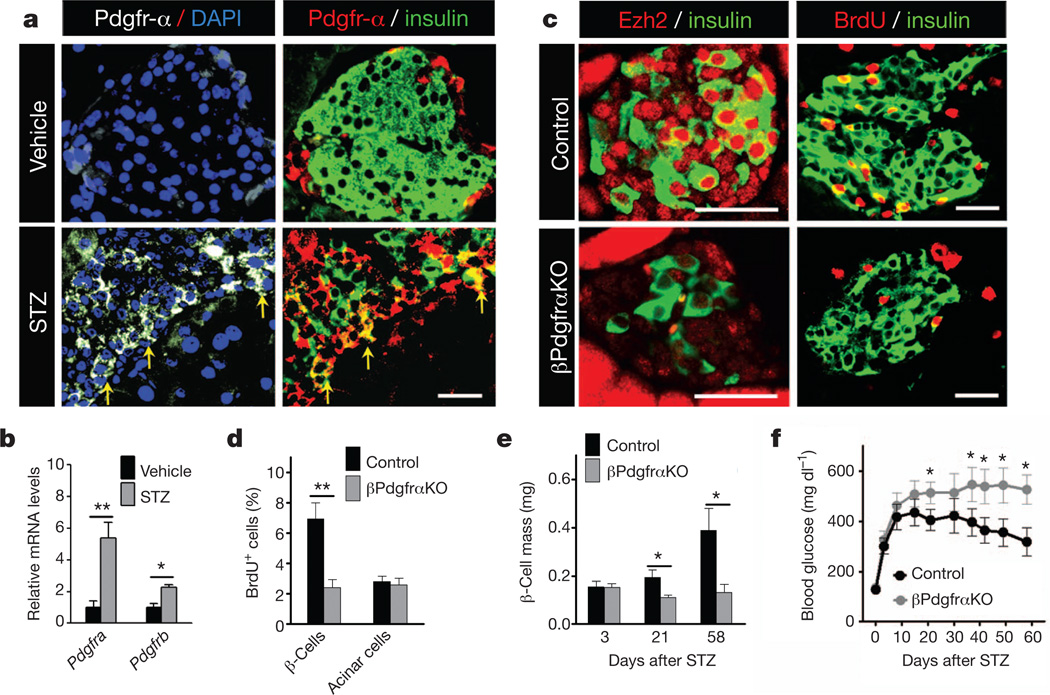
Destruction of β-cells by streptozotocin (STZ) in mice provokes β-cell proliferation with regeneration of β-cell mass11,13. Following STZ exposure, Pdgfra and Pdgfrb were induced in wild-type islets (Fig. 2a, b), suggesting possible roles for Pdgfr signalling in adult β-cell regeneration. Consistent with this view, β-cell mass in control mice gradually recovered after STZ treatment, accompanied by increased β-cell Ezh2 expression and proliferation; in contrast, βPdgfαKO β-cells failed to increase Ezh2 or proliferation to restore β-cell mass (Fig. 2c–e). βPdgfαKO mice developed severe diabetes that persisted throughout the course of our studies (Fig. 2f). In contrast, control mice became moderately diabetic immediately after STZ challenge, but achieved restored glucose control 3–4 weeks thereafter (Fig. 2f), coinciding with increases of β-cell mass (Fig. 2e). Thus, Pdgfr-α is also required in adult β-cells for compensatory proliferation in experimental diabetes.
Figure 2. Pdgfra loss impairs β-cell regeneration in STZ-induced diabetes.
a, b, Immunostaining of Pdgfr-α and insulin on pancreas sections (a), and relative mRNA levels of Pdgfra and Pdgfrb in islets (b) from 8-week-old wild-type mice 1 week after vehicle or STZ (100 mg kg −1 body weight) treatment. n = 3–5 mice per group. a, Yellow arrows mark Pdgfr-α induction in a subset of insulin+ cells. c, Representative images showing immunostaining for insulin, Ezh2 and BrdU on pancreatic sections from indicated 6–7-week-old mice 3 weeks after STZ (150 mg kg−1 body weight) treatment. d, Percentage of BrdU+ insulin+ cells (β-cells) or BrdU+ acinar cells 3 weeks after STZ challenge. n = 3 or 6 mice per group. e, f, Pancreatic β-cell mass (e) and blood glucose (f) levels during ad libitum feeding after STZ treatment. n = 3–8 mice (e) or 18–21 mice (f) per time point per group. *P < 0.05, **P < 0.01 for the comparison as indicated or versus control. Scale bars, 25 µm. Error bars denote s.e.m.
PDGFR-α promotes functional β-cell expansion
To testwhether enhanced Pdgfr signalling might promote β-cell expansion, we exposed islets from juvenile and adult mice to physiological concentrations of platelet-derived growth factor-AA (PDGF-AA). Exposure of 3-week-old islets to PDGF-AA increased phospho-Pdgfr-α levels and Ezh2 mRNA and protein levels, but not Ezh1 expression (Supplementary Fig. 4a–c). In contrast, juvenile islets exposed to mitogens like insulin or prolactin did not detectably alter Ezh2 mRNA levels (Supplementary Fig. 4d, e). Unlike in 3-week-old islets, levels of Ezh2 mRNA and protein were not significantly increased in 7- or 9-month-old adult islets exposed to PDGF-AA (Supplementary Fig. 4c, f). PDGF-AA exposure increased β-cell proliferation in juvenile islets, as assessed by BrdU incorporation, but not in adult islets (Supplementary Fig. 4g, h). Increased Ezh2 expression and β-cell BrdU incorporation were eliminated by simultaneous treatment with the receptor tyrosine kinase inhibitors Sunitinib20 or Vargatef21 (Supplementary Fig. 4a, c, g, h). Thus, PDGF-AA exposure was sufficient to stimulate β-cell replication in juvenile islets, but loss of competence for Pdgf prevented this response to PDGF-AA in adult islets.
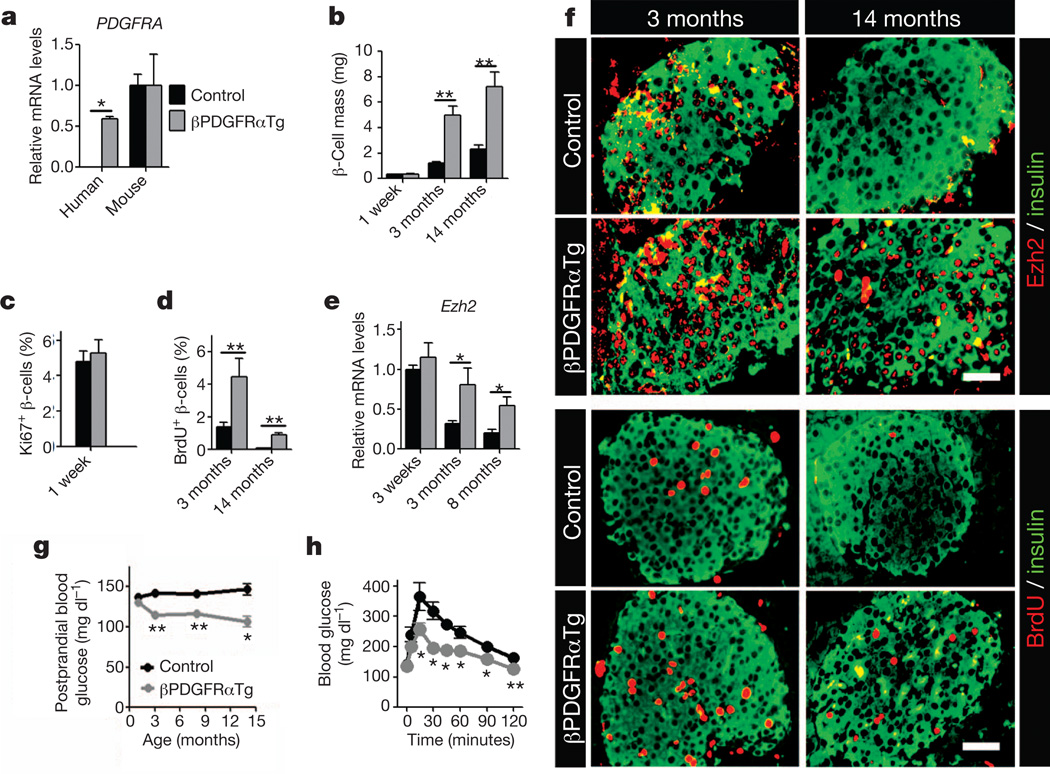
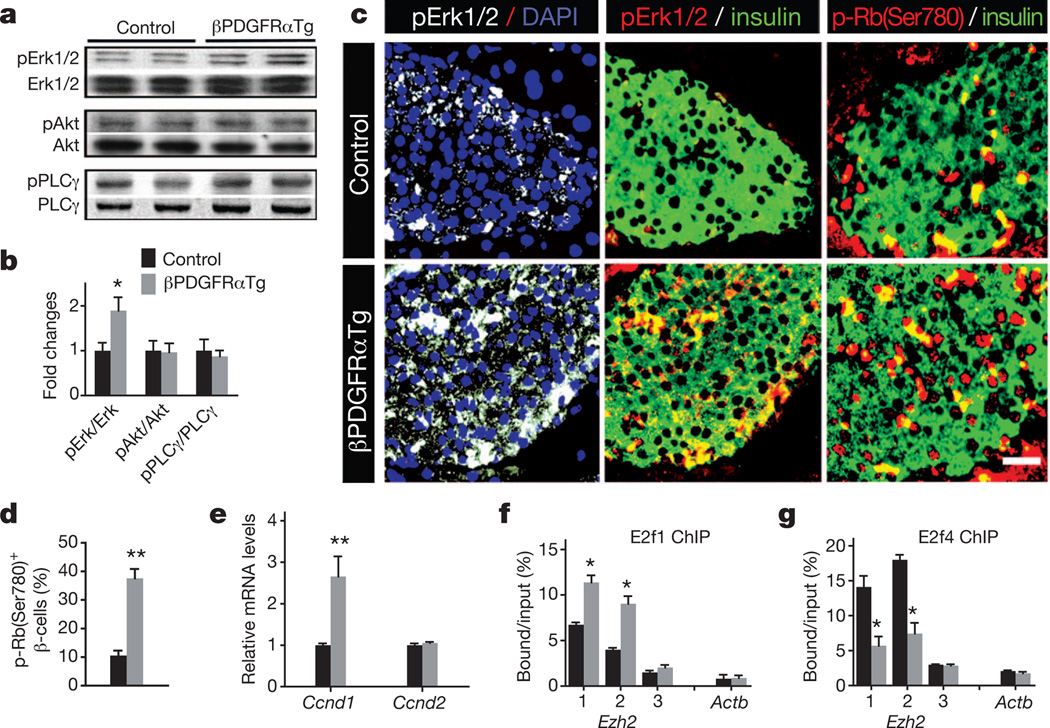
To investigate whether sustained PDGFR signalling permitted adult β-cell expansion in vivo, we intercrossed the RIP-Cre strain with mice harbouring a human PDGFRAD842V allele encoding a ligand-independent activated receptor inserted at the ROSA26 (R26) locus22. A loxP-flanked transcriptional stop sequence permits Cre-dependent PDGFRAD842V expression and activation of PDGF signalling in vivo22. In contrast to littermate controls, mice with the RIP-Cre; R26-PDGFRAD842V genotype (abbreviated to βPDGFRαTg) expressed human PDGFRA mRNA and PDGFR-a(D842V) protein in islet β-cells (Fig. 3a and Supplementary Fig. 5a). Expression of R26-PDGFRαD842V did not affect endogenous islet Pdgfra mRNA levels (Fig. 3a) or β-cell expansion and glucose control in neonatal or juvenile βPDGFRαTg mice (Fig. 3b, c and Supplementary Figs 5b and 6a–d). By 3 months, β-cell Ezh2 expression had declined in control mice, but β-cell Ezh2 expression in βPDGFRαTg mice was maintained at elevated levels, a difference that persisted for 8–14 months (Fig. 3e, f). mRNA levels of islet p16INK4a and p19Arf, both targets13 of Ezh2, were reduced in βPDGFRαTg mice compared to those in littermate controls (Supplementary Fig. 6f, g). In contrast, islet mRNA levels of Ezh1 in βPDGFRαTg and control mice were indistinguishable (Supplementary Fig. 6h). β-cell proliferation in 14-month-old βPDGFRαTg mice was increased ninefold compared to age-matched controls, a level of proliferation seen in 3-month-old control β-cells (Fig. 3d, f). β-cell mass was increased in βPDGFRαTg mice at 3 months, and remained increased at 14 months compared to age-matched controls (Fig. 3b). In contrast, total pancreatic mass and islet architecture in βPDGFRαTg mice and controls were indistinguishable (Supplementary Figs 5c and 6b). Thus, Pdgf signalling activation is sufficient to sustain adult β-cell expansion in vivo.
Figure 3. Activated PDGFR-α delays age-dependent Ezh2 loss and replication failure in pancreatic β-cells.
a, Relative mRNA levels of human PDGFRA and mouse Pdgfra in islets from littermate control and βPDGFRαTg mice at 3 months of age. n = 3–7 mice per group. b–d, Pancreatic β-cell mass (b), and β-cell proliferation, assessed by Ki67 expression (c) or BrdU incorporation (d) in βPdgfαKO and control mice at indicated ages. n = 3–5 mice per group. e, f, Relative mRNA levels of Ezh2 (e) in islets, and immunostaining (f) for Ezh2, BrdU and insulin on pancreatic sections from control and βPDGFRαTg mice at indicated ages. n = 3–7 mice per group. Scale bars, 25 µm. g, Postprandial blood glucose levels in mice at indicated ages n = 14–24 mice per group per time point. h, Glucose tolerance assessed in 14-month-old control and βPDGFRαTg mice. n = 6 or 10 mice per group. *P < 0.05, **P < 0.01 in comparisons indicated. Error bars denote s.e.m.
Prior studies indicate that β-cell expansion stimulated by mitogens can be complicated by disrupted β-cell function and impaired metabolic control3,4. To assess the physiological impact of prolonged adult β-cell proliferation in βPDGFRαTg mice, we measured systemic blood glucose and insulin levels. Blood glucose levels during fasting and ad libitum feeding and responses to insulin challenge were indistinguishable in βPDGFRαTg mice and littermate controls (Supplementary Fig. 6c–e). During re-feeding after overnight fast, βPDGFRαTg mice had a 25% reduction of blood glucose (Fig. 3g), associated with a modest elevation of plasma insulin levels (Supplementary Fig. 6i), and both changes were sustained for up to 14 months. After overnight fast, glucose clearance was enhanced in glucose-challenged 3- or 14-month-old βPDGFRαTg mice (Fig. 3h and Supplementary Fig. 6j). Thus, PDGFRaTg mice maintained normal glucose-regulated insulin release and glycaemic control, suggesting that β-cell function remained regulated in these mice.
PDGF-induced β-cell expansion requires Ezh2
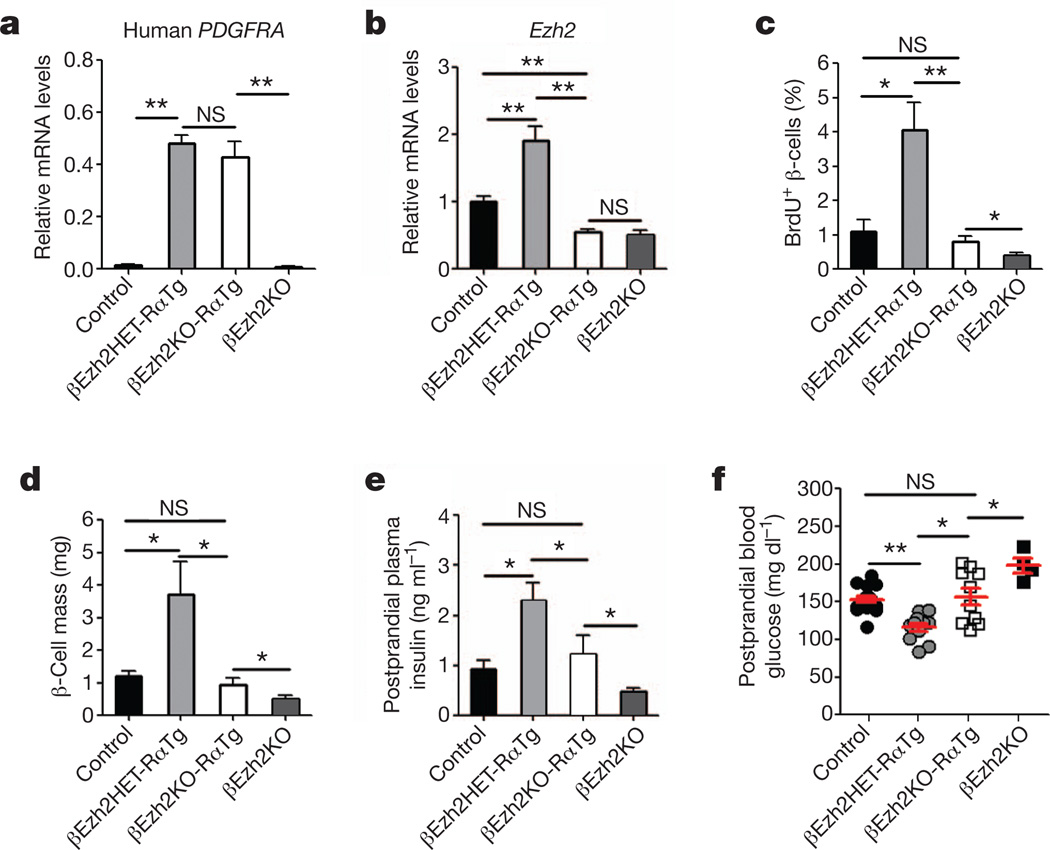
To test whether PDGFR-α-induced β-cell proliferation and expansion required Ezh2, we generated mice permitting conditional inactivation13 of Ezh2 in βPDGFRαTg β-cells. Intercrosses generated RIP-Cre; Ezh2f/f; R26-PDGFRαD842V mice (abbreviated to βEzh2KO-RαTg) and littermate or sibling RIP-Cre; Ezh2f/+; R26-PDGFRαD842V mice (abbreviated to βEzh2HET-RαTg) and RIP-Cre; Ezh2f/f mice (abbreviated to βEzh2KO). Islet PDGFRAD842V mRNA levels in both βEzh2KO-RαTg and βEzh2HET-RαTg mice were elevated and indistinguishable (Fig. 4a). By contrast, increased islet Ezh2 mRNA levels and β-cell Ezh2 protein were not observed in βEzh2KO-RαTg or βEzh2KO littermates (Fig. 4b and Supplementary Fig. 7), confirming Cre-mediated deletion of Ezh2 in these mice. As expected, β-cell BrdU incorporation and mass were increased in βEzh2HET-RαTg mice (Fig. 4c, d and Supplementary Fig. 7) compared to controls (either Ezh2f/f, R26-PDGFRαD842V or Ezh2f/+, R26-PDGFRαD842V genotype). However, neither β-cell BrdU incorporation nor mass were increased in βEzh2KO-RαTg mice compared to controls (Fig. 4c, d and Supplementary Fig. 7). Consistent with these findings, we detected modest postprandial blood glucose reduction and increased blood insulin levels in βEzh2HET-RαTg mice, but not in βEzh2KO-RαTg mice (Fig. 4e, f). Thus, Ezh2 is required for β-cell expansion and metabolic changes from PDGFR-α activation in β-cells.
Figure 4. PDGFR-α promotes β-cell expansion through Ezh2.
a, b, mRNA levels for human PDGFRA (a) and mouse Ezh2 (b) in islets from mice with indicated genotypes at 3–4 months of age. n = 3–5 mice per group. c–f, β-cell BrdU incorporation (c), β-cell mass (d), postprandial plasma insulin (e) and blood glucose (f) levels in 3–4-month-old mice with indicated genotypes. Each dot in f represents a measurement from an individual mouse. n = 4–5 mice for c and d, and n = 5–18 mice for e and f per genotype. *P < 0.05, **P < 0.01. NS, not significant in comparisons indicated. Error bars denote s.e.m.
β-cell PDGFR controls Ezh2 via Erk and Rb/E2f
To elucidate the signalling basis of altered β-cell proliferation in βPDGFRαTg, βPdgfαKO and ageing wild-type mice, we investigated Pdgf signal transduction factors. Pdgf receptors activate signalling elements16, including the mitogen-activated protein kinase/extracellular signal-regulated kinase (Mapk/Erk), phosphatidylinositol 3-kinase (PI3K)/Akt and phospholipase PLC-γ. Ezh2 induction in juvenile wild-type islets exposed to PDGF-AA was blocked by U0126, which inhibits Erk1/2 activation23, but not by LY294002 or U-73122, inhibitors of PI3K or PLC-γ signalling (Supplementary Fig. 8a). Similarly, increased β-cell BrdU incorporation in islets exposed to PDGF-AA was blocked by U0126, but not by LY294002 (Supplementary Fig. 8b), indicating that Erk mediates β-cell Pdgf signalling responses. We also detected increased phosphorylation of Erk1/2, but not phosphorylation of Akt or PLC-γ, in βPDGFRαTg islets compared to control islets (Fig. 5a, b). Immunohistology confirmed increased phospho-Erk1/2 levels in β-cells of βPDGFRαTg islets compared to controls (Fig. 5c). PDGF/ERK signalling activates cell cycle regulators to stimulate proliferation24,25, and βPDGFRαTg islets had elevated levels of Ccnd1 mRNA encoding cyclin D1, and increased β-cells with nuclear cyclin D1 and phosphorylated Rb protein (Fig. 5c–e and Supplementary Fig. 9a). In contrast, loss of β-cell Pdgf signalling in βPdgfαKO mice diminished islet levels of Ccnd1 mRNA, and reduced numbers of phospho-Erk1/2+ and phospho-Rb+ β-cells (Supplementary Fig. 9b, c). Rb protein phosphorylation regulates association of the E2f1 transcriptional activator and E2f4 transcription repressor with their targets26,27. We identified two conserved candidate E2f-binding elements near the Ezh2 promoter (Supplementary Fig. 10), and chromatin immunoprecipitation (ChIP) studies showed increased E2f1 association and reduced E2f4 association with these Ezh2 elements in βPDGFRαTg islets (Fig. 5f, g). In wild-type mice, levels of phospho-Erk1/2 and phospho-Rb declined with age in islet β-cells at a tempo similar to declining Ezh2 and Pdgfra expression (Supplementary Fig. 11a, b). ChIP studies also revealed that association of E2f1 with Ezh2 decreased, whereas association of E2f4 with Ezh2 elements increased in islets with age (Supplementary Fig. 11c, d). Thus, age-dependent attenuation of β-cell Pdgfr-α altered Erk and Rb/E2f signalling, resulting in reduced Ezh2 expression.
Figure 5. Pdgfr signalling governs Erk and Rb/E2f regulation of Ezh2 in islet β-cells.
a, Western blot analysis of total and phosphorylated Erk1/2, Akt and PLCγ in islet proteins from 3–4-month-old βPDGFRαTg and littermate control mice. Similar results were obtained from multiple independent experiments. b, Relative phosphorylated protein level compared to total protein was quantified by densitometry. c, Immunostaining for phospho-Erk1/2 (pErk1/2), phospho-Rb-Ser 780 (p-Rb(Ser780)) and insulin in 3-month-old littermate control and βPDGFRαTg pancreatic sections. Scale bar, 25 µm. d, Percentage of phospho-Rb-Ser 780+ insulin+ cells from the indicated mice quantified by morphometry. n = 4 mice per group. e, Cyclin D1 (Ccnd1) or cyclin D2 (Ccnd2) mRNA levels in 3-month-old control and βPDGFRαTg islets. n = 4 or 6 mice per group. f, g, ChIP analyses of the Ezh2 and β-actin (Actb) loci with antibodies to E2f1 (f) and E2f4 (g) using the indicated amplicons (see Supplementary Information) in 3–4-month-old littermate control and βPDGFRαTg islets. n = 3–4 independent experiments per antibody with independent islet samples. *P < 0.05, **P < 0.01 for control versus βPDGFRαTg. Error bars denote s.e.m.
To verify that Rb/E2f signalling regulates β-cell Ezh2, we examined triple mutant ROSA-CreERT2; Rbf/f; p130f/f; p107−/− mice (RbTriKO) lacking Rb family proteins28. After exposure to tamoxifen, β-cells in adult RbTriKO mice, lacking detectable Rb, p130 and p107, had increased Ezh2 protein and mRNA levels, compared to those from tamoxifen-exposed Rbf/f, p130f/f, p107−/− control littermates (Supplementary Fig. 12a–c). Islet ChIP studies revealed increased E2f1 association, and reduced E2f4 association with Ezh2 cis-regulatory elements (Supplementary Fig. 12d, e). Consistent with these findings, BrdU incorporation in RbTriKO β-cells was increased by 15-fold, culminating in a 400% increase of β-cell mass and hypoglycaemia that progressively worsened until death (Supplementary Fig. 12f–h). Thus, Rb proteins constrain Ezh2 expression and adult islet cell growth.
PDGF control of human β-cell proliferation
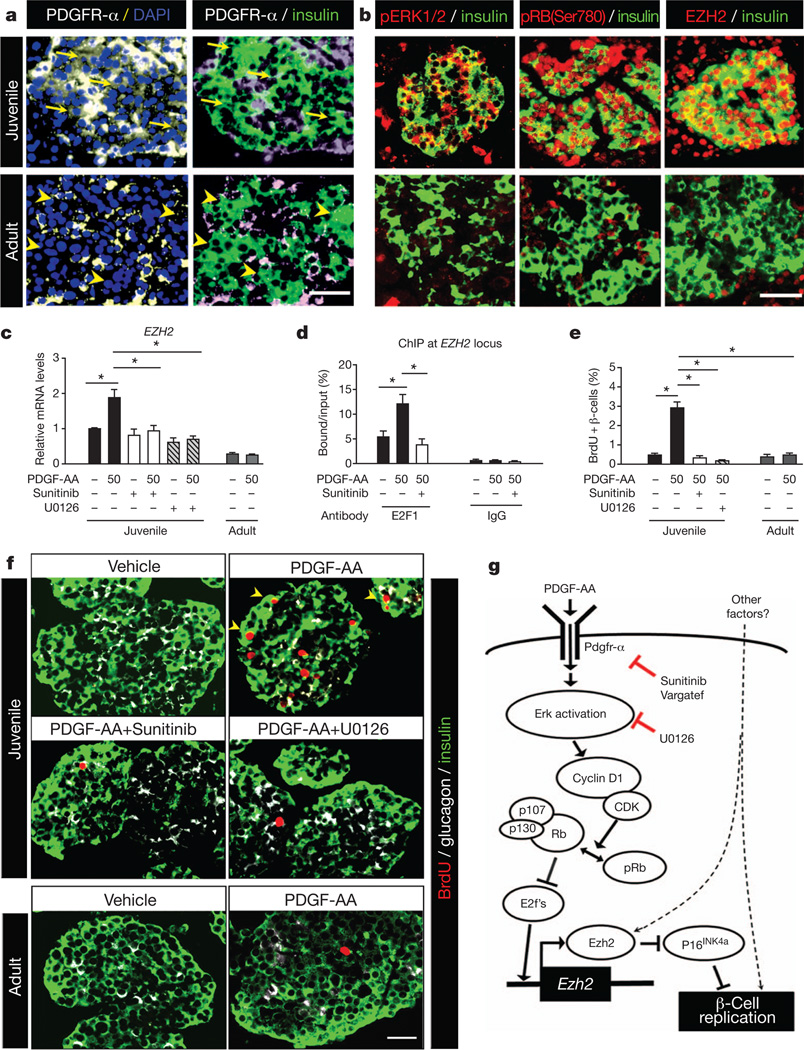
To investigate the relevance of our studies to human β-cells, we assessed PDGF signalling and competence in human islets. Like in juvenile mice, PDGFR-α protein and phospho-ERK1/2 levels were readily detected in islets from young human donors (Fig. 6a, b). We also detected abundant phospho-RB-Ser 780 and EZH2 protein in β-cell nuclei from young islets (Fig. 6b). In contrast, PDGFR-a was only detected in non-β-cells of islets from adult human donors, with little to no detectable phospho-ERK1/2, phospho-RB or EZH2 in β-cells (Fig. 6a, b), suggesting that PDGF signalling attenuation is a conserved feature of ageing islet β-cells. mRNA levels of EZH2, but not EZH1, were increased in juvenile human islets exposed to PDGF-AA, an effect blocked by Sunitinib or U0126 (Fig. 6c and Supplementary Fig. 13a). In contrast, basal EZH2 mRNA levels were lower in adult islets and not induced by PDGF-AA (Fig. 6c). Similar to juvenile mouse islets with activated Pdgf signalling, ChIP studies demonstrated PDGF-AA-stimulated association of E2F1 with the human EZH2 locus, an effect blocked by Sunitinib (Fig. 6d). Thus, evolutionarily conserved elements of the PDGF signalling pathway govern EZH2 expression and β-cell cycle regulators in human islets. Proliferating BrdU+ cells in juvenile islets stimulated by PDGF-AA were predominantly β-cells, verified by immuno-localization of BrdU+ nuclei within insulin+ cells and PDX1+ nuclei with confocal microscopy (Fig. 6f and Supplementary Fig. 13b, c). Compared to vehicle-exposed controls, juvenile islets exposed to PDGF-AA had a sixfold increase of β-cell BrdU incorporation, an effect eliminated by simultaneous exposure to Sunitinib or U0126 (Fig. 6e, f). In contrast, PDGF-AA did not alter β-cell proliferation in adult human islets (Fig. 6e, f). Thus, dynamic PDGF signalling competence may regulate declining β-cell proliferation in ageing human islets.
Figure 6. PDGFR-α regulates human β-cell EZH2 expression and proliferation.
a, b, Representative images showing immunostaining for PDGFR-α (a), phospho-ERK1/2 (pERK1/2), phospho-RB-Ser 780 (pRB(Ser780)), EZH2 (b) and insulin (a and b) on pancreatic sections from juvenile and adult human subjects. PDGFR-α was detected in juvenile β-cells (arrows) but not in adult β-cells (arrowheads). Scale bars, 25 µm.
c–f, Assessment of effects on human juvenile or adult islets after exposure to PDGF-AA (50 ng ml−1) for 2 days, with or without Sunitinib (2 µM) or U0126 (10 µM)co-treatment. c, Islet EZH2 mRNA levels after the indicated treatments. n = 3–5 independent experiments. d, EZH2 locus ChIP analysis with anti-E2F1 antibody or IgG in human juvenile islets. n = 3–4 for E2F1, n = 2 for IgG. e, f, Human islet β-cell proliferation changes after the indicated treatments. Average percentage of BrdU+ insulin+ cells (e) was quantified by morphometry from sectioned islets immunostained (f) for insulin (green), glucagon (white) and BrdU (red). n = 3–6 independent experiments. Scale bar, 25 µm.
g, Illustration summarizing how Pdgfr-α signalling regulates β-cell Ezh2 and proliferation by activating Erk/Rb/E2f pathways sensitive to the indicated inhibitors. *P < 0.05, **P < 0.01 as indicated. Error bars denote s.e.m.
Discussion
The elucidation and control of mechanisms governing pancreatic β-cell proliferation could transform treatments for diseases like diabetes. However, prior attempts to expand islets by modulating extrinsic or intrinsic growth regulators have been bedevilled by limited proliferation with loss of defining β-cell features3,4, indicating disruption of mechanisms that preserve fate in proliferating β-cells. The maintenance of cell fate and function in proliferating cells is the essence of epigenetic regulation. Here we focused on identifying the principal elements of a native signalling pathway regulating Ezh2, an essential epigenetic regulator of β-cell proliferation13. We found that β-cell Pdgfr signalling was required for Ezh2 induction, to sustain both physiological and regenerative β-cell proliferation.
During islet β-cell regeneration (this study), and in maternal islets in pregnancy (H.C. and S.K.K., unpublished results), we observed a twofold increase of islet Ezh2 mRNA levels. However, pathological loss of all Rb family proteins in RbTriKO islets led to a fourfold increase in Ezh2 mRNA, and a 15-fold increase in β-cell proliferation. RbTriKO mice became hypoglycaemic and developed other islet and metabolic phenotypes reminiscent of insulinoma pathogenesis. Thus, the physiological expansion of β-cells maintaining regulated function may require mechanisms limiting Ezh2 induction. Compared with β-cells from βEzh2KO mice, we also observed a modest enhancement of proliferation and expansion after PDGFR-α activation in β-cells lacking Ezh2. Insulin or prolactin, two mitogens capable of promoting β-cell proliferation1,2,29, did not induce β-cell Ezh2 expression in cultured islets. Thus, our findings here support the view that Ezh2-independent mechanisms regulate β-cell proliferation. Likewise, decline of β-cell proliferation in ageing βPDGFRαTg mice suggests that PDGF-independent pathways restrict β-cell expansion.
Proliferative responses to PDGF decline in some ageing cells30, and progenitor cell self-renewal is impaired by Pdgf signalling disruption31. However, the molecular basis for these observations and the roles of Pdgf signalling in islet β-cell growth and development were not established17,18,32. Here we showed that loss of β-cell Pdgfr-α signalling accelerated β-cell replication failure both in juvenile mice and in adult mice after conditional β-cell ablation. In vivo activation of β-cell PDGFR-α signalling increased Ezh2 expression and mitigated age-dependent decline of β-cell proliferation. Crucially, conditional Ezh2 inactivation in β-cells with activated PDGFR-α signalling prevented these changes. Thus, our conditional loss- and gain-of-function studies reveal how Pdgf signalling regulates age-dependent physiological β-cell proliferation and expansion. Our work also suggests that Mapk/Erk regulation links Pdgfr activation to Ezh2 induction and β-cell proliferation, consistent with studies demonstrating Erk regulation of Ezh2 in cancer cells33, and Erk activation that accompanies mouse β-cell expansion in pregnancy34. Further studies should clarify how Erk and other factors might integrate β-cell responses to Pdgf or other mitogens35.
Although the mechanisms underlying pancreatic β-cell expansion in physiological settings may differ between species36,37, age-dependent restriction of β-cell proliferation is a conserved feature in mice and humans9,10. Here, we provide unprecedented evidence that a conserved signalling pathway restricts β-cell proliferation in mice and humans. We suggest that β-cell competence for signalling factors like PDGF can underlie dynamic islet responses to mitogens. Such age-dependent signalling competence might explain earlier findings of inconsistent human β-cell responses to other mitogens5,6. PDGF ligands are measurable in serum, and regulation of such circulating signalling agonists may provide an additional level of growth control in competent islet β-cells. Prior studies correlated increased PDGFR and EZH2 expression in human endocrine neoplasias38, and disrupted PDGF signalling in β-cells of humans with type 2 diabetes39. Thus, our demonstration that PDGF signalling regulates EZH2,CDKN2A and β-cell proliferation in human islets combines several observations into a new molecular model for physiological and pathological β-cell growth. The factors governing β-cell expression of PDGF signalling regulators like PDGFR-a remain unknown, but we speculate that the identification of such factors may be useful for modulating human β-cell growth and function in diabetes and cancer.
METHODS SUMMARY
Animal studies and mouse islet culture experiments
Details for various mouse strain generation, in vivo manipulation and measurement, mouse islet isolation and culture and FACS-based β-cell purification are provided in Supplementary Data.
Histology, immunohistochemistry and molecular studies
Standard histology and immunostaining protocols for various proteins on pancreas or islet sections were described previously13. β-cell mass and proliferation were morphometrically quantified13 by independent researchers blinded to sample identity. Details of these studies and real-time RT–PCR, western blot and ChIP analyses are provided in Supplementary Data.
Human pancreas and islet studies
Four juvenile and four adult pancreases, and eight juvenile and three adult individual islet preparations from healthy human deceased organ donors (mean age 3.0 ± 0.6 years for juveniles; mean age 44.3 ± 5.3 years for adults) were used in this study. As in the mouse studies, similar procedures and experiments were performed on human pancreatic sections or islet cultures as described in Supplementary Data.
Statistical analyses
Results are shown as mean ± standard error of the mean (s.e.m.). Statistical analysis was performed by one-way ANOVA, two-way repeated measures ANOVA, or unpaired Student’s t-test, as appropriate, with significance set at P < 0.05.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
METHODS
Animals
Transgenic mice harbouring a floxed Pdgfra allele40 encoding Pdgfr-α were purchased from the Jackson Laboratory. This strain of mice was intercrossed with RIP-Cre mice41 expressing Cre recombinase from rat insulin 2 gene promoter elements to generate RIP-Cre, Pdgfraf/f mice (designated as βPdgfrαKO) and their littermate controls (including RIP-Cre, Pdgfrαf/+ mice designated as βPdgfrαHet) on a mixed 129/Sv; C57BL/6 genetic background.
R26hPDGFRαPM transgenic mice that harbour a mutated human PDGFRAD842V allele encoding a ligand-independent activated receptor targeted immediately after a loxP-flanked transcriptional stop sequence at the ROSA26 locus have been described previously22. This strain of mice was intercrossed with RIP-Cre mice to generate RIP-Cre; R26-PDGFRAD842V mice (designated as βPDGFRαTg) together with their littermate controls on a mixed 129/Sv; C57BL/6 genetic background. Mice with specific expression of mutant human PDGFRAD842V, but lacking Ezh2 in β-cells (designated as βEzh2KO-RαTg), were generated from subsequent intercrosses between βEzh2KO13 and βPDGFRαTg mice.
Tamoxifen-inducible, compound triple-mutant mice lacking retinoblastoma pocket proteins Rb, p130 and p107 (ROSA26-CreERT2; Rbf/f; p130f/f; p107−/−, designated as RbTriKO) were produced in the Sage group (Stanford University). RbTriKO mice harbour a germline-deleted p107 null allele (p107−/−), and loxP-flanked Rb and p130 conditional alleles which can be excised by tamoxifen-sensitive CreERT2 recombinase targeted into the ROSA26 locus28. Littermate mice (Rbf/f; p130f/f; p107−/−) lacking ROSA26-CreERT2 were used as controls. To delete Rb and p130 alleles, RbTriKO and littermate control mice aged 3–5 months received intraperitoneal injections of tamoxifen (Sigma, 1.5 mg per mouse per day) dissolved in ethanol/corn oil (Sigma) on five consecutive days.
MIP-EGFP mice42 were obtained from the laboratory of M. Hara. C57BL/6 mice were purchased from the National Cancer Institute (NCI). All mutant mice used in this study were genotyped by PCR of tail genomic DNA for the human PDGFRAD842V allele, mouse Pdgfraf, Ezh2f, Rbf, p130f, p107− alleles, and transgenes encoding EGFP, RIP-Cre or ROSA-CreERT2 as described previously13,22,28,40–42. Mice used in this study were age- and gender-matched littermates including both sexes. All mice were housed in the animal facility of Stanford University on a 12-h light/dark cycle with ad libitum access to water and normal chow except when otherwise indicated. All animal experiments and methods were approved by the Institutional Animal Care and Use Committee of Stanford University.
Physiological studies
We performed glucose physiology studies as previously reported13. Briefly, at the ages or times indicated in the text and figures, or after tamoxifen administration, we monitored body weight and blood glucose levels in ad libitum fed mice, or after 16 h overnight fasting, or 2 h after re-feeding (‘2 h postprandial’). We drew blood from tail vein punctures or from the aorta when mice were killed, for measuring blood glucose levels using a Contour glucose meter (Bayer), or for measuring plasma insulin levels by mouse insulin ELISA kits (ALPCO Diagnostics).
Glucose tolerance tests were performed on mice after a 16 h overnight fast, and the blood glucose levels were determined immediately before (0) and 5, 10, 20, 30, 45, 60, 90 and 120 min after intraperitoneal injection of glucose (2 g kg−1 body weight). For measurement of plasma insulin levels during these glucose tolerance tests, mice were injected with glucose at a dose of 3 g kg−1 body weight, and we collected tail vein blood at 0, 15 and 45 min after glucose injection followed by mouse insulin ELISA (ALPCO diagnostics). For insulin tolerance tests, mice were fasted for 6 h then received intraperitoneal injection of 0.75 units kg−1 of body weight of insulin (Sigma); glucose levels at 0, 10, 20, 30, 40, 60 and 90 min after injection were then measured.
For detecting in vivo β-cell proliferation by BrdU incorporation, mice were either fed with water containing BrdU (1mg ml−1) or intraperitoneally injected with BrdU/PBS solution (50mg kg−1 body weight) for specified schedules as follows: βPdgfrαKO mice, 2 days of intraperitoneal injection of BrdU/PBS; βPdgfrαKO mice with STZ treatment, 1 week of BrdU water feeding; βPDGFRαTg or βEzh2KO-RαTg mouse studies, 6–7 days of BrdU water feeding; RbTriKO mice, 4 days of BrdU water feeding. After BrdU chase, mouse pancreases were collected and subjected to immunohistology followed by morphometric analyses described later.
STZ-induced diabetes
For measuring islet Pdgfra and Pdgfrb induction during STZ-induced diabetes, male wild-type C57BL/6 mice, aged 8 weeks, were injected intraperitoneally with 100 mg kg−1 body weight of freshly dissolved streptozotocin (STZ, Sigma) in 0.1 mol l−1 sodium citrate (pH 4.5) or with the same volume of sodium citrate (Vehicle). Six days later, islets or pancreases were recovered from the mice for gene expression analyses by real-time RT–PCR or immunohistology studies. To test the roles of Pdgfra in islet regeneration in STZ diabetes, βPdgfrαKO or control mice (including βPdgfraHαt), aged 7–8 weeks, were treated with a single intraperitoneal STZ injection at a dose of 150 mg kg−1 body weight. After injection, blood glucose values were measured on day 3 and day 6 in the first week, and thereafter once per week. After 2 weeks of STZ treatment, subsets of βPdgfrαKO and control littermates were fed with BrdU-containing drinking water (1mg ml−1) for 1 week for BrdU incorporation analyses. Three days, 21 days and 58 days after STZ treatment, subsets of mice were killed, and blood samples and pancreases were harvested for measurement of plasma insulin levels by ELISA, and for pancreatic β-cell proliferation and mass quantification following immuno-staining of insulin and BrdU.
Islet isolation and culture studies
Mouse pancreatic islets were purified as described previously13 and used immediately for assays described in the text. To study Ezh2 induction in cultured islets exposed to mitogens, freshly isolated islets from 2–3-week old, and 7–12-month old C57BL/6 mice were recovered and equilibrated overnight at 37 °C, 5% CO2 in RPMI-1640 medium supplemented with 0.5% fetal bovine serum (FBS), 0.2% bovine albumin (BSA) and 2% penicillin/streptomycin. Afterwards, islets were distributed into microplates or dishes, and treated with recombinant human PDGF-AA, prolactin or insulin (Sigma), with or without addition of pharmacological inhibitors, including Sunitinib (Selleck), Vargatef (Selleck), U0126 (Sigma), LY294002 (Sigma) or U-73122 (Sigma) at the indicated concentrations. Two days later, islets were harvested for RNA or protein extraction for real-time RT–PCR or western blot analyses. To detect phosphorylation of Pdgfr-α by western blot, islets were exposed to PDGF-AA (with or without indicated pharmacological inhibitors) for 90 min before islet harvesting and protein extraction. To detect in vitro islet β-cell proliferation stimulated by PDGF-AA, a final concentration of 50 µM BrdU was introduced into each culture dish 24 h before harvest, followed by standard histological processing. Immunostaining of BrdU and insulin was performed on processed islets, and β-cell proliferation rate was determined by quantifying percentage of insulin+ cells with BrdU+. Culture experiments of human islets were performed using a similar procedure described later.
β-cell purification by FACS
Freshly purified islets from MIP-GFP mice at 2 weeks or 5 months of age were pooled, and dispersed into a suspension of single cells by incubation with 0.05% trypsin/0.53mM EDTA solution at 37 °C for 10 min as described previously37,43. After dissociation, cells were washed three times in PBS containing 2% FBS, and re-suspended in FACS buffer (HBSS/0.2% BSA/1% HEPES) containing propidium iodide (to exclude dead cells). FACS was performed on a FACS AriaII (BD Biosciences), and MIP-GFP+ β-cells with >95% purity were harvested and immediately lysed for RNA purification and real-time RT–PCR analysis.
Real-time RT–PCR and western blot analysis
Total RNA from freshly isolated or cultured islets after indicated treatments were purified using the Absolutely RNA miniprep purification kit (Stratagene) according to the manufacturer’s instructions. RNA concentration was measured with a RiboGreen RNA quantification assay (Invitrogen). One-step quantitative RT–PCR was performed and analysed using an ABI Prism 7300 detection system (Applied Biosystems) with TaqMan one-step RT–PCR Master Mix Reagents and appropriate amounts (10–100 ng) of islet total RNAas the template. We calculated the ratio of mRNA for the gene of interest to the amount of internal control mRNA of peptidylprolyl isomerase A (cyclophilin A, PPIA), and then normalized the ratio for each gene to its median. Primer and probe sequences are listed in Supplementary Tables 1 and 2.
For western blots, total islet protein was prepared from freshly isolated or cultured islets after indicated treatments as previously described13. Equal amounts of protein were resolved on SDS–PAGE and transferred to polyvinylidine fluoride membranes (Amersham Pharmacia) for immunoblotting with specific antibodies, including rabbit polyclonal anti-human Ezh2 (1:1,000, Millipore), rabbit polyclonal anti-phospho-PDGFR-α (Tyr 720) (1:1,000, Santa Cruz Biotechnologies), rabbit monoclonal anti-PDGFR-α (1:1,000, Cell Signaling), mouse monoclonal anti-β-actin (1:4,000, Sigma), rabbit monoclonal anti-Akt (1:1,500, Cell Signaling), rabbit monoclonal anti-phospho-Ark (Ser 473) (1:1,000, Cell Signaling), rabbit monoclonal anti-p44/42 MAPK (Erk1/2) (1:1,500, Cell Signaling), rabbit monoclonal anti-phospho-p44/42MAPK (Erk1/2) (Thr 202/Tyr 204) (1:1,000, Cell Signaling), rabbit monoclonal anti-PLCγ1 (1:1,000, Cell Signaling) and rabbit monoclonal anti-phospho-PLCc1 (Tyr 783) (1:1,000, Cell Signaling). Signal was visualized using ECL detection (Amersham Pharmacia) on Kodak film after further incubation with HRP-conjugated secondary antibodies. For phospho-PDGFR-α (Tyr 720) and PDGFR-α detection, signal was amplified by incubating the membrane with biotinylated anti-rabbit-IgG (1:2,000), followed with an incubation with HRP-avidin (1:2,000, Vector Laboratories) and then ECL visualization.
Histology, immunofluorescence and immunohistochemistry
We performed standard histological paraformaldehyde fixation, paraffin-embedding and immunostaining protocol as previously described13. Briefly, immunohistochemical analysis was performed on 5-µm-thick sections of pancreatic tissues or islet sections after antigen retrieval (DAKO) and using the following primary antibodies: guinea pig anti-insulin (1:400, Sigma), mouse anti-glucagon (1:200, Sigma), rabbit anti-somatostatin (1:200, Dakocytomation), rabbit anti-pancreatic polypeptide (1:200, Dakocytomation), rabbit anti-Ezh2 (1:100, Epigentek), rabbit anti-PDGFR-α (1:50, Novus Biologicals), sheep anti-PDGFR-β (1:100, Sigma), rabbit anti-phospho-PDGFR-α (Tyr 720) (1:50, Santa Cruz Biotechnologies), rabbit anti-phospho-p44/42 MAPK (Erk1/2) (Thr 202/Tyr 204) (1:50, Cell Signaling), rabbit anti-phospho-Rb (Ser 780) (1:100, Cell Signaling), rabbit anti-cyclin D1 (1:50, Cell Signaling), rabbit anti-cyclin D2 (1:50, Cell Signaling), mouse anti-PDX1 (1:50; gift from H. Edlund), mouse monoclonal anti-Ki67 (1:100, Novocastra) and mouse monoclonal anti-BrdU (1:100, Sigma). We detected immune complexes with secondary antibodies conjugated with either Cy3, fluorescein isothiocyanate (Jackson ImmunoResearch) or horseradish peroxidase (Vector Laboratories). After staining, images were directly collected on an AxioM1 microscope equipped with a CCD digital camera (Carl Zeiss), or on a Leica SP2 inverted confocal microscope.
For measuring pancreatic β-cell mass or BrdU by morphometry, three to six mice for each group were analysed. At least five sections separated by more than 300 µm(pancreases) or 50 µm (cultured islets) were immunostained and assessed for each sample. Images were analysed with an ImagePro program by observers blinded to genotype, and pancreatic β-cell mass and percentage of BrdU+ β-cells were calculated as described previously13.
Pancreatic islet ChIP analysis
ChIPs were performed using a chromatin immunoprecipitation kit from Millipore as described previously13. Briefly, freshly isolated mouse pancreatic islets or cultured human islets after the indicated treatments were pooled, and fixed with 2% formaldehyde for cross-linking for 20 min at room temperature (25 °C). After washing, islets were dissolved in SDS lysis buffer containing a proteinase inhibitor cocktail followed by sonication to shear the chromatin. Precleared chromatin from 150 to 300 islets was used for each ChIP sample with incubation of 1 to 5 µg of the appropriate antibodies at 4 °C overnight. The antibodies used in the ChIP assays included mouse anti-E2F1 (Millipore), rabbit anti-E2F4 (Santa Cruz Biotechnologies), rabbit or mouse polyclonal IgG (Millipore). After incubation, the immunoprecipitated chromatin DNA was harvested, cross-link reversed, and purified. After measurement of DNA concentration by PicoGreen DNA assay (Invitrogen), equivalent amounts of chromatin DNA from every sample was quantified by real-time qPCR in ABI Prism 7300 detection system (Applied Biosystems). The sequences of the PCR primers and probes are listed in Supplementary Table 3.
Human pancreas and islet studies
Human pancreases or islets from organ donors were procured by arrangement with the National Disease Resource Interchange and the University of Alabama, Birmingham with appropriate consent. Institutional review board approval for research use of tissue was obtained from Stanford University School of Medicine. For pancreas studies, fresh human pancreases were processed in our laboratory into paraffin sections using standard histological protocols. Four pancreases from juvenile donors (1, 2, 3, 7 years old) and four pancreases from adult donors (22, 34, 55, 56 years old) were used for immunohistology studies here. Similar immunohistology methods and antibodies as those used for mouse pancreas tissues were used to detect PDGFR-α, phospho-ERK1/2 (pERK1/2), phospho-RB (Ser 780) (pRB(Ser780), EZH2 and insulin on human pancreas sections. Experiments were repeated onmultiple pancreas sections for every donor pancreas and similar results were obtained.
For islet studies, human islets were isolated either at Pittsburgh University Medical Center (R.B.), or the University of Alabama, Birmingham (S. Bryant and A. Thompson). Eight independent human islet preparations from juvenile donors at the age of 8 months, 1.0, 1.5, 2, 3, 4, 5 and 6 years, and three adult islet preparations from donors at the age of 39, 48 and 56 years were used in this study. After isolation, fresh islets were shipped to our laboratory, and were hand-picked and transferred to RPMI-1640 medium supplemented with 0.5% FBS, 0.2% BSA and 2% penicillin/streptomycin for an overnight recovery before experiments. Similar to the experiments performed with mouse islets, equilibrated human islets were exposed to recombinant human PDGF-AA, without or with addition of pharmacological inhibitors, including Sunitinib (Selleck) and U0126 (Sigma) for 2 days, followed by islet RNA purification for real-time RT–PCR analyses of gene expression. To detect in vitro human islet β-cell proliferation stimulated by PDGF-AA, human islets were exposed to 50 µM BrdU for 24 h. Afterwards, human islets were processed in a similar way as in mouse islet studies for immunostaining of insulin, glucagon, PDX1 and BrdU. β-cell proliferation rate was determined by quantifying percentage of insulin+ cells with BrdU+. More than 50 islets per group were counted for determining cultured human islet β-cell proliferation rate.
Supplementary Material
Acknowledgements
We thank A. Bhushan, A. Stewart, A. Powers, P. Beachy, M. White, X. Chen and X. Li for helpful discussions and advice, A. Tarakhovsky, P. Herrera and M. Hara for mice, A. Powers, A. Thompson and S. Bryant for human islet sample procurement, and members of the S.K.K. laboratory for comments on the manuscript. H.C. was supported by the NIH Ruth L. Kirschstein NRSA/Stanford Regenerative Medicine Training Program. J.S. was supported by NIH-NCI RO1 CA114102. H.S. was supported by the Stem Cell Network NRW and Deutsche Krebshilfe. Work in the S.K.K. laboratory was supported by a gift from the Dewey family fund, and grants from the Juvenile Diabetes Research Foundation, Snyder Foundation, Stinehart Foundation, the NIH Beta Cell Biology Consortium (UO1 DK89532 to S.K.K. and UO1 DK89572 to A. Powers) and by the Howard Hughes Medical Institute(HHMI). S.K.K. is an Investigator of the HHMI.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Contributions H.C., X G., Y.L. and J.W. performed experiments. H.C., S.E.W., J.S. and H.S. generated mice. R.B. isolated human islets. H.C. and S.K.K. conceived the project, generated hypotheses, analysed data and wrote the manuscript.
Author Information The authors declare no competing financial interests.
References
- 1.Heit JJ, Karnik SK, Kim SK. Intrinsic regulators of pancreatic -cell proliferation. Annu. Rev. Cell Dev. Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 2.Vasavada RC, et al. Growth factors and β-cell replication. Int. J. Biochem. Cell Biol. 2006;38:931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Vasavada RC, et al. Targeted expression of placental lactogen in the β-cells of transgenic mice results in β-cell proliferation, islet mass augmentation, and hypoglycemia. J. Biol. Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Ocana A, et al. Hepatocyte growth factor overexpression in the islet of transgenic mice increases β-cell proliferation, enhances islet mass, and induces mild hypoglycemia. J. Biol. Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- 5.Beattie GM, et al. A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes. 2002;51:3435–3439. doi: 10.2337/diabetes.51.12.3435. [DOI] [PubMed] [Google Scholar]
- 6.Parnaud G, et al. Proliferation of sorted human and rat β-cells. Diabetologia. 2008;51:91–100. doi: 10.1007/s00125-007-0855-1. [DOI] [PubMed] [Google Scholar]
- 7.Dor Y, et al. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 8.Kushner JA, et al. Cyclins D2 and D1 are essential for postnatal pancreatic β-cell growth. Mol. Cell. Biol. 2005;25:3752–3762. doi: 10.1128/MCB.25.9.3752-3762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teta M, et al. Very slow turnover of β-cells in aged adult mice. Diabetes. 2005;54:2557–2567. doi: 10.2337/diabetes.54.9.2557. [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, et al. β-cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. doi: 10.2337/db07-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krishnamurthy J, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 12.Zindy F, et al. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene. 1997;15:203–211. doi: 10.1038/sj.onc.1201178. [DOI] [PubMed] [Google Scholar]
- 13.Chen H, et al. Polycomb protein Ezh2 regulates pancreatic β-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao R, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 15.van der Vlag J, Otte AP. Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation. Nature Genet. 1999;23:474–478. doi: 10.1038/70602. [DOI] [PubMed] [Google Scholar]
- 16.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 17.Swenne I, et al. Effects of platelet-derived growth factor and somatomedin-C/ insulin-like growth factor I onthe deoxyribonucleic acid replication of fetal rat islets of Langerhans in tissue culture. Endocrinology. 1988;122:214–218. doi: 10.1210/endo-122-1-214. [DOI] [PubMed] [Google Scholar]
- 18.Welsh M, et al. Coexpression of the platelet-derived growth factor (PDGF) B chain and the PDGF beta receptor in isolated pancreatic islet cells stimulates DNA synthesis. Proc. Natl Acad. Sci. USA. 1990;87:5807–5811. doi: 10.1073/pnas.87.15.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su I, et al. Polycomb group protein Ezh2 controls actin polymerization and cell signaling. Cell. 2005;121:425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Mendel DB, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 21.Hilberg F, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 22.Moenning A, et al. Sustained platelet-derived growth factor receptor a signaling in osteoblasts results in craniosynostosis by overactivating the phospholipase C-γ pathway. Mol. Cell. Biol. 2009;29:881–891. doi: 10.1128/MCB.00885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncia JV, et al. MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg. Med. Chem. Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 24.Uhrbom L, Nerio E, Holland EC. Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nature Med. 2004;10:1257–1260. doi: 10.1038/nm1120. [DOI] [PubMed] [Google Scholar]
- 25.Furstoss O, Manes G, Roche S. Cyclin E and cyclin A are likely targets of Src for PDGF-induced DNA synthesis in fibroblasts. FEBS Lett. 2002;526:82–86. doi: 10.1016/s0014-5793(02)03120-4. [DOI] [PubMed] [Google Scholar]
- 26.Bracken AP, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22:5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moberg K, Starz MA, Lees JA. E2F4 switches from p130 to p107 and pRB in response to cell cycle reentry. Mol. Cell. Biol. 1996;16:1436–1449. doi: 10.1128/mcb.16.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viatour P, et al. Hematopoietic stemcell quiescence is maintained by compound contributions of the retinoblastomagene family. Cell Stem Cell. 2008;3:416–428. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brelje TC, Parsons JA, Sorenson RL. Regulation of islet β-cell proliferation by prolactin in rat islets. Diabetes. 1994;43:263–273. doi: 10.2337/diab.43.2.263. [DOI] [PubMed] [Google Scholar]
- 30.Scherping SC, Jr., et al. Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect. Tissue Res. 1997;36:1–8. doi: 10.3109/03008209709160209. [DOI] [PubMed] [Google Scholar]
- 31.Pallante BA, et al. Bone marrow Oct3/4+ cells differentiate into cardiacmyocytes via age-dependent paracrine mechanisms. Circ. Res. 2007;100:e1–e11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 32.LeBras S, Czernichow P, Scharfmann R. A search for tyrosine kinase receptors expressed in the rat embryonic pancreas. Diabetologia. 1998;41:1474–1481. doi: 10.1007/s001250051094. [DOI] [PubMed] [Google Scholar]
- 33.Fujii S, et al. MEK–ERK pathway regulates EZH2 overexpression in association with aggressive breast cancer subtypes. Oncogene. 2011 Apr 18; doi: 10.1038/onc.2011.118. [DOI] [PubMed] [Google Scholar]
- 34.Gupta RK, et al. Expansion of adult β-cell mass in response to increased metabolic demand is dependent on HNF-4α. Genes Dev. 2007;21:756–769. doi: 10.1101/gad.1535507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miettinen PJ, et al. Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal β-cell growth. Diabetes. 2006;55:3299–3308. doi: 10.2337/db06-0413. [DOI] [PubMed] [Google Scholar]
- 36.Butler AE, et al. Adaptive changes in pancreatic β-cell fractional area and β-cell turnover in human pregnancy. Diabetologia. 2010;53:2167–2176. doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieck S, Kaestner KH. Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010;21:151–158. doi: 10.1016/j.tem.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebert M, et al. Induction of platelet-derived growth factor A and B chains and overexpression of their receptors in human pancreatic cancer. Int. J. Cancer. 1995;62:529–535. doi: 10.1002/ijc.2910620507. [DOI] [PubMed] [Google Scholar]
- 39.Nyblom HK, et al. Apoptotic, regenerative, and immune-related signaling in human islets from type 2 diabetes individuals. J. Proteome Res. 2009;8:5650–5656. doi: 10.1021/pr9006816. [DOI] [PubMed] [Google Scholar]
- 40.Tallquist MD, Soriano P. Cell autonomous requirement for PDGFRα in populations of cranial and cardiac neural crest cells. Development. 2003;130:507–518. doi: 10.1242/dev.00241. [DOI] [PubMed] [Google Scholar]
- 41.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 42.Hara M, et al. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- 43.Sugiyama T, et al. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl Acad. Sci. USA. 2007;104:175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.