Abstract
Current paradigms assume that gas bubbles cannot be formed within yeasts although these workhorses of the baking and brewing industries vigorously produce and release CO2 gas. We show that yeasts produce gas bubbles that fill a significant part of the cell. The missing link between intracellular CO2 production by glycolysis and eventual CO2 release from cells has therefore been resolved. Yeasts may serve as model to study CO2 behavior under pressurized conditions that may impact on fermentation biotechnology.
Keywords: carbon dioxide, cell inclusions, fermentation, intracellular gas bubbles, NanoSAM, yeast
The fermentation process is at the heart of some of the most important biotechnological processes. This is demonstrated by the production of breads and alcoholic beverages where the fermenting capabilities of yeasts are exploited to produce ethanol and CO2. These conditions lead to cells capable of increased ethanol and CO2 production (Van Maris et al., 2001).
Although the yeast fermentation process is well established, no sign of CO2 bubbles inside cells has been reported. The lack of efforts to search for CO2 bubbles inside cells probably stems from the extensive work of Hemmingsen et al. (1990), whose research suggested that gas bubbles do not form within most types of cells even during gas supersaturation. This is ascribed to cytoplasmic resistance to gas bubble formation because of the increased structuring of water. Not even protein-enveloped gas vesicles that provide buoyancy to the cyanobacteria (Walsby, 1994) have been reported within yeast cells.
We used Nano scanning auger microscopy (NanoSAM) in both the scanning auger microscopy (SAM) and scanning electron microscopy (SEM) modes coupled with Argon-etching to show the gas bubble status inside individual brewer's yeast cells grown in fermentable and nonfermentable media, respectively.
The brewer's yeast, Saccharomyces pastorianus WS 34-70 (preserved at Cara Technology Limited, Leatherhead Enterprise Centre, Leatherhead, Surrey, UK), and the baker's yeast, Saccharomyces cerevisiae CBS 1171 NT (preserved at the Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands), were cultivated in 500-mL conical flasks containing 100 mL media. Cells were grown to stationary phase at 25 °C while shaking at 160 r.p.m. in fermentable and nonfermentable media (Sherman, 2002). Fermentable media used was Glucose Yeast Malt broth (10 g L−1 glucose, 3 g L−1 yeast extract, 3 g L−1 malt extract, and 5 g L−1 peptone). The nonfermentable medium used, favoring respiration, was Yeast Peptone Glycerol broth (30 mL L−1 glycerol, 10 g L−1 yeast extract, and 20 g L−1 peptone). Samples were drawn from cultures in growth and stationary phases and subjected to Light Microscopy (Axioplan, Zeiss, Germany) to test purity and observe granular appearance. Cells were harvested by centrifugation at 1450 g for 5 min, washed with equal volumes of de-ionized water, and prepared for SEM, NanoSAM, and transmission electron microscopy (TEM) according to the study by Swart et al. (2010) and Kock et al. (2011). Experiments were performed in at least triplicate, and reproducible results were obtained. The brewer's yeast was analyzed by Light Microscopy, SEM, NanoSAM, and TEM, while the baker's yeast was analyzed by Light Microscopy and TEM as described. In addition, a Microcystis aeruginosa culture was subjected to TEM analysis as described by Swart et al. (2010).
To trace CO2 bubbles inside cells, the metal salt ZnSO4.7H2O (Merck, Germany) was added to the culture media, containing brewer's yeasts in fermenting mode, to a final concentration of 2 mg L−1 where after cells were prepared as aforementioned for NanoSAM analysis.
With this unique new application of nanotechnology, we could demonstrate the presence of a maze of coalescing bubbles that filled a significant part of fermenting cells (Fig. 1a; Supporting Information, Movie S1). A significant increase in the number and size of cross-sectioned bubbles were observed on surfaces of fermenting yeasts after Argon-etching (Fig. 1a and b; Table S1).
Figure 1.
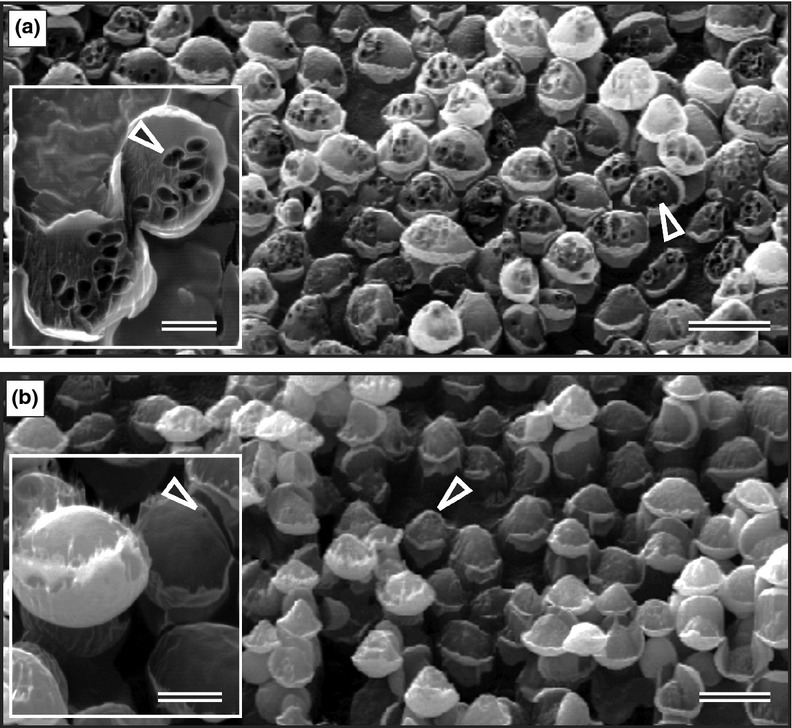
Bubbles (▸) within brewer's yeasts observed by NanoSAM Argon-etched SEM. (a) Fermenting cells with increased bubble formation. Insert: Cross-sectioned bubbles on etched surface. (b) Respiring yeasts with decreased bubble formation. Insert: Cross-sectioned bubbles on etched surface. Scale bars: (a, b)= 6 μm; (a, b) inserts = 2 μm.
To verify these striking results by an independent and nonrelated technique, we used the same TEM method with which we visualized protein-coated gas vesicles in a cyanobacterium (Fig. S1a), to show the naked cross-sectioned bubbles in brewer's yeast (Fig. S1b–d). The TEM-observed bubbles were of similar size to the bubbles observed in NanoSAM-SEM Argon-etched cells (Fig. a and b; Table S1). A similar trend in bubble formation was found in the model baker's yeast, S. cerevisiae, when grown in fermentable and nonfermentable media (Table S1). We could identify bubbles as granules in both the baker's and brewer's yeasts using Light Microscopy directly on living cells (Fig. S1e and f), thereby verifying both the NanoSAM- and TEM-demonstrated bubble formation in cells. This is reminiscent of gas vesicles observed by Light Microscopy in the cyanobacteria (Walsby, 1994). These results imply a wide distribution of the CO2 bubble formation phenomenon in fermenting Saccharomyces species. We found increased bubble production in young as well as older fermenting cells, suggesting that bubble production and fermentation were not strictly linked to cell age (Movie S1).
As expected, we observed ‘galvanized’ bubbles inside brewer's yeasts after the addition of zinc in the form of ZnSO4.7H2O to the growth medium. This suggested that zinc reacted with carbonic acid to form insoluble or weakly soluble metal bicarbonate at neutral pH in the cytoplasm (Ryan & Bauman, 1978). Carbonic acid should be produced at higher concentrations nearer to the boundary of the bubble if CO2 were present inside it, because of the reaction CO2 + H2O ⇌ H2CO3 in the cytoplasm (Fig. S1g and h). This mechanism may sequester metals to protect cells against toxicity when an excess of metal is present (Eide, 2006).
Based on the above and the fact that the observed empty bubbles were not collapsed by reported high intracellular osmotic pressure that may reach 2.1 MPa (Vella et al., 2012), we conclude that these intracellular bubble-like holes are gas bubbles containing CO2. We suggest that intracellular CO2 may eventually be secreted by pressure through the yeast cell wall to affect pressure homeostasis. This in turn should result in vigorous bubble release under diminished pressure (decompression) of the external environment, resulting in their coalescence and enlargement to visible bubbles of millimeter and centimeter size as is generally experienced in products of fermentation such as leavened bread, traditional beer, and champagne. Internal cell pressure is probably needed in these yeasts to keep bubble size at a minimum to decrease any adverse effects on cell function. Alternatively, these bubbles may be in nonpressurized transit as they are shipped out of the cell upon production.
An important question that needs to be answered is what stabilizes the bubbles in the cell? According to the study by Blasco et al. (2011), there are several cellular components of yeasts that are involved in foam formation and stabilization in fermented beverages. These surface active compounds may also be responsible for bubble stabilization inside the cell. Additionally, the potential meliorating effects of zinc and other inorganic ions on intracellular CO2 gas bubble structures warrant further investigation. The effects, control, and kinetics of intracellular gas bubbles at molecular and cellular levels as well as their impact on biotechnological processes should now be assessed. More generally, the yeast-bubble phenomenon may serve as a model that will provide a better understanding of the origins and effects of CO2 in biology, food, medicine, physics as well as the environment. We expect that this work will advance research on gas exchange in prokaryotic and eukaryotic cells and, for example, diving mammals where gas bubbles are formed in tissues under elevated pressurized conditions (Hooker et al., 2012).
Acknowledgments
This work was supported by the National Research Foundation (NRF) and the Claude Leon Foundation Postdoctoral Fellowship, South Africa. We acknowledge contributions of L. van der Westhuizen and D. Struwig, Science Writers, University of the Free State, South Africa.
Statement
Re-use of this article is permitted in accordance with the Terms and Conditions set out athttp://wileyonlinelibrary.com/onlineopen#OnlineOpen_Terms
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Bubble (▸) and gas vesicle (→) analyses in the brewer's yeast and cyanobacterium, Microcystis, respectively.
NanoSAM-SEM video showing sequential Argon-etching through a dense maze of gas bubbles inside fermenting brewer's yeast cells of different ages that is older mother cell (center) with attached younger daughter cells.
Cell size (diameter) and gas bubble (Bub) number and diameter dimensions inside brewer's and baker's yeasts grown in fermentable and non-fermentable media.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Blasco L, Viñas M, Villa TG. Proteins influencing foam formation in wine and beer: the role of yeast. Int Microbiol. 2011;14:61–71. doi: 10.2436/20.1501.01.136. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transport and the cellular trafficking of zinc. Biochim Biophys Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Hemmingsen BB, Ducoeur LC, Grapp SJ, Skaug V, Hemmingsen EA. Gas supersaturation tolerances in amoeboid cells before and after ingestion of bubble-promoting particles. Cell Biochem Biophys. 1990;17:37–51. doi: 10.1007/BF02989803. [DOI] [PubMed] [Google Scholar]
- Hooker SK, Fahlman A, Moore MJ, et al. Deadly diving? Physiological and behavioral management of decompression stress in diving mammals. Proc Biol Sci. 2012;279:1041–1050. doi: 10.1098/rspb.2011.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock JLF, Swart CW, Pohl CH. The anti-mitochondrial antifungal assay for the discovery and development of new drugs. Expert Opin Drug Discov. 2011;6:671–681. doi: 10.1517/17460441.2011.575358. [DOI] [PubMed] [Google Scholar]
- Ryan MP, Bauman JE. Thermodynamics of the zinc bicarbonate ion pair. Inorg Chem. 1978;17:3329–3331. [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 2002;350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- Swart CW, Swart HC, Coetsee E, Pohl CH, Van Wyk PWJ, Kock JLF. 3-D architecture and elemental composition of fluconazole treated yeast asci. Sci Res Essays. 2010;5:3411–3417. [Google Scholar]
- Van Maris AJA, Bakker BM, Brandt M, Boorsma A, Teixeira de Mattos MJ, Grivell LA, Pronk JT, Blom J. Modulating the distribution of fluxes among respiration and fermentation by over expression of HAP4 in Saccharomyces cerevisiae. FEMS Yeast Res. 2001;1:139–149. doi: 10.1111/j.1567-1364.2001.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Vella D, Ajdari A, Vaziri A, Boudaoud A. The indentation of pressurized elastic shells: from polymeric capsules to yeast cells. J R Soc Interface. 2012;9:448–455. doi: 10.1098/rsif.2011.0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE. Gas vesicles. Microbiol Rev. 1994;58:94–144. doi: 10.1128/mr.58.1.94-144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bubble (▸) and gas vesicle (→) analyses in the brewer's yeast and cyanobacterium, Microcystis, respectively.
NanoSAM-SEM video showing sequential Argon-etching through a dense maze of gas bubbles inside fermenting brewer's yeast cells of different ages that is older mother cell (center) with attached younger daughter cells.
Cell size (diameter) and gas bubble (Bub) number and diameter dimensions inside brewer's and baker's yeasts grown in fermentable and non-fermentable media.


