Abstract
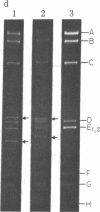
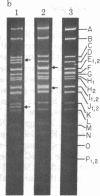
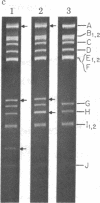
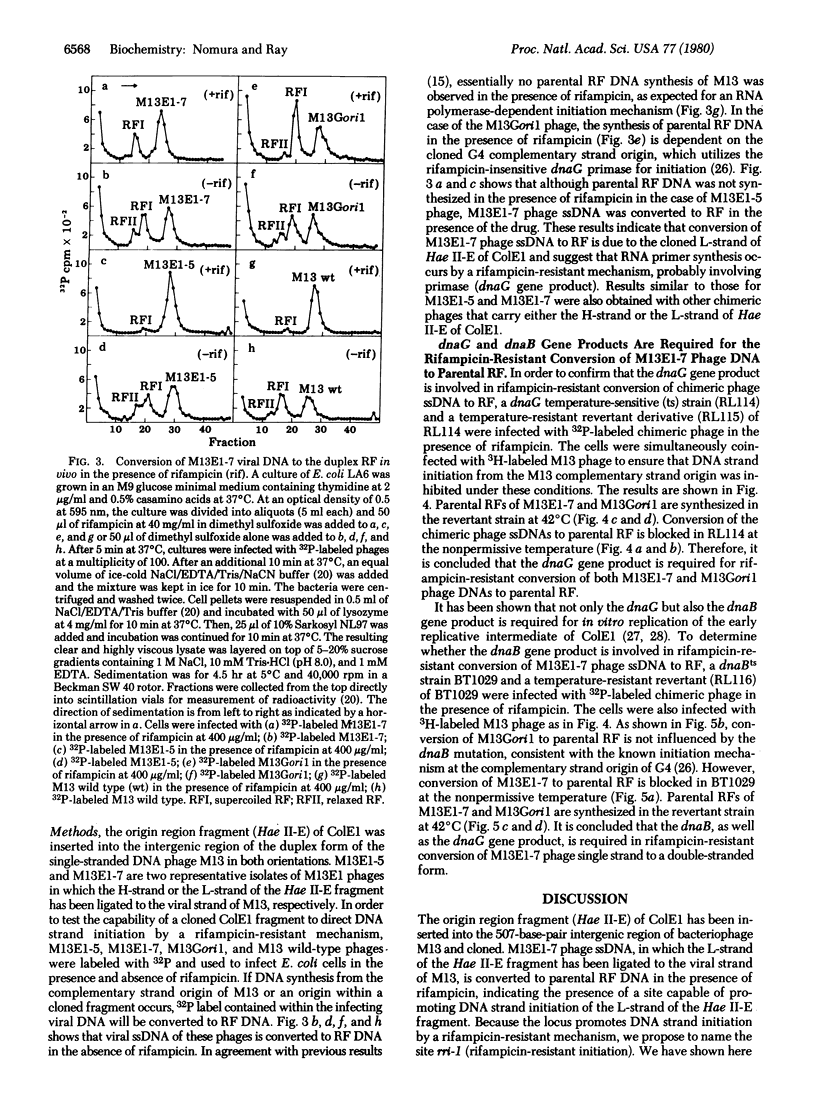
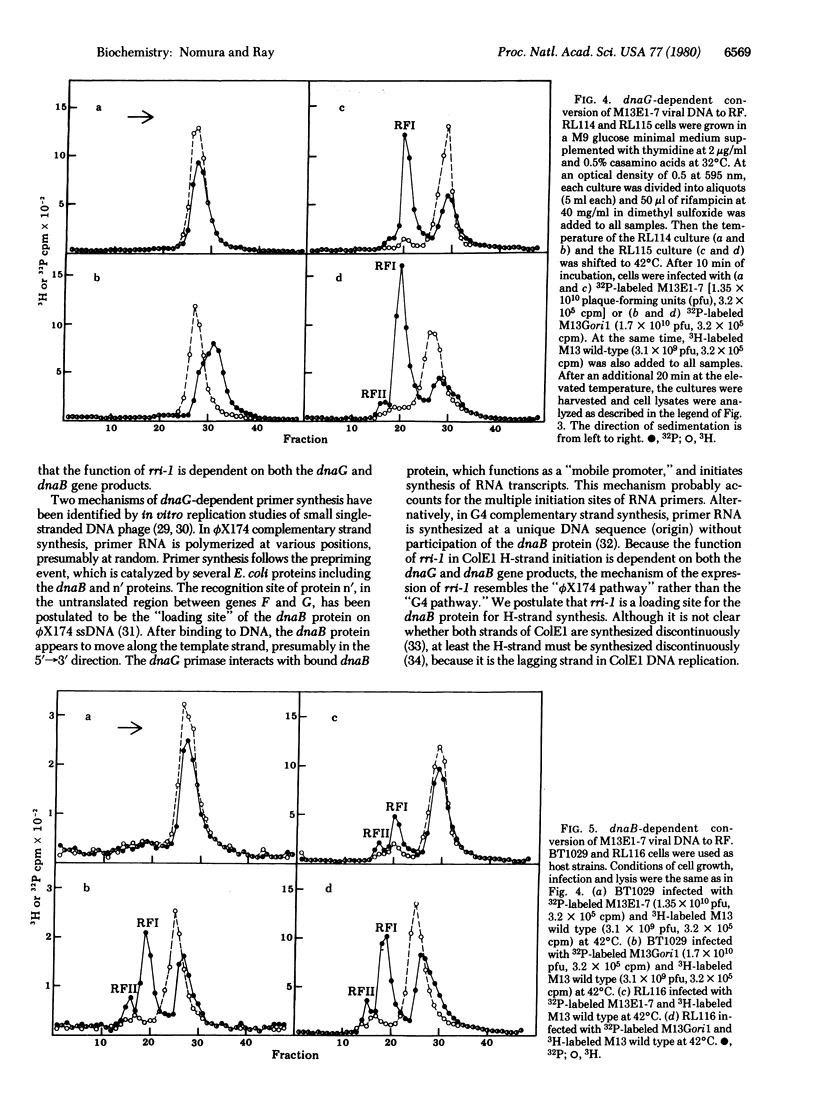
In order to investigate initiation of H-strand (lagging strand) replication of the plasmid ColE1, the origin region fragment (Hae II-E) of ColE1 was inserted into the intergenic region of filamentous DNA phage M13 and cloned. A site capable of promoting DNA strand initiation on a single-stranded DNA template has been detected on the L-strand (leading strand) of the cloned fragment. The site, named rri-1 rifampicin-resistant initiation), directs conversion of chimeric phage single-stranded DNA to parental replicative form in the presence of rifampicin, which blocks the function of the complementary strand origin of M13. The function of rri-1 is dependent on both the dnaG and dnaB gene products. It is postulated that rri-1 might be an initiation site for synthesis of the lagging DNA strand during unidirectional replication of ColE1 DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Tomizawa J. Ribonucleotide-deoxyribonucleotide linkages at the origin of DNA replication of colicin E1 plasmid. J Mol Biol. 1978 Mar 25;120(1):137–143. doi: 10.1016/0022-2836(78)90300-5. [DOI] [PubMed] [Google Scholar]
- Bouché J. P., Rowen L., Kornberg A. The RNA primer synthesized by primase to initiate phage G4 DNA replication. J Biol Chem. 1978 Feb 10;253(3):765–769. [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Ray D. S. Replication of bacteriophage M13. XIII. Structure and replication of cloned M13 miniphage. J Mol Biol. 1978 Oct 25;125(2):107–121. doi: 10.1016/0022-2836(78)90340-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Williams P., Helinski D. R. Plasmid ColE1 DNA replication in Escherichia coli strains temperature-sensitive for DNA replication. Mol Gen Genet. 1975;136(4):273–289. doi: 10.1007/BF00341713. [DOI] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S. Replication of bacteriophage M13. VI. Attachment of M13 DNA to a fast-sedimenting host cell component. Virology. 1971 Mar;43(3):647–664. doi: 10.1016/0042-6822(71)90289-3. [DOI] [PubMed] [Google Scholar]
- Hines J. C., Ray D. S. Construction and characterization of new coliphage M13 cloning vectors. Gene. 1980 Nov;11(3-4):207–218. doi: 10.1016/0378-1119(80)90061-x. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Replication of colicin E1 plasmid DNA in minicells from a unique replication initiation site. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2256–2259. doi: 10.1073/pnas.71.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J., LaVerne L. S., Ray D. S. Cloning and expression of the Escherichia coli replication origin in a single-stranded DNA phage. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6250–6254. doi: 10.1073/pnas.76.12.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni J., Ray D. S. Cloning of a functional replication origin of phage G4 into the genome of phage M13. J Mol Biol. 1979 Dec 25;135(4):863–878. doi: 10.1016/0022-2836(79)90516-3. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Katz L., Helinski D. R. Unidirectional replication of plasmid ColE1 DNA. Nature. 1974 Sep 27;251(5473):337–340. doi: 10.1038/251337a0. [DOI] [PubMed] [Google Scholar]
- McMacken R., Ueda K., Kornberg A. Migration of Escherichia coli dnaB protein on the template DNA strand as a mechanism in initiating DNA replication. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4190–4194. doi: 10.1073/pnas.74.10.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. R., Shlomai J., Kobori J., Bates D. L., Rowen L., McMacken R., Ueda K., Kornberg A. Enzymatic conversion of single-stranded phiX174 and G4 circles to duplex forms: discontinuous replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):289–293. doi: 10.1101/sqb.1979.043.01.035. [DOI] [PubMed] [Google Scholar]
- Oka A., Takanami M. Cleavage map of colicin E1 plasmid. Nature. 1976 Nov 11;264(5582):193–196. doi: 10.1038/264193a0. [DOI] [PubMed] [Google Scholar]
- Ray D. S., Schekman R. W. Replication of bacteriophage M13. I. Sedimentation analysis of crude lysates of M13-infected bacteria. Biochim Biophys Acta. 1969 Apr 22;179(2):398–407. [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. Replication of colicin E1 plasmid DNA in cell extracts. II. Selective synthesis of early replicative intermediates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1403–1407. doi: 10.1073/pnas.71.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. An Escherichia coli replication protein that recognizes a unique sequence within a hairpin region in phi X174 DNA. Proc Natl Acad Sci U S A. 1980 Feb;77(2):799–803. doi: 10.1073/pnas.77.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Lanka E., Schuster H. Replication of small plasmids in extracts of Escherichia coli: involvement of the dnaB and dnaC protein in the replication of early replicative intermediates. Mol Gen Genet. 1978 Jul 4;162(3):243–249. doi: 10.1007/BF00268849. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Scherzinger E., Lanka E. Replication of the colicin E1 plasmid in extracts of Escherichia coli: uncoupling of leading strand from lagging strand synthesis. Mol Gen Genet. 1979;177(1):113–120. doi: 10.1007/BF00267260. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Structure and replication of the colicin E1 plasmid. Curr Top Microbiol Immunol. 1978;83:93–156. doi: 10.1007/978-3-642-67087-9_3. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA in cell extracts. Origin and direction of replication. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2260–2264. doi: 10.1073/pnas.71.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. Two distinct mechanisms of synthesis of DNA fragments on colicin E1 plasmid DNA. Nature. 1975 Sep 18;257(5523):253–254. doi: 10.1038/257253a0. [DOI] [PubMed] [Google Scholar]
- Van Den Hondel C. A., Schoenmakers J. G. Cleavage maps of the filamentous bacteriophages M13, fd, fl, and ZJ/2. J Virol. 1976 Jun;18(3):1024–1039. doi: 10.1128/jvi.18.3.1024-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Nüsslein V., Otto B., Klein A., Bonhoeffer F., Herrmann R., Gloger L., Schaller H. Isolation and characterization of thermosensitive Escherichia coli mutants defective in deoxyribonucleic acid replication. J Bacteriol. 1973 Mar;113(3):1381–1388. doi: 10.1128/jb.113.3.1381-1388.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P. M., Hulsebos T. J., Schoenmakers J. G. Nucleotide sequence of the filamentous bacteriophage M13 DNA genome: comparison with phage fd. Gene. 1980 Oct;11(1-2):129–148. doi: 10.1016/0378-1119(80)90093-1. [DOI] [PubMed] [Google Scholar]