Abstract
Small renal masses (SRMs; ≤ 4 cm in dimension) have rapidly risen in incidence in recent decades and pose an increasingly common management dilemma in urology. SRMs are biologically heterogeneous and a wide variety of treatments exist with favorable oncologic outcomes. Active surveillance (AS) has emerged as a viable option for those not desiring surgery or those who are suboptimal candidates for surgery, with < 2% of patients progressing to metastatic disease in retrospective and prospective studies. This article reviews the current data regarding AS for SRM, operational considerations for an AS program, and criteria for safely selecting patients for this treatment strategy.
Key Words: Small renal mass, Renal cell carcinoma, Active surveillance
Small renal masses (SRMs; ≤ 4 cm in dimension) are an increasingly common clinical entity encountered by practicing urologists. Epidemiological studies indicate that SRMs account for nearly one-half of all newly diagnosed renal masses, largely based on incidental diagnosis during abdominal imaging.1 However, although these lesions have a radiographic appearance of malignancy (contrast enhancement of solid component), extirpative surgical series have demonstrated benign pathology in 20% to 30% of tumors ≤ 4 cm,2 and for those lesions that are renal cell carcinoma (RCC), the majority of tumors are low grade3 and unlikely to develop metastases.4 Given the rapid increase in the incidence of SRMs, the known biological heterogeneity of these masses, and a wide variety of treatment options, a number of rational approaches to their management, including extirpative surgery (radical and partial nephrectomy), ablative therapies (cryotherapy and radiofrequency ablation), and active surveillance (AS), exists.
Against this backdrop, a growing body of literature has emerged regarding AS for select patients with SRMs. A number of retrospective analyses, meta-analyses, and prospective studies quote the risk of metastatic progression while on AS to be < 2%.5–10 However, much of the data supporting AS are retrospective and must be evaluated with caution because such studies are limited by selection and reporting bias. Those undergoing AS frequently include older, sicker patients; outcomes are based on a composite of benign and malignant masses; and untreated patients who develop metastases and/or die from renal cancer may be lost to follow-up. Reflecting this theme, the 2009 American Urologic Association (AUA) “Guideline for Management of the Clinical T1 Renal Mass” recommends AS for high-surgical-risk patients and as an option for healthy patients desiring to avoid treatment and willing to assume the oncological risk of delaying intervention.11
Epidemiological Trends in SRMs
The incidence of kidney cancer has surged over the past few decades, from 28,000 in 1997 to 58,000 in 2010; the increased use of axial imaging12 has led to increased detection. This increasing incidence has been accompanied by a dramatic stage migration, with SRMs accounting for the largest proportion of the incident rise in renal malignancies and nearly 40% of all renal tumors diagnosed.1 The interpretation of these trends is complicated by the concomitant observation of relatively stable deaths from kidney cancer in the United States (11,000–13,000 from 1997–2010), only decreasing modestly in the past few years.12 It is believed that the rising incidence of SRMs reflects a combination of early-stage malignancies destined to become clinically significant advanced cancers and lesions of benign histology or indolent behavior of unclear clinical significance. Although multiple variables contribute to the perplexing trends in kidney cancer diagnosis and mortality, it is almost certain that a number of treated SRMs lack lethal potential, raising the question of possible overdiagnosis and overtreatment.
Interestingly, autopsy series indicate that, whereas renal tumors are present in 2% to 3% of the population and SRMs in ≤ 1%, approximately 30% and 12% of SRMs have locally advanced disease and metastases, respectively.13 A study comparing a historical autopsy series (1955–1960) to a contemporary series (1991–2001) found that, although the overall proportion of renal masses found at autopsy were similar, the number of masses found incidentally at autopsy is declining and the rate of occult kidney cancers per 100 autopsies had not changed—implying that better detection prior to death may not necessarily translate into the improved detection of clinically significant tumors.14
The Natural History of SRMs
As stated previously, extirpative surgical series indicate that 20% to 30% of SRMs are benign entities2 and of the lesions that are RCC, 70% to 80% are low-grade, early-stage lesions believed to have little malignant potential.3,4,7,15 Supporting the indolent nature of these tumors, several meta-analyses have demonstrated a slow interval growth rate for most tumors under surveillance, on the order of 0.2 to 0.3 cm/year with 23% to 33% of tumors demonstrating a zero growth rate while under observation.5–7 In addition, reports of metastases while on surveillance for SRMs are rare.7 Therefore, sufficient retrospective data suggest that most SRMs behave in an indolent fashion and can be safely observed.
The remaining 20% to 30% of SRMs are malignant tumors with potentially aggressive features; 15% to 25% of SRM RCCs are high-grade lesions (Fuhrman grade 3–4). Locally advanced disease (≥ pT3) has been documented in 10% to 40% of SRMs, and 3% to 12% present with or will develop metastatic disease.3,15,16 Although a small proportion of patients may present with synchronous metastatic disease and an SRM, the existing literature implies that the risk of developing metastatic disease while undergoing AS for a SRM is even smaller—on the order of 1%.6,7 Consequently, synchronous and metachronous metastases may be different entities and patients who present without distant disease are more likely to have indolent tumors with little metastatic potential. Therefore, an efficacious AS program should recognize the heterogeneity of SRM biology and seek to distinguish indolent lesions from aggressive tumors based on clinical parameters so that ideally, no patients die of RCC but rather of competing causes.
Efficacy and Oncologic Outcomes for Patients Undergoing AS
Despite a lack of Level I evidence, a number of robust, retrospective series demonstrate favorable outcomes for contemporary patients undergoing AS. More than 70 peer-reviewed articles appear within Medline on the topic of AS for SRM and a recent meta-analysis included 18 retrospective series comprising 880 patients.7 A number of retrospective AS cohorts demonstrated a 0% to 5.7% risk of progression to metastasis while on surveillance with prospective studies and meta-analyses showing an overall rate of metastasis on the order of 1%.5–10 Although a direct comparison of AS to intervention is lacking, historic recurrence rates and cancer-specific survival following treatment (regardless of the intervention) are in the range of 90% to 95% and 95% to 99% at 5 years, respectively—indicating both the indolent nature of T1 lesions and the difficulty in comparing AS and primary treatment options.11
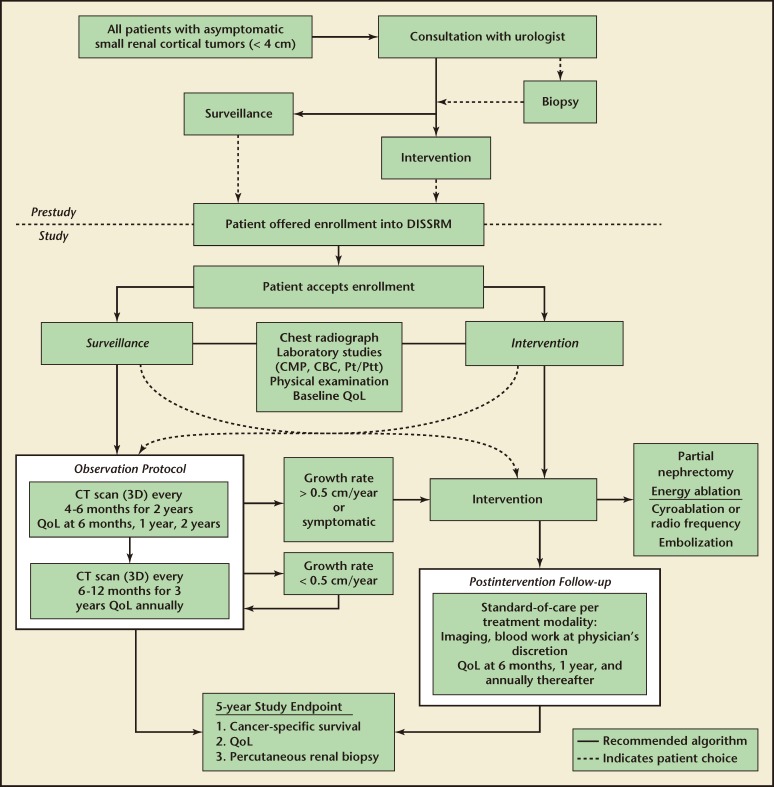
The Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) Registry is administered through Johns Hopkins University (Baltimore, MD) and was developed as a multi-institutional prospective clinical study to report the outcomes of patients undergoing AS and immediate intervention for newly diagnosed SRMs (Figure 1). The registry is powered based on noninferiority principles to demonstrate equivalent cancer-specific survival rates at 5 years for AS and immediate intervention. Through 34 months of enrollment, with a median follow-up of 1 year (range, 3–32 months), 3 of 89 patients undergoing AS died of causes not related to RCC and no patient developed metastases or died of disease. Three of 187 patients undergoing immediate intervention have died, 1 of RCC. The patient who died of RCC had a tumor with sarcomatoid features resected with negative margins that recurred distantly. Although lacking a comparison arm, a similar prospective cohort of 82 AS patients showed one patient (1.2%) progressing to metastatic disease, seven patients (8.6%) dying of competing causes, and no patients dying from RCC over a median follow-up of 36 months.8 Although immature, results from the DISSRM Registry and similar prospective studies promise to improve our understanding and utilization of AS in select patients.
Figure 1.
Management algorithm for patients with small renal masses (≤ 4 cm) according to the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM). Registry protocol. 3D, three-dimensional; CBC, complete blood count; CMP, comprehensive metabolic panel; CT, computed tomography; Pt/Ptt, prothrombin time/partial thromboplastin time; QoL, quality of life.
Selection Criteria
Although a number of groups make general recommendations for the selection of patients for AS including increased age, decreased life expectancy, suitability for surgery, and decreased risk of metastatic disease,7,11,17 there is a paucity of data supporting or defining specific objective criteria for selection of patients for AS. Some of the important considerations include patient and tumor characteristics as they may impact life expectancy, malignant/metastatic risk, the likelihood of renal replacement therapy after treatment, and the feasibility of nephron-sparing surgery (NSS). Several studies indicate that AS is safe in the elderly18,19 and/or patients with extensive comorbidities precluding surgery.20 Prognostic models created from extirpative series indicate that age and sex modulate the likelihood of having a benign SRM, with younger women and older men having an increased likelihood of a benign pathology.21,22 It is also known from data extrapolated from patients with Von Hippel-Lindau disease, surgical series, and the aforementioned retrospective AS cohorts that the risks of RCC, high-grade RCC, and metastatic disease increase dramatically when tumors reach 3 cm.4,15,16,23 Tumor complexity, measured by various statistics including RENAL nephrometry score, may enable some prediction of tumor histology and grade,24 and can be used to determine the appropriateness of NSS; indeed, low-complexity tumors are generally more suitable for NSS.25 In addition, although the majority of patients present incidentally, the presence of symptoms (predominantly hematuria or flank pain) can indicate advanced disease.26 Finally, patients with chronic kidney disease (CKD) or contributing comorbidities (diabetes mellitus, hypertension, smoking) are at the highest risk of renal replacement therapy following intervention and may benefit from a period of AS.
One of the objectives of the DISSRM Registry was to develop a scoring system based on existing literature5–8 and empiric evidence to assist in the identification of patients most suitable for AS. Major criteria are considered to be age > 65 years, Eastern Cooperative Oncology Group (ECOG) score > 1, Charlson Comorbidity Index Score > 2, greatest tumor diameter < 3 cm, and moderate to severe CKD. Minor criteria are prior abdominal surgery, incidental presentation, nephrometry score > 10, and minor CKD or a contributing comorbidity. By assigning 2 points to each major criterion and 1 point to each minor criterion, scoring was applied to the cohort to characterize the distribution of scores within this population. At 3 years of enrollment, 89 patients electing AS had at least 1 major criterion and 85% had 2 or more major criteria. Nearly 50% of patients undergoing AS had a DISSRM score ≥ 7 and only 1 patient had a score < 4, whereas 20% of patients undergoing intervention had a DISSRM score < 4 and 69% had a score < 7. Therefore, based on early reports of this registry, the DISSRM score is a promising means to risk stratify patients for AS versus intervention wherein patients with a score ≥ 7 can be considered favorable candidates for AS, those with a score ≤ 3 may be favorable candidates for surgery, and those with an intermediate score (4–6) may benefit from either management strategy.10 As the registry continues to accrue patients and these criteria are further tested, they will be refined to better select patients for AS.
Operational Considerations
Following a thorough consultation, a patient and physician may choose AS as the best option for the management of an SRM. The AUA recommends that this consultation include a discussion of the small but real risk of cancer progression, loss of a window of opportunity for NSS, lack of curative treatments for metastatic RCC (mRCC), limitations of renal biopsy, and deficiencies in the current literature.11 However, no guidelines or recommendations exist for imaging modality, timing of surveillance images, and the use of renal biopsy or triggers for intervention.
The main trigger for intervention is believed to be growth rate (GR). Oda and colleagues found a greater GR in the primary tumor of patients with mRCC when compared with localized tumors (1.7 cm vs 0.54 cm/year; P = .02).27 Kato and associates demonstrated a significantly higher GR in high-grade RCC compared with low-grade tumors (0.93 cm/year vs 0.28 cm/year; P = .01.28 Of patients reported to develop metastases while on AS, GR has been high, ranging from 1.3 to 2.9 cm/year.6,8 However, a number of studies have demonstrated zero or slow (< 0.5 cm/year) GR for tumors of malignant pathology.5,6 In addition, benign lesions, specifically oncocytoma, have been shown to demonstrate rapid growth.29 A number of biological factors may modulate GR and add confusion to the utility of GR in observing patients with SRMs. For instance, in the prospective study by Mason and colleagues, larger tumors (> 2.45 cm) demonstrate a faster GR than smaller tumors.8 However, several retrospective analyses have failed to find a relationship between primary tumor size and GR.6,20 Kouba and colleagues demonstrated that patients aged < 60 years had more rapidly growing tumors than those aged > 60 years.9 Finally, changes in tumor volume have been touted as more accurately reflecting growth kinetics and the biologic aggressiveness of an SRM; however, consistent with Gompertzian growth kinetics, smaller tumors are demonstrated to grow faster volumetrically.30 A recent pooled analysis of the AS literature demonstrated increased age, larger greatest initial tumor dimension and estimated volume, and higher linear and volumetric GR to predict metastatic progression.7 Although a number of important factors may indicate the malignant potential of an SRM, it is clear that progression to metastatic disease is exceptionally low in tumors that demonstrate slow or no GR and remain < 3 cm. Conversely, although tumors may demonstrate variable GR, the majority that progress to metastasis exceed 3 cm and often become cT1b (> 4 cm) tumors prior to or at the diagnosis of metastasis.
In the retrospective literature, on average, patients undergo five to six imaging evaluations over a period of 29 to 41 months yielding an approximate average rate of imaging every 6 months.7 The majority of retrospective studies use computed tomography (CT) and magnetic resonance imaging (MRI), with ultrasonography (US) used sparingly. The prospective study by Mason and colleagues recommended CT, MRI, or US imaging every 6 months.8 The DISSRM protocol recommends a high-quality axial image (CT or MRI with contrast) at enrollment to be followed by CT, MRI, or US every 4 to 6 months for 2 years and then every 6 to 12 months thereafter (Figure 1).10 It is our experience that, given conflicting reports regarding the risk of secondary malignancy,31 few patients are willing to undergo serial exposure to ionizing radiation in the form of CT scan. As GR is the main trigger for delayed intervention, we approve of serial US examination with confirmation of a change in growth with axial imaging if indicated. To better determine the aggressiveness of a new lesion, we recommend the first serial image be performed within 4 to 6 months with the caveat that GR may be exacerbated by even a small change in tumor diameter seen over a short period of time. It is known that tumor diameter measurements may vary by up to 3 mm between and among observers.32 Consequently, wide fluctuation is seen and little prognostic value is gained by small changes in tumor diameter seen on the first surveillance image. In addition, small fluctuations in tumor diameter may disproportionately impact estimates of tumor volume. Therefore, we recommend a second interval image to more accurately gauge GR and indicate the need for intervention depending on patient suitability for AS.
Percutaneous renal biopsy (PRB) can determine SRM pathology and impact the decision for AS or intervention. Historical series demonstrate variable accuracy on the order of 70%, nondiagnostic rates around 30%, and rates of serious complications around 5%, preventing its widespread acceptance. A recent meta-analysis by Volpe and colleagues reviewed 49 publications regarding the use of PRB in the diagnosis and management of renal tumors.33 They found a low rate of complications and improved rates of detection with sensitivity ranging from 70% to 100%, specificity at 100%, and a cumulative accuracy > 90% for needle core biopsies. A recent publication by a center performing a high-volume of PRB found biopsy tissue to aid in the diagnosis of nearly 90% of patients.34 However, PRB is unreliable for tumor grade35 and performs less well in tumors < 3 cm.36 Because most SRMs are low-grade indolent RCC and can safely undergo a period of AS, one could argue that there is little clinical utility in PRB for patients with a clear indication for AS or intervention. However, we find that PRB may provide additional information in patients without a clear indication for surgery or AS (DISSRM score 3–7) to aid in the decision and consultation process. In addition, we recognize that PRB provides tissue which, in addition to blood and urine, may provide biomarkers to improve the detection and surveillance of SRM.
Conclusions
SRMs ≤ 4 cm are commonly seen in clinical practice and represent a large proportion of newly diagnosed renal masses. Given recent epidemiologic trends and studies of the natural history of SRMs, most are believed to be indolent tumors with little potential for metastatic progression. AS has emerged as an alternative to extirpative or ablative treatments for these masses and should involve an informed decision by patient and physician based on patient and tumor characteristics and the calculated risk of metastatic progression. Ongoing prospective studies, including the DISSRM Registry, will provide additional information regarding the use and timing of serial imaging in patients undergoing AS.
Main Points.
Small renal masses (SRMs; ≤ 4 cm in dimension) are an increasingly common clinical scenario for practicing urologists and physicians with contemporary epidemiological studies indicating that SRMs account for nearly one-half of all renal masses diagnosed.
SRMs are biologically heterogeneous with 20% to 30% being benign lesions; 70% to 80% of malignancies are low-grade; early stage lesions are believed to behave in an indolent manner; and 20% to 30% are potentially aggressive tumors.
Active surveillance (AS) has emerged as a viable option for the management of SRMs with < 2% of patients progressing to metastatic disease in retrospective and prospective studies.
Ongoing prospective studies, including the Delayed Intervention and Surveillance for Small Renal Masses Registry, will provide additional information regarding the use and timing of serial imaging in patients undergoing AS.
Footnotes
The authors report no real or apparent conflicts of interest.
References
- 1.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176(6 Pt 1):2397–2400. doi: 10.1016/j.juro.2006.07.144. discussion 2400. [DOI] [PubMed] [Google Scholar]
- 2.Kutikov A, Fossett LK, Ramchandani P, et al. Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology. 2006;68:737–740. doi: 10.1016/j.urology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Rothman J, Egleston B, Wong YN, et al. Histopathological characteristics of localized renal cell carcinoma correlate with tumor size: a SEER analysis. J Urol. 2009;181:29–33. doi: 10.1016/j.juro.2008.09.009. discussion 33–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson RH, Hill JR, Babayev Y, et al. Metastatic renal cell carcinoma risk according to tumor size. J Urol. 2009;182:41–45. doi: 10.1016/j.juro.2009.02.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunkle DA, Egleston BL, Uzzo RG. Excise, ablate or observe: the small renal mass dilemma-a meta-analysis and review. J Urol. 2008;179:1227–1233. doi: 10.1016/j.juro.2007.11.047. discussion 1233–1234. [DOI] [PubMed] [Google Scholar]
- 6.Chawla SN, Crispen PL, Hanlon AL, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–431. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 7.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118:997–1006. doi: 10.1002/cncr.26369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason RJ, Abdolell M, Trottier G, et al. Growth kinetics of renal masses: analysis of a prospective cohort of patients undergoing active surveillance. Eur Urol. 2011;59:863–867. doi: 10.1016/j.eururo.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Kouba E, Smith A, McRackan D, et al. Watchful waiting for solid renal masses: insight into the natural history and results of delayed intervention. J Urol. 2007;177:466–470. doi: 10.1016/j.juro.2006.09.064. discussion 470. [DOI] [PubMed] [Google Scholar]
- 10.Pierorazio PM, Hyams ES, Trock BJ, et al. A multiinstitutional prospective clinical trial of Delayed Intervention and Surveillance for Small Renal Masses: the DISSRM registry. 2012 doi: 10.1016/j.eururo.2015.02.001. (submitted for publication) [DOI] [PubMed] [Google Scholar]
- 11.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–1279. doi: 10.1016/j.juro.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 12.American Cancer Society, authors. Cancer Facts and Figures 2010. [Accessed March 9, 2012]. http://www.cancer.org/acs/groups/content/@nho/documents/document/acspc-024113.pdf.
- 13.Kozłowska J, Okoń K. Renal tumors in postmortem material. Pol J Pathol. 2008;59:21–25. [PubMed] [Google Scholar]
- 14.Mindrup SR, Pierre JS, Dahmoush L, Konety BR. The prevalence of renal cell carcinoma diagnosed at autopsy. BJU Int. 2005;95:31–33. doi: 10.1111/j.1464-410X.2005.05243.x. [DOI] [PubMed] [Google Scholar]
- 15.Thompson RH, Kurta JM, Kaag M, et al. Tumor size is associated with malignant potential in renal cell carcinoma cases. J Urol. 2009;181:2033–2036. doi: 10.1016/j.juro.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kates M, Korets R, Sadeghi N, et al. Predictors of locally advanced and metastatic disease in patients with small renal masses [published online ahead of print September 20, 2011] BJU Int. doi: 10.1111/j.1464-410X.2011.10553.x. [DOI] [PubMed] [Google Scholar]
- 17.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 18.Abouassaly R, Lane BR, Novick AC. Active surveillance of renal masses in elderly patients. J Urol. 2008;180:505–508. doi: 10.1016/j.juro.2008.04.033. discussion 508–509. [DOI] [PubMed] [Google Scholar]
- 19.Beisland C, Hjelle KM, Reisaeter LA, Bostad L. Observation should be considered as an alternative in management of renal masses in older and comorbid patients. Eur Urol. 2009;55:1419–1427. doi: 10.1016/j.eururo.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Lamb GW, Bromwich EJ, Vasey P, Aitchison M. Management of renal masses in patients medically unsuitable for nephrectomy-natural history, complications, and outcome. Urology. 2004;64:909–913. doi: 10.1016/j.urology.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Pierorazio PM, Murphy AM, Benson MC, McKiernan JM. Gender discrepancies in the diagnosis of renal cortical tumors. World J Urol. 2007;25:81–85. doi: 10.1007/s00345-006-0124-9. [DOI] [PubMed] [Google Scholar]
- 22.Lane BR, Babineau D, Kattan MW, et al. A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol. 2007;178:429–434. doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 23.Duffey BG, Choyke PL, Glenn G, et al. The relationship between renal tumor size and metastases in patients with von Hippel-Lindau disease. J Urol. 2004;172:63–65. doi: 10.1097/01.ju.0000132127.79974.3f. [DOI] [PubMed] [Google Scholar]
- 24.Kutikov A, Smaldone MC, Egleston BL, et al. Anatomic features of enhancing renal masses predict malignant and high-grade pathology: a preoperative nomogram using the RENAL Nephrometry score. Eur Urol. 2011;60:241–248. doi: 10.1016/j.eururo.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simhan J, Smaldone MC, Tsai KJ, et al. Objective measures of renal mass anatomic complexity predict rates of major complications following partial nephrectomy. Eur Urol. 2011;60:724–730. doi: 10.1016/j.eururo.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee CT, Katz J, Fearn PA, Russo P. Mode of presentation of renal cell carcinoma provides prognostic information. Urol Oncol. 2002;7:135–140. doi: 10.1016/s1078-1439(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 27.Oda T, Miyao N, Takahashi A, et al. Growth rates of primary and metastatic lesions of renal cell carcinoma. Int J Urol. 2001;8:473–477. doi: 10.1046/j.1442-2042.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Suzuki T, Suzuki Y, et al. Natural history of small renal cell carcinoma: evaluation of growth rate, histological grade, cell proliferation and apoptosis. J Urol. 2004;172:863–866. doi: 10.1097/01.ju.0000136315.80057.99. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi S, Fernandes KA, Finelli A, et al. Most renal oncocytomas appear to grow: observations of tumor kinetics with active surveillance. J Urol. 2011;186:1218–1222. doi: 10.1016/j.juro.2011.05.080. [DOI] [PubMed] [Google Scholar]
- 30.Crispen PL, Boorjian SA, Lohse CM, et al. Outcomes following partial nephrectomy by tumor size. J Urol. 2008;180:1912–1917. doi: 10.1016/j.juro.2008.07.047. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Punnen S, Haider MA, Lockwood G, et al. Variability in size measurement of renal masses smaller than 4 cm on computerized tomography. J Urol. 2006;176(6 Pt 1):2386–2390. doi: 10.1016/j.juro.2006.07.142. discussion 2390. [DOI] [PubMed] [Google Scholar]
- 33.Volpe A, Kachura JR, Geddie WR, et al. Techniques, safety and accuracy of sampling of renal tumors by fine needle aspiration and core biopsy. J Urol. 2007;178:379–386. doi: 10.1016/j.juro.2007.03.131. [DOI] [PubMed] [Google Scholar]
- 34.Leveridge MJ, Finelli A, Kachura JR, et al. Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol. 2011;60:578–584. doi: 10.1016/j.eururo.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Blumenfeld AJ, Guru K, Fuchs GJ, Kim HL. Percutaneous biopsy of renal cell carcinoma underestimates nuclear grade. Urology. 2010;76:610–613. doi: 10.1016/j.urology.2009.09.095. [DOI] [PubMed] [Google Scholar]
- 36.Rybicki FJ, Shu KM, Cibas ES, et al. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003;180:1281–1287. doi: 10.2214/ajr.180.5.1801281. [DOI] [PubMed] [Google Scholar]