Abstract
The most recent guidelines on prostate cancer screening from the American Urological Association (2009), the National Comprehensive Cancer Network (2011), and the European Association of Urology (2011), as well as treatment and advances in disease monitoring, have increased the androgen deprivation therapy (ADT) population and the duration of ADT usage as the first-line treatment for metastatic prostate cancer. According to the European Association of Urology, gonadotropin-releasing hormone (GnRH) agonists have become the leading therapeutic option for ADT because they avoid the physical and psychological discomforts associated with orchiectomy. However, GnRH agonists display several shortcomings, including testosterone (T) surge (“clinical flare”) and microsurges. T surge delays the intended serologic endpoint of T suppression and may exacerbate clinical symptoms. Furthermore, ADT manifests an adverse-event spectrum that can impact quality of life with its attendant well-documented morbidities. Strategies to improve ADT tolerability include a holistic management approach, improved diet and exercise, and more specific monitoring to detect and prevent T depletion toxicities. Intermittent ADT, which allows hormonal recovery between treatment periods, has become increasingly utilized as a methodology for improving quality of life while not diminishing chronic ADT efficacy, and may also provide healthcare cost savings. This review assesses the present and potential future role of GnRH agonists in prostate cancer and explores strategies to minimize the adverse-event profile for patients receiving ADT.
Key words: Prostate cancer; Androgen deprivation therapy; Gonadotropin-releasing hormone, agonists
The management of prostate cancer from initial screening to the treatment of castrate-resistant prostate cancer (CRPC) has a myriad of options and associated controversies. There are a multitude of questions and related controversies involving initial screening and the therapeutic management of low-risk disease. Does prostate-specific antigen (PSA) screening save lives? Should Gleason 6 histopathology be redefined as a nonmalignant process, due to its exceedingly low risk for the development of recurrent disease after definitive localized therapy? Are we overtreating low-risk prostate cancer? What is the optimal treatment for clinically localized prostate cancer?
Androgen deprivation therapy (ADT) has been incorporated into the treatment of prostate cancer since the 1940s, when Huggins and Hodges first reported that surgical and medical castration promotes regression of metastatic prostate cancer with dramatic disease palliation.1 Nonetheless, controversies related to ADT for prostate cancer are abundant. When should ADT be initiated, if at all, during PSA relapse (ie, biochemical recurrence? Is there an advantage to initiating maximal androgen blockade (MAB) versus monotherapy? Does ADT increase cardiovascular mortality? Does intermittent androgen deprivation (IAD) therapy improve quality of life without compromising survival? What level of testosterone (T) suppression is most consistent with castration? Does the level of T suppression achieved following administration of gonadotropin-releasing hormone (GnRH) agonists influence survival? Are all GnRH agonists the same with regard to T suppression? Do GnRH antagonists offer any advantage? What are the optimal dosaging intervals and modes of administration for ADT?
Until recently, there was less controversy regarding the management of CRPC because the therapeutic armamentarium was limited. There are now increasing numbers of choices available for treating CRPC in addition to GnRH agonists. In 2012, chemotherapy, immune therapy, and secondary hormonal therapy were approved for the treatment of CRPC.2 For example, in 2010, the US Food and Drug Administration (FDA) approved sipuleucel-T (Provenge®; Dendreon Corporation, Seattle, WA), a cancer treatment vaccine using a patient’s own immune cells. GnRH antagonists, by immediately stopping luteinizing hormone (LH) secretion, produce rapid T suppression without the initial LH and T surge. Other clinical trials are investigating new treatments such as molecularly targeted agents and biomarkers. However, there are unanswered questions regarding the cost/benefit analysis and safety of these new treatments as well as a paucity of data regarding the most ideal sequencing and/or combination implementation strategies.
ADT is first-line treatment for advanced/metastatic prostate cancer and recommended for use before, during, or after definitive radiotherapy for intermediate- and high-risk localized prostate cancer. ADT is also commonly used for shorter intervals to reduce prostate gland volume in patients contemplating brachytherapy, cryotherapy, and high-intensity focused ultrasound therapy, especially when gland volume exceeds 50 g; however, this utilization does not have an FDA- or European Medicines Agency-approved indication. ADT does not have an FDA-labeled indication for PSA relapse. ADT may be accomplished by surgical castration (bilateral orchiectomy) or via pharmacologic therapy, for example, GnRH agonists and antagonists. According to the American Urological Association (AUA) guidelines, “primary ADT may be employed with the goal of providing symptomatic control of prostate cancer for patients in whom definitive treatment with surgery or radiation is not possible or acceptable.”3 According to the European Association of Urology (EAU), GnRH agonists have become the leading therapeutic option for ADT because they avoid the physical and psychological discomforts associated with orchiectomy.4,5 However, GnRH agonists display several shortcomings including T surge (“clinical flare”) and microsurges.4
Irrespective of how ADT is achieved, T suppression causes adverse effects, such as hot flushes, osteoporosis, and possible cardiometabolic effects.6 The introduction of PSA testing has increased the prevalence of prostate cancer patients diagnosed at earlier stages. The consequent increase in ADT utilization highlights the importance of strategies to help reduce side effects associated with T suppression, as well as strategies to avoid unnecessary screening, overdetection, and overtreatment.
Some of the above controversies related to ADT in the management of prostate cancer are addressed, specifically, PSA screening for prostate cancer and its impact upon ADT utilization; new insights related to the adverse-event profile associated with ADT; the role of intermittent hormone therapy (IHT); measuring T levels and whether the level of T suppression following GnRH agonists influences survival; and differences between GnRH agonists. In addition, this article assesses the potential future role of GnRH agonists in prostate cancer therapy. Novel strategies to minimize the risk of adverse effects of T suppression are also reviewed.
GnRH Agonists
GnRH is a decapeptide that is produced by the hypothalamus and regulates serum T levels through its effects on LH release by the pituitary gland.7 The various commercially available GnRH agonists are all modifications of the GnRH decapeptide by amino acid substitutions or chemical alterations of existing amino acids. GnRH agonists can cause a T flare in response to increased stimulation of LH; continuous stimulation of the GnRH receptors promotes desensitization of the GnRH receptors, resulting in T suppression.8 Commercially available GnRH agonists differ in their duration of action (1 month to 1 year), route of administration (intramuscular or subcutaneous injection or subcutaneous implant), and requirement for reconstitution.
It is generally thought that GnRH agonists have similar efficacy and side effects because all of the commercially available agents have been shown to effectively reduce serum T levels to < 50 ng/dL, which historically was the level thought to be consistent with surgical castration.9 Using modern assay techniques, it is now recognized that the median T level achieved following surgical castration is ∼15 ng/dL, with a range between 10 to 30 ng/dL.9 In a review of the literature, Perachino and colleagues10 reported that between 13% and 42% of men with prostate cancer fail to achieve castrate levels of androgens (< 20 ng/dL as per the study, as compared with standard < 50 ng/dL) after initiating leutinizing hormone-releasing hormone (LHRH) therapy, depending on the upper limit of serum T. The clinical benefits of maintaining T levels < 20 ng/dL versus < 50 ng/dL have not been prospectively studied. A prospective, randomized, and carefully designed trial contemplating clinical progression and specific mortality is necessary as the primary endpoint would be required to confirm these findings and reassess the cutoff level.
Mechanism of Action/Pharmacological Profile
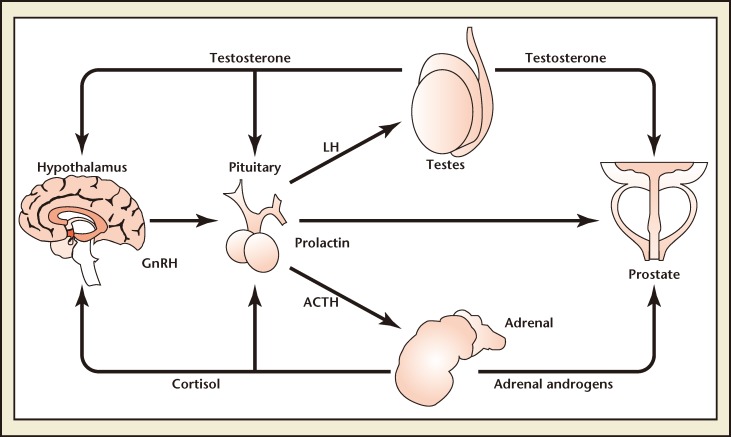
GnRH, which is secreted in pulses from the hypothalamus, stimulates release of LH, along with adrenocorticotropic hormone and prolactin, from the pituitary gland. LH subsequently stimulates secretion of T, predominantly by the testes (Figure 1).11,12 Sustained pituitary overstimulation eventually downregulates and desensitizes GnRH receptors, causing a decrease in hormone levels.13 The overall effect of ADT on hormone levels in prostate cancer differs between treatments. Orchiectomy reduces T and dihydrotestosterone (DHT) but is accompanied by significant rises in both LH and follicle-stimulating hormone (FSH).14,15 GnRH agonists cause an initial surge in LH, FSH, T, and DHT; over time these hormones are suppressed. 16 However, FSH gradually rises during GnRH agonist treatment and results in a FSH “escape.”17
Figure 1.
Gonadotropin-releasing hormone (GnRH), secreted in pulses from the hypothalamus, stimulates release of luteinizing hormone (LH) from the pituitary gland, along with adrenocorticotropic hormone (ACTH) and prolactin. LH subsequently stimulates secretion of testosterone, predominantly by the testes. Damber JE, Acta Oncologica, 2005;44:605-609, copyright © 2005, Informa Healthcare. Reproduced with permission of Informa Healthcare.74
Hence, these distinct modes of action of GnRH agonists produce different clinical effects. The initial agonist-induced T surge can exacerbate clinical symptoms (clinical flare) in advanced prostate cancer.18 An appreciable proportion of patients (∼ 12%) receiving GnRH agonists fail to achieve castrate T levels ≤ 50 ng/dL.19 T microsurges associated with repeat injections also occur with agonists.19 In a study with goserelin, microsurges (T surges above a castration threshold of 18.5 ng/dL after ≥ 1 repeat injection) occurred in 17.7% to 27% of patients.20 The clinical implications of microsurges are currently unclear.
Loss of GnRH receptor sensitivity during long-term agonist therapy can allow renewed T production manifesting as a late breakthrough T escape.19,21
GnRH agonists usually cause partial FSH suppression.22,23 FSH stimulates prostate cancer cell growth in vitro.24 FSH receptors are present on prostate tumors25 and the surface of tumor blood vessels 26; they are expressed at higher levels on prostate versus normal tissue.27 FSH signaling may also contribute to progression of CRPC.28 FSH promotes RANK (receptor activator of nuclear factor κB) expression on CD141+ cells, indicating the acquisition of osteoclast precursor cell characteristics.29 The exact significance of the role of FSH is still being defined. Long-term T control has been suggested to reduce mortality risk among patients with metastatic disease.10 In 129 patients with metastatic prostate cancer receiving a GnRH agonist, those with high T levels at 6 months had a 1.33-fold increase in mortality risk.10
How May Changing Prostate Cancer Screening Guidelines Impact Use of AdT?
PSA is the most utilized biomarker for diagnosing prostate cancer. It is a serine protease inhibitor that was discovered and purified in 1979.30 Thirteen years later, two large studies reported the utility of using PSA screening for prostate cancer.31,32 In one study, approximately 15% of men of 1249 over the age of 50 years were found to have an elevated PSA, defined by a serum level > 4.0 ng/mL. Prostate cancer was diagnosed in slightly more than 30% of men with an elevated PSA. Soon thereafter, PSA screening gained widespread acceptance in the United States. According to Zeliadt and colleagues, it has been estimated that approximately 50% of the male US population between the ages of 55 and 74 years undergo PSA screening over a 6- to 7-month period.33
Prior to the widespread acceptance of PSA screening, the overwhelming majority of prostate cancers were advanced at the time of diagnosis.34 PSA screening has resulted in dramatic stage migration. The overwhelming majority of cases diagnosed today are clinically localized, suggesting that there is no clinical or radiologic evidence that the cancer has metastasized beyond the prostate.34 Based on the protracted natural history of the disease, one could speculate that it would require decades for PSA screening to maximally impact mortality rates for prostate cancer. Beginning in the early to mid-1990s, mortality rates from prostate cancer have consistently been on the decline.35 Since the peak mortality in 1991, there has been a 40% reduction in prostate cancer mortality that many have attributed to PSA screening.36
In 2011, two large screening studies were reported with conflicting conclusions. The PLCO (Prostate, Lung, Colon and Ovarian) study randomized men to PSA screening versus no mandated PSA screening. 37 With a median follow-up of 6.3 years, there was no significant prostate cancer survival advantage attributable to PSA screening. This study has been used to condemn PSA screening, implying it is an instrument that subjects men unnecessarily to biopsies and ineffective treatment. A critical review shows this study was methodologically flawed. First, half of the men in the unscreened group underwent PSA screening before randomization. Second, half of the men in the unscreened group underwent subsequent PSA testing. Third, among the men with an elevated PSA, many did not undergo biopsy. Fourth, a median follow-up of 6.3 years is grossly inadequate to determine screening impact on mortality. Follow-up information has continued to show no statistically significant difference between prostate cancer mortality rates in the intervention arm and the control arm.38
The European Randomized Study of Screening for Prostate Cancer (ERSPC) had less contamination than the PLCO study because a smaller proportion of men in the unscreened cohort underwent screening prior to randomization or during the study.39 The median follow-up was 9 years. Overall, prostate cancer mortality was reduced by 20%. Upon correcting for contamination, PSA screening decreased prostate cancer mortality by 31% in actually screened patients.40
The Scandinavian Prostate Cancer Screening Study was recently reported and received far less fanfare than the PLCO and ERSPC studies.41 The Scandinavian study is the most informative and relevant PSA screening study because contamination was low and follow-up was 14 years. The Scandinavian study reported a 40% reduction in prostate cancer mortality attributable to PSA screening, which is consistent with the declining prostate cancer mortality statistics seen in the United States.36
Despite the compelling prostate cancer survival advantage of prostate cancer screening, the US Preventative Task Force (USPTF) made a general recommendation against PSA screening because they interpreted the literature to show that PSA screening produced more harm than benefit.42 The debate regarding the value of PSA screening played out in the lay press for several weeks. How the primary care physician will react to the controversy regarding PSA screening is unclear. There is also uncertainty as to whether the Center for Medicare Services (CMS) will continue to reimburse for PSA screening; if the USPTF recommends against PSA screening, then CMS may decide to cease PSA reimbursement. The ultimate decision regarding coverage for PSA screening will certainly influence the proportion of men who will be screened in the future.
A randomized study comparing radical prostatectomy (RP) versus watchful waiting for localized disease diagnosed in the pre-PSA screening era reported that 40% of the men undergoing RP received ADT.43 There is no doubt that prostate screening decreases prostate cancer mortality, but this occurs at the expense of subjecting many men with low-risk disease to unnecessary treatment. Rather than summarily abandoning prostate cancer screening, there is a need to rationally risk stratify newly diagnosed cancers in order to maintain the reduction in prostate cancer mortality while limiting unnecessary treatment.
There has been a decline in the use of ADT for prostate cancer due in part to fewer men developing metastatic disease as the result of screening and subsequent curative localized therapies. There has also been a higher threshold for administering these treatments due to increased awareness of potentially significant adverse events. If the diagnostic milieu is turned back to the pre-screening era, this may ironically, and unfortunately, result in more ADT utilization. More men will once again present with locally advanced or metastatic disease that is no longer amenable to localized cure and will be more appropriately managed with ADT.
Adverse Effects of ADT
T suppression is associated with bone loss,44 which may also be influenced by other factors such as obesity, age, and sedentary lifestyle. Moreover, ADT and attendant bone demineralization is associated with an increased risk of skeletal fracture.45 Skeletal fractures are of particular concern, given their documented correlation with decreased overall survival in men with prostate cancer.46
ADT has also been correlated with several metabolic complications. GnRH agonists can increase central abdominal weight gain and overall fat body mass and decrease lean body mass and muscle size,47 as well as decrease insulin sensitivity.48 ADT can also increase total and low-density lipoprotein cholesterol and triglycerides.49 It is believed metabolic changes associated with ADT may have significant consequences for cardiovascular health; GnRH agonists have been associated with increased risk of incident diabetes, possibly coronary heart disease, acute myocardial infarction, and sudden cardiac death.50 The FDA recently highlighted an increased risk of diabetes, heart attack, stroke, and sudden death with GnRH agonists based on a review of published studies; warnings of such risks must now be added to GnRH agonist labels.51 In contrast, most but not all studies appear to indicate that orchiectomy is not associated with greater risk of cardiovascular events.50,52,53 The final verdict on the cardiovascular risks of ADT is still clouded by a lack of level 1 evidence, required by long-term, prospective, blinded trials. These findings are confirmed by Nguyen and colleagues,54 where in a pooled analysis of randomized trials of unfavorable-risk prostate cancer, ADT use was not associated with an increased risk of cardiovascular death but was associated with a lower risk of prostate cancer-specific mortality and all-cause mortality.
By assessing a patient’s susceptibility to such effects, a comprehensive (holistic) treatment program can be tailored to maximize ADT efficacy while protecting against adverse effects.55 Patients receiving ADT should be counseled to help them recognize, prevent, and manage side effects; they should be encouraged towards a healthy lifestyle including a heart-healthy diet and manageable regular exercise program.19 Measures to promote bone health include weight-bearing (resistance) exercise, smoking cessation, vitamin D and calcium supplementation, and moderate alcohol consumption.55,56 Bisphosphonates (which increase bone mineral density [BMD] in patients treated with ADT6) should be considered in patients with fractures or BMD T scores of −22.5 or less.57 Based on one study, denosumab, a monoclonal antibody agonist RANK ligand, has increased BMD and reduced the incidence of new vertebral fractures among men receiving ADT for nonmetastatic prostate cancer.58 Denosumab was FDA approved in November 2011 for prevention of osteoporosis for men receiving ADT. Clinicians should carefully assess fracture risk (eg, via the World Health Organization fracture risk assessment tool [FRAX]) and BMD should be monitored at regular intervals via dual-energy X-ray absorptiometry when deemed appropriate to clinically alter therapeutic options.56
The morbidities of ADT should be considered in the context of the existing comorbidities of the patient when choosing palliative ADT. As per the AUA guidelines, ADT may be used for the palliation of symptomatic patients with more extensive or poorly differentiated tumors, whose life expectancy is too short to benefit from treatment with curative intent.3 When making treatment decisions about ADT, physicians and patients should discuss and review existing guidelines for lifestyle modifications, and the increased risk of adverse effects such as osteoporosis, fracture risks, obesity, alteration in lipids, diabetes and cardiovascular disease.5,59 Patients should be monitored with periodic follow-up evaluations including assessment of blood pressure, lipid profile, and glucose level. Patients with cardiac disease should receive appropriate secondary preventive measures as recommended by existing guidelines.5,53
Intermittent Hormonal Therapy
IAD has been touted as a possible alternative for some patients to minimize ADT side effects while maintaining anti-tumor efficacy.60 Although some evidence suggests that IAD performs at least as well as continuous androgen deprivation (CAD) in terms of overall survival, and perhaps better in terms of side effects, IAD still remains experimental and unproven regarding long-term implications of disease progression and survival impact.61 In fact, many organizations such as the National Comprehensive Cancer Network have been skeptical in their practice guidelines regarding IAD, stating that “the long term efficacy [of IAD] remains unproven.”5
In light of the experimental nature of IAD in the United States, optimal thresholds for stopping/resuming ADT are empirical, and the best candidates for IAD have not been completely defined. According to Gomella and colleagues,62 during IAD, active treatment periods are separated by periods without treatment. On-treatment periods usually last 6 to 9 months or until a PSA nadir < 4 ng/mL.62 Off-treatment periods are more variable, with treatment reinstated if PSA increases. In contrast, the EAU does not consider IAD an investigational therapy, and has formulated guidelines for locally advanced or relapsing disease; these suggest stopping treatment only if there is no clinical progression (a clear PSA response: PSA < 4 ng/mL in metastatic disease, or 0.5 ng/mL in relapsing disease), and resuming treatment if there is either clinical progression or a PSA value above a predetermined fixed threshold (usually 4 ng/mL in nonmetastatic patients or 10–15 ng/mL in metastatic patients).4 As there is no consensual standardization of IAD protocols and guidelines in the United States, it has been difficult to compare data and conclusions from clinical trials.
However, the potential advantages of IAD, which include improved quality of life, the theoretical possibility of delaying hormone resistance, and possible reduction in healthcare costs, warrant further exploration.63 American clinical trials have documented the efficacy of IAD. An intergroup, phase III, randomized, controlled trial study from Klotz and colleagues61 showed that IAD was not inferior to complete androgen blockade with respect to overall survival in men with rising PSA after radical therapy for prostate cancer.61 This study outcome is similar to two previous pivotal trials and a systematic review of IAD based on the EAU guidelines.61,64,65
Based on limited randomized studies, whereas CAD appears appropriate for patients with advanced metastatic prostate cancer, according to a study by Shore and Crawford IAD may be appropriate for many patients who reach castrate T levels (< 20 ng/dL) and a PSA nadir of < 4.0 ng/dL during induction therapy.63 However, the clinical benefits of maintaining T levels < 20 ng/dL versus < 50 ng/dL have not been prospectively studied. Carefully designed, prospective, randomized, phase III trials are needed for further assessment, with results clarifying issues such as selection of the most appropriate patients to receive IAD, optimal thresholds for stopping/resuming therapy, suitable ADT agents, and confirmation of the efficacy of IAD to mitigate serious adverse events.
Does T Level Influence Survival Following ADT?
Controversy previously existed regarding the clinical significance of circulating androgens following treatment with GnRH agonists. There is evidence that very low levels of T and its metabolites may elicit prostate cancer progression. Although the treatment is controversial, some experts believe that MAB (medical or surgical castration in combination with an antiandrogen) achieves superior survival over GnRH agonists alone.66 It is unclear whether this modest observed survival advantage is attributable to prevention of the T flare or T escape, or suppression of adrenal androgens. There is new evidence that prostate cancers themselves are capable of synthesizing endogenous T.67 A recent clinical study showing that treatment with a CYP17 inhibitor such as abiraterone, either alone or with glucocorticoids, resulted in significant antitumor activity in patients with androgen independent progression (AIP) both who had and had not received chemotherapy.68
The goal of pharmacologic castration is to achieve T suppression comparable with surgical castration. Historically, it was assumed that surgical castration achieved T levels < 1.5 pmol/L because this was the lower limit for assaying T levels at the time.69 Therefore, GnRH agonists were assumed to achieve equivalence to surgical castration if they achieved T levels < 50 ng/dL. Using newer chemiluminescent techniques,70 it was subsequently shown in a single study that surgical castration achieves median T levels equivalent to 15 ng/dL.9 Ideally, this should be the goal of GnRH agonists. There are two recent studies suggesting that consistent T suppression < 50 ng/dL following GnRH agonists can be associated with superior survival. Morote and colleagues21 and Perachino and colleagues10 hypothesized that progression and survival following administration of GnRH agonists is related to the degree of T suppression.
Morote and colleagues21 conducted a review based on a retrospective analysis of 73 nonmetastatic prostate cancer patients who were treated with ADT (GnRH agonist) and had both their serum PSA and T levels measured between 8 and 12 weeks after administration of the GnRH agonist. Over one-third of patients (38.4%) also received continued treatment with bicalutamide (MAB). All men were treated with a 3-month depot GnRH agonist for at least 1 year and had at least three serum T levels measured.21 All patients received bicalutamide, 50 mg, daily for 2 weeks prior to initiating GnRH agonist treatment. 21 Of the 73 evaluated, daily bicalutamide was maintained in 28 men throughout the course of ADT. In the subset of daily bicalutamide patients, the mean follow-up was 54 months (range, 13–240 months). Overall, 32% and 25% of men had T levels between 20 and 50 ng/dL and > 50 ng/dL, respectively.21 The endpoint was development of AIP, defined as three consecutive rising PSA levels. During a followup period (a mean follow-up of 51 months; range, 12–240 months), androgen-independent progression (AIP) events were identified and correlated with breakthrough T increases of 50 ng/dL (classic level) and 20 ng/dL (surgical castration level). The lowest serum T threshold that was able to significantly distinguish groups related with survival free of AIP was 32 ng/dL.21 A univariate analysis was performed to determine if age, initial clinical stage, biopsy Gleason score, administration of MAB, initial PSA, and T break- through > 50 ng/mL predicted the survival free of AIP (Table 1).21
Table 1.
Univariate Analysis Relating Dichotomic Variables Included in the Study and Survival Free of Androgen-Independent Progression
| Log Rank | P Value | |
| Age (≤ 70 years vs > 70 years) | 3.27 | .0723 |
| Initial clinical stage (T1-2 vs T3-4) | 0.01 | .9095 |
| Biopsy Gleason score (2–6 vs 7–10) | 6.71 | .0096 |
| LHRH agonist (± bicalutamide) | 0.99 | .3188 |
| Initial PSA (≤ 20 ng/mL or less vs > 20 ng/mL) | 0.10 | .7483 |
| Testosterone breakthrough increases > 20 ng/dL | 1.78 | .1819 |
| Testosterone breakthrough increases > 50 ng/dL | 7.74 | .0054 |
LHRH, luteinizing hormone-releasing hormone; PSA, prostate-specific antigen.
Reproduced with permission from Morote J et al.21
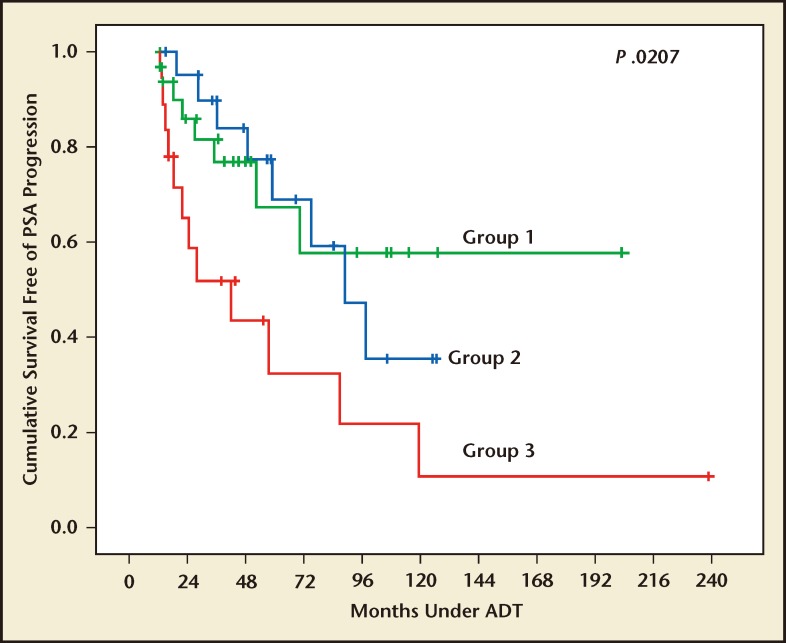
Survival free of AIP was compared for three groups: Group 1, all T levels < 20 ng/dL; Group 2, any T between 20 and 50 ng/dL; and Group 3, any T > 50 ng/dL. The mean time to develop AIP for Groups 1, 2, and 3 was 106 months, 90 months, and 72 months, respectively. 21 The Kaplan-Meier plots showing survival free of AIP for the three groups (Figure 2) confirms that the level of serum T suppression is a predictor of survival free of AIP. The mean survival free of AIP in patients with breakthrough increases greater than 32 ng/dL was 88 months, whereas it was 137 months in those without breakthrough increases. According to Morote and colleagues, these results show that a routine measurement of serum T should become part of clinical practice when evaluating the effects of hormonal therapy. A reasonable option to detect these breakthrough increases would be to monitor T levels at PSA determination.
Figure 2.
Survival free of androgen-independent progression (AIP) according to serum testosterone behavior. Group 1, patients with all three serum testosterone determinations less than < 20 ng/dL. Group 2, patients with breakthrough increases between 20 and 50 ng/dL. Group 3, patients with breakthrough increases . 50 ng/dL. ADT, androgen deprivation therapy; PSA prostate-specific antigen. Reproduced with permission from Morote J et al.21
In order to confirm findings of the study by Morote and colleagues, 21 a prospective, randomized, and carefully designed trial to assess clinical progression and mortality as primary endpoints would be required to reassess T cutoff level, as the clinical benefits of maintaining T levels < 20 ng/dL versus < 50 ng/dL have not been prospectively studied. Key issues remain unresolved-standardization of assays, a universally accepted definition of optimal castrate T levels, and clear evidence regarding the clinical benefits of androgen suppression, continuous or intermittent protocol, and maintenance of acceptable castrate levels of T.
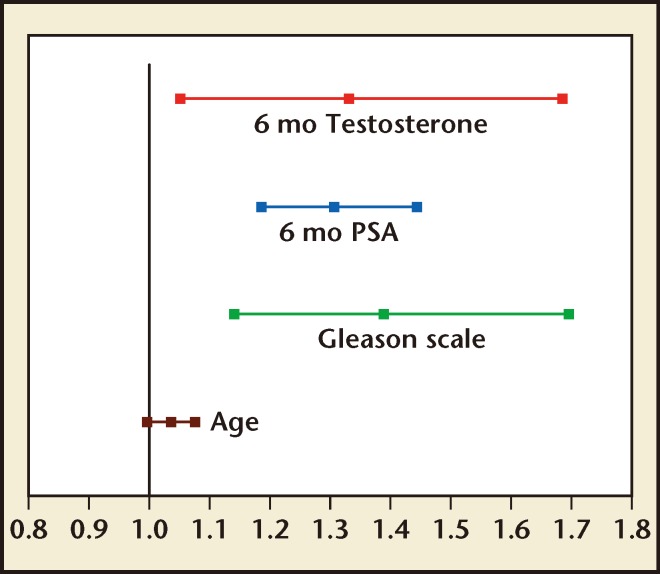
Perachino and colleagues10 followed T levels after initiation of ADT with GnRH agonists. The study was based on a retrospective review of 129 newly diagnosed ADT-naive patients with metastatic bone-only prostate cancer who were treated with a 3-month depot of goserelin every 12 weeks. Serum PSA and T were measured on the same day of goserelin administration. The mean and range of follow-up was 47.5 months and 6 to 120 months, respectively. Serum T and PSA data were taken retrospectively from patients on 3 months of ADT (n = 129) every 12 weeks for the duration of the study. After a mean follow-up of 47.5 months, 55% (n = 71) of patients died and 45% (n = 58) of patients survived. Overall, 25% and 31% of men receiving goserelin exhibited a T level > 50 ng/dL or between 20 and 50 ng/dL, respectively. A Cox regression model was utilized to determine predictors of prostate cancer survival. Gleason score, 6-month serum PSA, and 6-month T were independent predictors of cancer-specific survival. The hazard ratio and related 95% confidence interval are shown in Figure 3. PSA values were shown as natural logarithms and serum T levels as squared values, respectively, and represented on a logarithmic survivor function plot which showed a continuous direct relationship between serum T levels and cancer-specific survival.
Figure 3.
Hazard ratio and related 95% confidence interval. PSA prostate-specific antigen. Reproduced with permission from Perachino M et al.10
This study suggests a direct correlation between the risk of death and T levels during ADT. A prospective, randomized, and carefully designed trial contemplating clinical progression and specific mortality as the primary endpoint would be required to confirm these findings and reassess the cutoff level, as the clinical benefits of maintaining T levels < 20 ng/dL versus < 50 ng/dL have not been prospectively studied.
Do GnRH Agonists Have Unique Properties?
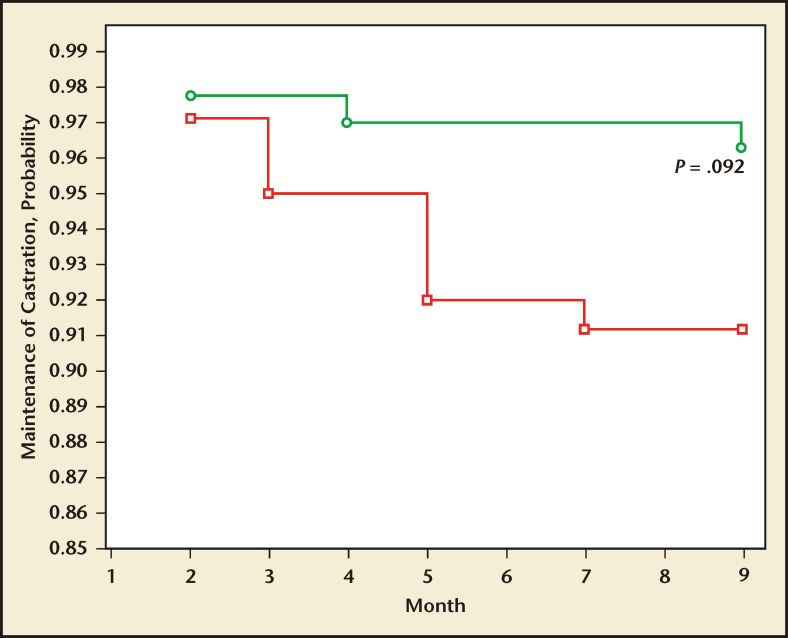
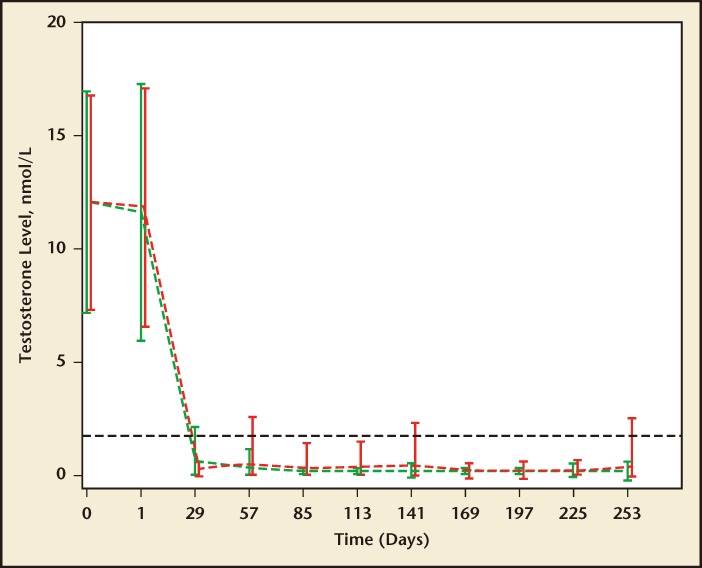
Heyns and colleagues71 compared T suppression in 140 and 137 men receiving monthly leuprolide acetate versus triptorelin pamoate, respectively. The primary endpoint of the trial was the percentage of men whose serum T declined and remained at or below castration level (1.735 nmol/L or 50 ng/dL) during the 9-month treatment duration. The probability of maintenance of castration T levels is shown at monthly intervals throughout the 9-month study (Figure 4). A Kaplan-Meier survival analysis for the maintenance of castration levels measured 3.75 mg triptorelin pamoate or 7.5 mg leuprolide. The cumulative maintenance of castration levels were 96% and 91% for triptorelin pamoate and leuprolide, respectively (P = .092). In this study, a greater proportion of men maintained medical castration with triptorelin pamoate at 29 days.
Figure 4.
The maintenance of castration in men treated with triptorelin pamoate, 3.75 mg (green line, open circles), or leuprolide acetate, 7.5 mg (red line, open squares), for 9 months (Kaplan-Meier survival analysis). Reproduced with permission from Heyns CF et al.71
Secondary endpoints (9-month survival rate, mean LH concentrations during treatment, median bone pain values as measured by the visual analog scale, quality of life, median PSA concentrations) were equivalent in both groups except for the 9-month overall survival rate, which was 97% in the triptorelin pamoate group versus 90.5% in the leuprolide acetate group (P = .033). Mean and cumulative castration maintenance rates between 29 and 253 days were equivalent between the treatment groups. Mean serum T levels were analyzed every 28 days in the leuprolide acetate and triptorelin pamoate treatment groups. These levels fell below the predefined levels for medical castration (≤ 50 ng/dL in this trial) at 29 days and 57 days for 91% and 98% of subjects in the triptorelin pamoate group and 99% and 97% of the subjects in the leuprolide group, respectively. Although the mean difference was significant between the leuprolide acetate and triptorelin pamoate treatment groups at 29 days, it was not significant at 57 days (Figure 5).
Figure 5.
Mean serum testosterone levels were analyzed every 28 days in 277 men receiving androgen deprivation with either leuprolide acetate or triptorelin pamoate. These levels fell below the predefined levels for medical castration (50 ng/dL) at 29 days and 57 days for 91% and 98% of subjects in the triptorelin pamoate group and 99% and 97% of the subjects in the leuprolide group. Although the mean difference was significant between the groups at 29 days, it was not significant at 57 days. Reproduced with permission from Heyns CF et al.71
The results from Heyns and colleagues71 indicate that triptorelin pamoate may induce castration at a slightly slower rate than leuprolide acetate, but triptorelin pamoate maintains castration at least as effectively as leuprolide. A possible reason for this outcome may be dosing differences between triptorelin pamoate and leuprolide acetate. The repeated exposure to the higher leuprolide acetate dose (7.5 mg), as compared with the lower triptorelin pamoate dose (3.75 mg) may more likely cause an escape resulting from weak desensitization of pituitary GnRH receptors.71 The hypothesis is supported by an insignificant trend throughout the study, evidenced where there was more LH stimulation with leuprolide than triptorelin pamoate at day 85 (98% vs 94%) and 169 days (98% vs 93%).71 Additional data support this claim with individual patients; there were fewer triptorelin pamoate than leuprolide patients who achieved castration at 29 days but had T escape at least once between months 2 and 9 of the study (4 vs 11, respectively); this result correlated with the pharmacological data. Assessment of T levels over 24 hours in a patient subset showed that 3 of 15 patients treated with leuprolide escaped castrate T levels as compared to none of the 14 patients treated with triptorelin pamoate.
Moreover, there is no evidence that the slower onset of castration caused deleterious effects. The higher 9-month survival rate in the triptorelin pamoate than in the leuprolide group is intriguing, but longterm data are required to determine the clinical significance of this observation. It has been observed that men on depot GnRH agonists, at castrate levels of T (as previously defined, < 50 ng/dL), may demonstrate subtle increases in PSA activity as serum T moves from nadir levels upward to 50 ng/dL.9
The information obtained from Heyns and colleagues71 is significant, as few comparative studies have examined the ability of different GnRH agonists to lower serum T levels. Yri and colleagues72 retrospectively compared serum T levels in 40 and 25 men receiving 3-month depot of either leuprolide acetate or goserelin acetate, respectively. Four of the men receiving leuprolide (10%) and none on goserelin failed to achieve castration levels of T. The failure rate of achieving castrate levels was not significant as per the study rates. Castrate levels of T in this study was 81 ng/dL, which is higher than the 50 ng/dL that has been used in other studies. (The 81 ng/dL corresponds with the upper limit of normal that was seen in women in one study.73) This study showed that although most patients reached castrate T levels, its retrospective nature prevented a true comparison between the effectiveness of leuprolide and goserelin.
Summary
According to EAU guidelines, ADT is the mainstay of treatment for advanced prostate cancer and largely comprised of GnRH agonists.4 Avoiding T surges may help avoid cancer stimulation and worsening of clinical status, as well as providing more rapid relief of cancer-related symptoms.74 PSA recurrence often precedes clinically detectable recurrence by years, and effective PSA control is associated with improved overall survival.75–77
As ADT is associated with a range of side effects (eg, bone loss, metabolic and possible cardiovascular complications), a variety of strategies should be considered to effectively manage these effects. In particular, this should include adoption of a more comprehensive treatment approach with counseling on diet and exercise as well as periodic monitoring of bone density and metabolic and cardiovascular parameters. In addition, some patients may benefit from IAD, which minimizes ADT adverse events; allowing hormonal recovery between treatment periods may improve quality of life. IAD may provide efficacy comparable with CAD but with improved tolerability. Nevertheless, consensus guidelines regarding a universally accepted definition of optimal castrate T levels, as well as evidence regarding the clinical benefits, safety, and tolerability of optimal androgen suppression, remain for further study and discussion. Because the clinical benefits of maintaining T levels < 20 ng/dL versus < 50 ng/dL have not been prospectively studied, further prospective, well-designed studies are needed to prove the hypothesis.
Main Points.
Androgen deprivation therapy (ADT) is first-line treatment for advanced/metastatic prostate cancer and recommended for use before, during, or after definitive radiotherapy for intermediate- and high-risk localized prostate cancer.
Prostate-specific antigen is the most utilized biomarker for diagnosing prostate cancer.
There has been a decline in the use of ADT for prostate cancer due in part to fewer men developing metastatic disease as the result of screening and subsequent curative localized therapies. There has also been a higher threshold for administering these treatments due to increased awareness of potentially significant adverse events.
Gonadotropin-releasing hormone agonists have been associated with increased risk of incident diabetes, possibly coronary heart disease, acute myocardial infarction, and sudden cardiac death. Patients receiving ADT should be counseled to help them recognize, prevent, and manage side effects; they should be encouraged towards a healthy lifestyle including a heart-healthy diet and manageable regular exercise program.
Although some evidence suggests that intermittent androgen deprivation (IAD) performs at least as well as continuous androgen deprivation in terms of overall survival, and perhaps better in terms of side effects, IAD still remains experimental and unproven regarding long-term implications of disease progression and survival impact. However, the potential advantages of IAD, which include improved quality of life, the theoretical possibility of delaying hormone resistance, and possible reduction in healthcare costs, warrant further exploration.
Consensus guidelines regarding a universally accepted definition of optimal castrate testosterone levels, as well as evidence regarding the clinical benefits, safety, and tolerability of optimal androgen suppression, remain for further study and discussion.
Footnotes
Dr. Herbert Lepor is a member of the Speakers’ Bureau for Amgen, is a consultant for Watson, and is consultant/advisor to Serenity Pharma and Quanterix. Dr. Neal D. Shore is a consultant/researcher for Ferring Pharmaceuticals, Watson, Endo Pharmaceuticals, Amgen, Janssen, Medivation, and Sanofi Oncology. The authors thank Lloyd Zimmerman, MD, MPH, for assistance in preparing this manuscript.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 2.Garcia JA, Rini BI. Castration-resistant prostate cancer: many treatments, many options, many challenges ahead. Cancer. 2012;118:2583–2593. doi: 10.1002/cncr.26582. [DOI] [PubMed] [Google Scholar]
- 3.American Urological Association, authors. Prostate Cancer: Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. [Accessed April 4, 2012]. http://www.auanet.org/content/clinical-practice-guidelines/clinical-guidelines/main-reports/proscan07/content.pdf.
- 4.Heidenreich A, Bolla M, Joniau S, et al. Guidelines on Prostate Cancer. [Accessed April 4, 2012]. http://www.uroweb.org/gls/pdf/08_Prostate_CancerJuly6th.pdf.
- 5.NCCN Clinical Practice Guides in Oncology: Prostate Cancer. [Accessed April 4, 2012]. http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 6.Sharifi N, Gulley JL, Dahut WL. An update on androgen deprivation therapy for prostate cancer. Endocr Relat Cancer. 2010;17:R305–R315. doi: 10.1677/ERC-10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schally AV, Kastin AJ, Arimura A. Hypothalamic follicle-stimulating hormone (FSH) and luteinizing hormone (LH)-regulating hormone: structure, physiology and clinical studies. Fertil Steril. 1971;22:703. [PubMed] [Google Scholar]
- 8.Auclair C, Kelly PA, Labrie F, et al. Inhibition of testicular luteinizing hormone receptor level by treatment with a potent luteinizing hormone-releasing hormone agonist of human chorionic gonadotropin. Biochem Biophys Res Commun. 1977;76:855–862. doi: 10.1016/0006-291x(77)91579-0. [DOI] [PubMed] [Google Scholar]
- 9.Oefelein MG, Feng A, Scolieri MJ, et al. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000;56:1021. doi: 10.1016/s0090-4295(00)00793-7. [DOI] [PubMed] [Google Scholar]
- 10.Perachino M, Cavalli V, Bravi F. Testosterone levels in patients with metastatic prostate cancer treated with luteinizing hormone-releasing hormone therapy: prognostic significance? BJU Int. 2009;105:648–651. doi: 10.1111/j.1464-410X.2009.08814.x. [DOI] [PubMed] [Google Scholar]
- 11.Huhtaniemi I, White R, McArdle CA, Persson BE. Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab. 2009;20:43–50. doi: 10.1016/j.tem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Drudge-Coates L. GnRH blockers: a changing paradigm in the management of prostate cancer. Int J Urol Nursing. 2009;3:85–92. [Google Scholar]
- 13.Crawford ED. Hormonal therapy in prostate cancer: historical approaches. Rev Urol. 2004;6((suppl 7)):S3–S11. [PMC free article] [PubMed] [Google Scholar]
- 14.Varenhorst E, Wallentin L, Carlström K. The effects of orchidectomy, estrogens, and cyproterone acetate on plasma testosterone, LH, and FSH concentrations in patients with carcinoma of the prostate. Scand J Urol Nephrol. 1982;16:31–36. doi: 10.3109/00365598209179637. [DOI] [PubMed] [Google Scholar]
- 15.Andò S, Giacchetto C, Canonaco M, et al. Effects of castration on androstenedione, testosterone and dihydrotestosterone plasma levels in adult male rats. Horm Res. 1986;23:122–127. doi: 10.1159/000180299. [DOI] [PubMed] [Google Scholar]
- 16.Labrie F, Bélanger A, Luu-The V, et al. Gonadotropin-releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005;26:361–379. doi: 10.1210/er.2004-0017. [DOI] [PubMed] [Google Scholar]
- 17.Bhasin S, Berman N, Swerdloff RS. Follicle-stimulating hormone (FSH) escape during chronic gonadotropin-releasing hormone (GnRH) agonist and testosterone treatment. J Androl. 1994;15:386–391. [PubMed] [Google Scholar]
- 18.Mahler C. Is disease flare a problem? Cancer. 1993;72((17 suppl)):3799–3802. doi: 10.1002/1097-0142(19931215)72:12+<3799::aid-cncr2820721707>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Tombal B, Berges R. Optimal control of testosterone: a clinical case-based approach of modern androgen-deprivation therapy. Eur Urol Suppl. 2008;7:15–21. [Google Scholar]
- 20.Zinner NR, Bidair M, Centeno A, Tomera K. Similar frequency of testosterone surge after repeat injections of goserelin (Zoladex) 3.6 mg and 10.8 mg: results of a randomized open-label trial. Urology. 2004;64:1177–1181. doi: 10.1016/j.urology.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 21.Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol. 2007;178:1290–1295. doi: 10.1016/j.juro.2007.05.129. [DOI] [PubMed] [Google Scholar]
- 22.Mahler C, Verhelst J, Chaban M, Denis L. Prolactin and pituitary gonadotropin values and responses to acute luteinizing hormone-releasing hormone (LHRH) challenge in patients having long-term treatment with a depot LHRH analogue. Cancer. 1991;67:557–559. doi: 10.1002/1097-0142(19910201)67:3<557::aid-cncr2820670304>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Khan MS, O’Brien A. An evaluation of pharmacokinetics and pharmacodynamics of leuprorelin acetate 3M-depot in patients with advanced and metastatic carcinoma of the prostate. Urol Int. 1998;60:33–40. doi: 10.1159/000030200. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Josef E, Yang SY, Ji TH, et al. Hormone-refractory prostate cancer cells express functional follicle- stimulating hormone receptor (FSHR) J Urol. 1999;161:970–976. [PubMed] [Google Scholar]
- 25.Huhtaniemi I. Are gonadotropins tumorigenic—a critical review of clinical and experimental data. Mol Cell Endocrinol. 2010;329:56–61. doi: 10.1016/j.mce.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 27.Mariani S, Salvatori L, Basciani S, et al. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J Urol. 2006;175:2072–2077. doi: 10.1016/S0022-5347(06)00273-4. [DOI] [PubMed] [Google Scholar]
- 28.Porter AT, Ben-Josef E. Humoral mechanisms in prostate cancer: a role for FSH. Urol Oncol. 2001;6:131–138. doi: 10.1016/s1078-1439(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 29.Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011;53:141–144. doi: 10.1016/j.cyto.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang MC, Valenzuela LA, Murphy GP, Chu TM. Purification of a human prostate specific antigen. J Urol. 2002;167:960–964. doi: 10.1016/s0022-5347(02)80311-1. [Reprinted from Invest Urol. 1976;17:159–163.] [DOI] [PubMed] [Google Scholar]
- 31.Catalona WJ, Smith DD, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1992;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- 32.Brawer MK, Chetner MP, Beatie J, et al. Screening for prostatic carcinoma with prostate-specific antigen. J Urol. 1992;147:841–845. doi: 10.1016/s0022-5347(17)37401-3. [DOI] [PubMed] [Google Scholar]
- 33.Zeliadt SB, Hoffman RM, Etzioni R, et al. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103:520–523. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 34.Newcomer LM, Sanford JL, Blumenstein BA, Brawer MK. Temporal trends in rates of prostate cancer: declining incidence of advanced stage disease 1974 to 1994. J Urol. 1997;158:1427–1430. doi: 10.1016/s0022-5347(01)64231-9. [DOI] [PubMed] [Google Scholar]
- 35.McDavid K, Lee J, Fulton JP, et al. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep. 2004;119:174–186. doi: 10.1177/003335490411900211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SEER Cancer Statistics Review 1975–2007. Table 23.6: Cancer of the Prostate (Invasive) [Accessed January 17, 2012]. www.seer.cancer.gov/csr/1975_2007/download_csr_datafile.php/sect_23_table.06.csv.
- 37.Andriole GL, Crawford ED, Grubb RL, III, et al. PLCO Project Team, authors. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andriole GL, Crawford ED, Grubb RL, III, et al. PLCO Project Team, authors. Prostate Cancer Screening in the Randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators, authors. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 40.Roobol MJ, Kerkhof M, Schröder FH, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2009;56:584–591. doi: 10.1016/j.eururo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Göteborg randomized populationbased prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:762–771. doi: 10.7326/0003-4819-155-11-201112060-00375. [DOI] [PubMed] [Google Scholar]
- 43.Bill-Axelson A, Holmberg L, Ruutu M, et al. SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 44.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–186. [PubMed] [Google Scholar]
- 45.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 46.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–1007. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 47.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 48.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 49.Shahani S, Braga-Basaria M, Basaria S. Androgen deprivation therapy in prostate cancer and metabolic risk for atherosclerosis. J Clin Endocrinol Metab. 2008;93:2042–2049. doi: 10.1210/jc.2007-2595. [DOI] [PubMed] [Google Scholar]
- 50.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 51.US Food and Drug Administration (FDA), authors FDA Drug Safety Communication 10-20-2010. [Accessed January 17, 2012]. http://www.fda.gov/Drugs/DrugSafety/ucm229986.htm.
- 52.Alibhai SM, Duong-Hua M, Sutradhar R, et al. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine GN, D’Amico AV, Berger P, et al. Androgendeprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society and American Urological Association: endorsed by the American Society for Radiation Oncology. Circulation. 2010;121:833–840. doi: 10.1161/CIRCULATIONAHA.109.192695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen PL, Je Y, Schutz FAB, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 55.Ebeling PR. Clinical practice. Osteoporosis in men. N Engl J Med. 2008;358:1474–1482. doi: 10.1056/NEJMcp0707217. [DOI] [PubMed] [Google Scholar]
- 55.Northhouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a family intervention for prostate cancer patients and their spouses. Cancer. 2007;110:2809–2818. doi: 10.1002/cncr.23114. [DOI] [PubMed] [Google Scholar]
- 56.Grossmann M, Hamilton EJ, Gilfillan C, et al. Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust. 2011;194:301–306. doi: 10.5694/j.1326-5377.2011.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 57.Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–899. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 58.Smith MR, Egerdie B, Hernández Toriz N, et al. Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–755. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998–2006. doi: 10.1016/j.juro.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tunn U. The current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlight. BJU Int. 2007;99((suppl 1)):19–22. doi: 10.1111/j.1464-410X.2007.06596.x. [DOI] [PubMed] [Google Scholar]
- 61.Klotz L, O’Callaghan GJ, Ding K, et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with PSA progression after radical therapy: NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013. J Clin Oncol. 2011;29((suppl 7)) Abstract 3. [Google Scholar]
- 62.Gomella LG, Singh J, Lallas C, Trabulsi EJ. Hormone therapy in the management of prostate cancer: evidence-based approaches. Ther Adv Urol. 2010;2:171–181. doi: 10.1177/1756287210375270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shore ND, Crawford ED. Intermittent androgen deprivation therapy: redefining the standard of care? Rev Urol. 2010;12:1–11. [PMC free article] [PubMed] [Google Scholar]
- 64.Calais da Silva FE, Bono AV, Whelan P, et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 65.Abrahamsson PA. Potential benefits of intermittent androgen suppression therapy in the treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2010;57:49–59. doi: 10.1016/j.eururo.2009.07.049. [DOI] [PubMed] [Google Scholar]
- 66.Schmitt B, Bennett C, Seidenfeld J, et al. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst Rev. 2000;2:CD001526. doi: 10.1002/14651858.CD001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mohler JH, Gregory CW, Ford OH, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 68.de Bono JS, Logothetis CJ, Molina A, et al. COUAA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem. 1987;33:1372–1375. [PubMed] [Google Scholar]
- 70.Wheeler MJ, D’Souza A, Matadeen J, Croos P. Ciba Corning ACS:180 testosterone assay evaluated. Clin Chem. 1996;42:1445–1449. [PubMed] [Google Scholar]
- 71.Heyns CF, Simonin MP, Grosgurin P, et al. for the South African Triptorelin Study Group, authors. Comparative efficacy of triptorelin pamoate and leuprolide acetate in men with advanced prostate cancer. BJU Int. 2003;92:226–231. doi: 10.1046/j.1464-410x.2003.04308.x. [DOI] [PubMed] [Google Scholar]
- 72.Yri OE, Bjoro T, Fossa SD. Failure to achieve castration levels in patients using leuprolide acetate in locally advanced prostate cancer. Eur Urol. 2006;49:54–58. doi: 10.1016/j.eururo.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 73.Sherwin BB. Randomized clinical trials of combined estrogen-androgen preparations: effects on sexual functioning. Fertil Steril. 2002;77((suppl 4)):S49–S52. doi: 10.1016/s0015-0282(02)03002-9. [DOI] [PubMed] [Google Scholar]
- 74.Damber JE. Endocrine therapy for prostate cancer. Acta Oncologica. 2005;44:605–609. doi: 10.1080/02841860510029743. [DOI] [PubMed] [Google Scholar]
- 75.Williams SG, Duchesne GM, Millar JL, Pratt GR. Both pretreatment prostate-specific antigen level and posttreatment biochemical failure are independent predictors of overall survival after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2004;60:1082–1087. doi: 10.1016/j.ijrobp.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 76.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 77.Hussain M, Goldman B, Tangen C, et al. Prostatespecific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450–2456. doi: 10.1200/JCO.2008.19.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]