Abstract
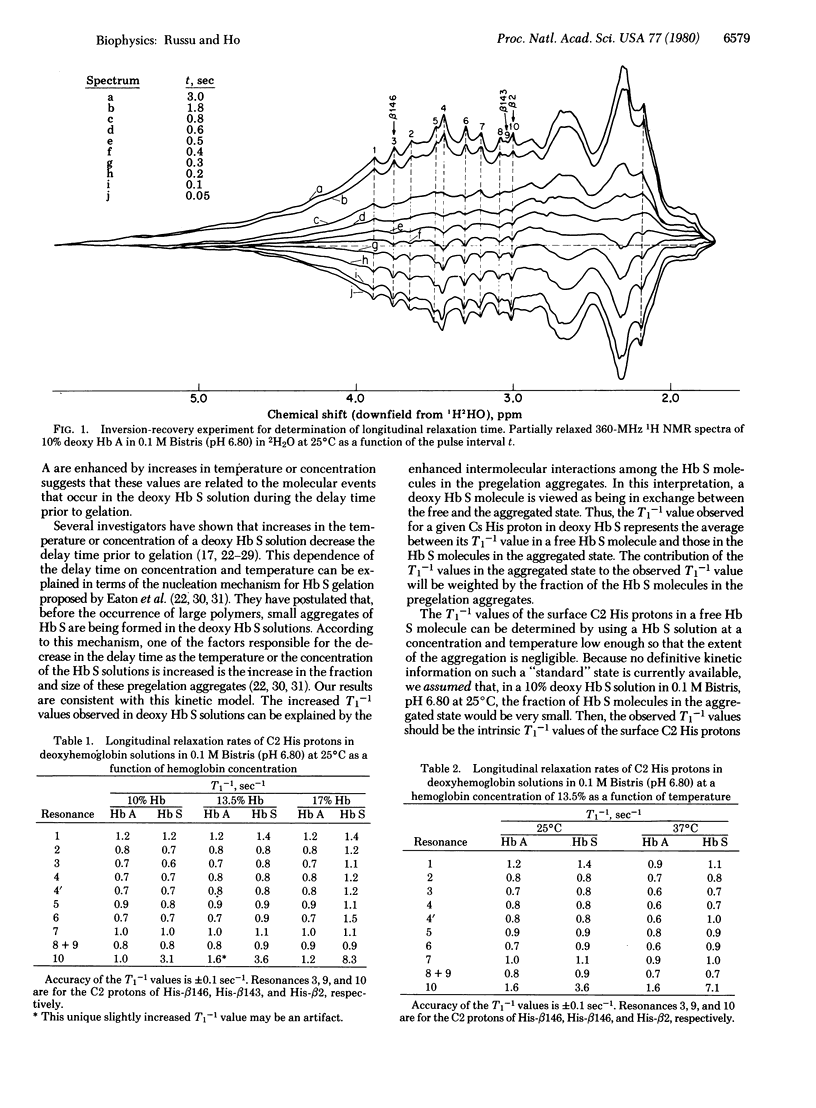
Proton nuclear magnetic resonance longitudinal-relaxation-rate measurements have been used to investigate the molecular events that occur during the early stages of the polymerization process of sickle hemoglobin. The longitudinal relaxation rates (T1-1) of the C2 protons of 11 observable surface histidyl residues in normal human adult and sickle hemoglobin in the deoxy state were measured in 0.1 M bis[(2-hydroxyethyl)imino]tris(hydroxymethyl)methane (pH 6.8) in 2H2O. These proton resonances in hemoglobin occur at a position 1.5-5.0 ppm downfield from that of residual water in 2H2O. The T1-1 values for the C2 protons of several surface histidyl residues in sickle hemoglobin in the deoxy state were sensitive to the temperature and the concentration of hemoglobin, factors known to have a profound effect on the polymerization process of sickle hemoglobin. For hemoglobin concentrations of 13.5% or less and temperatures of 25 degrees C or less, the T1-1 values in sickle hemoglobin solutions were the same as the corresponding values in normal hemoglobin, except for the C2 proton of beta 2 histidine, which had a larger T1-1 value. When the temperature or the hemoglobin concentration was increased (i) several additional histidine resonances in sickle hemoglobin solutions had larger T1-1 values than the corresponding ones in normal hemoglobin and (ii) the differences between the T1-1 values (sickle versus normal hemoglobin) of these histidine resonances as well as that of the beta 2 histidine resonance gradually increased. It is proposed that these results reflect the formation of small aggregates in the deoxygenated sickle hemoglobin solutions before gelation. In this model, the histidyl residues for which the T1-1 values are greatly increased in sickle hemoglobin solutions as compared with those in normal hemoglobin are viewed as being located in or near the "contact" areas between sickle hemoglobin molecules within the pregelation aggregates. Thus, this magnetic resonance technique can also be used to identify the intermolecular contacts in the polymerization of sickle hemoglobin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Asakura T., McConnell M. L. Formation of nuclei during delay time prior to aggregation of deoxyhemoglobin S in concentrated phosphate buffer. Biochim Biophys Acta. 1979 Oct 24;580(2):405–410. doi: 10.1016/0005-2795(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Adachi K., Asakura T. Nucleation-controlled aggregation of deoxyhemoglobin S. Possible difference in the size of nuclei in different phosphate concentrations. J Biol Chem. 1979 Aug 25;254(16):7765–7771. [PubMed] [Google Scholar]
- Benesch R. E., Kwong S., Benesch R., Edalji R. Location and bond type of intermolecular contacts in the polymerisation of haemoglobin S. Nature. 1977 Oct 27;269(5631):772–775. doi: 10.1038/269772a0. [DOI] [PubMed] [Google Scholar]
- Berman M., Benesch R., Benesch R. E. The removal of organic phosphates from hemoglobin. Arch Biochem Biophys. 1971 Jul;145(1):236–239. doi: 10.1016/0003-9861(71)90031-2. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (first of three parts). N Engl J Med. 1978 Oct 5;299(14):752–763. doi: 10.1056/NEJM197810052991405. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J., Ross P. D., Tschudin R. G., Becker E. D. Comparison of sickle cell hemoglobin gelation kinetics measured by NMR and optical methods. Biochem Biophys Res Commun. 1976 Mar 22;69(2):538–547. doi: 10.1016/0006-291x(76)90554-4. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Fung L. W., Ho C., Roth E. F., Jr, Nagel R. L. The alkylation of hemoglobin S by nitrogen mustard. High resolution proton nuclear magnetic resonance studies. J Biol Chem. 1975 Jun 25;250(12):4786–4789. [PubMed] [Google Scholar]
- Fung L. W., Lin K. L., Ho C. High-resolution proton nuclear magnetic resonance studies of sickle cell hemoglobin. Biochemistry. 1975 Jul 29;14(15):3424–3430. doi: 10.1021/bi00686a021. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Kinetics and mechanism of deoxyhemoglobin S gelation: a new approach to understanding sickle cell disease. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4864–4868. doi: 10.1073/pnas.71.12.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Breen J. J., Roberts G. C., Ho C. Direct measurement of the pK values of an alkaline Bohr group in human hemoglobin. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1246–1249. doi: 10.1073/pnas.70.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom T. R., Ho C. Functional nonequivalence of and hemes in human adult hemoglobin. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1707–1710. doi: 10.1073/pnas.69.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfa R., Steinhardt J. A temperature-dependent latent-period in the aggregation of sickle-cell deoxyhemoglobin. Biochem Biophys Res Commun. 1974 Aug 5;59(3):887–893. doi: 10.1016/s0006-291x(74)80062-8. [DOI] [PubMed] [Google Scholar]
- Pearson H., Gust D., Armitage I. M., Huber H., Roberts J. D., Stark R. E., Vold R. R., Vold R. L. Nuclear magnetic resonance spectroscopy: reinvestigation of carbon-13 spin-lattice relaxation time measurements of amino acids. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1599–1601. doi: 10.1073/pnas.72.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Calorimetric and optical characterization of sickle cell hemoglobin gelation. J Mol Biol. 1975 Aug 5;96(2):239–253. doi: 10.1016/0022-2836(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Russu I. M., Ho N. T., Ho C. Role of the beta 146 histidyl residue in the alkaline Bohr effect of hemoglobin. Biochemistry. 1980 Mar 4;19(5):1043–1052. doi: 10.1021/bi00546a033. [DOI] [PubMed] [Google Scholar]
- Sykes B. D., Hull W. E., Snyder G. H. Experimental evidence for the role of cross-relaxation in proton nuclear magnetic resonance spin lattice relaxation time measurements in proteins. Biophys J. 1978 Feb;21(2):137–146. doi: 10.1016/S0006-3495(78)85514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman M. R., Cottam G. L. Kinetics of the polymerization of hemoglobin S: studies below normal erythrocyte hemoglobin concentration. Biochem Biophys Res Commun. 1976 Dec 6;73(3):639–645. doi: 10.1016/0006-291x(76)90858-5. [DOI] [PubMed] [Google Scholar]
- Willard J. M., Davis J. J., Wood H. G. Phosphoenolpyruvate carboxytransphosphorylase. IV. Requirement for metal cations. Biochemistry. 1969 Aug;8(8):3137–3144. doi: 10.1021/bi00836a002. [DOI] [PubMed] [Google Scholar]


