Abstract
We have previously demonstrated that WNT3 and Frizzled7 (FZD7) expression levelswere upregulated in hepatocellular carcinoma (HCC) and that they directly interact to activate the canonical Wnt/β–catenin pathway in HCC cell lines. In this study, we investigated the functional consequences of WNT3 and FZD7 expression levels in non-transformed hepatic cells to address the question of whether WNT3/FZD7-mediated signal transduction could be involved in cellular transformation. After stable transfection of WNT3 and FZD7, the activation of the Wnt/β–catenin pathway was confirmed by western blot, immunostaining and quantitative real-time reverse transcriptase–PCR (qRT–PCR) analysis in two non-transformed hepatocyte-derived cell lines. In vitro characteristics of the malignant phenotype were measured, including cell proliferation, migration, invasion and anchorage-independent growth in soft agar. Stable expression of WNT3 and FZD7 in the two cell lines led to cellular accumulation of β-catenin and expression of downstream target genes activated by this pathway. In the stable WNT3/FZD7-expressing clones, hepatic cell proliferation, migration, invasion as well as soft agar colony formation were enhanced compared with the non-transformed control cells. The epithelial–mesenchymal transition (EMT) factors, Twist, Snail and Vimentin, were increased in cells expressing WNT3 and FZD7. However, the WNT3/FZD7-expressing cells did not form tumors in vivo. We conclude that activation of the WNT3/FZD7 canonical pathway has a role in the early stages of hepatocarcinogenesis by promoting the acquisition of a malignant phenotype with features of EMT.
Keywords: HCC, WNT, Frizzled, β-catenin, hepatic transformation, EMT
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of death by cancer worldwide.1 Hepatocarcinogenesis is a complex, multistep process, which develops in a normal hepatocyte owing to the accumulation of aberrant genetic and epigenetic changes and activation of cellular-transformation signaling pathways.2 Although these alterations are highly heterogeneous, the deregulated activation of the canonical Wnt/β-catenin pathway has been found in >50% of these HCC tumors.3 Activation of this pathway is initiated by the binding of a WNT extracellular ligand to a membrane-associated Frizzled (FZD) receptor along with a low-density lipoprotein receptor-related protein (LRP) co-receptor and leads to the accumulation of the β-catenin in the cytoplasm by inhibition of its destruction complex. The β-catenin can then translocate to the nucleus to form a transcription complex with the T-cell factor/lymphoid enhancer factor proteins followed by activation of target genes that control cell proliferation, migration, invasion and cell-cycle progression, which are involved in the establishment and maintenance of the tumor phenotype.
The Wnt pathway is activated normally during the early stages of embryogenesis and has a role in stem cells renewal and differentiation.4 Aberrant activation of the Wnt signaling in various tumor types may be caused by mutation in Axin, Adenomatous polyposis coli (APC) or β-catenin genes. In a recent study by Guichard et al.,5 these mutually exclusive mutations were found in about 50% of 125 HCC tumors (CTNNB1, 32.8% APC, 1.6% AXIN1, 15.2%). The signaling pathway can also be activated by the deregulated expression of the upstream components of the cascade. We have previously demonstrated that WNT3 and FZD7 were overexpressed in 30–90% of HCC6, 7 and that they physically interact to activate the canonical Wnt/β-catenin pathway in HCC cell lines. The biologic effect was noted to be an increase in cell proliferation.8 These results provide evidence that the WNT3/FZD7-mediated signal transduction cascade may have an oncogenic role in the development of the HCC malignant phenotype. Interestingly, WNT3 and FZD7 are not only commonly overexpressed in HCC tumors but also upregulated in the surrounding peri-tumor region compared with the normal human liver tissue. In hepatitis B virus- and C virus-related tumors, WNT3 was upregulated in 25–59% of peri-tumor tissues.6, 8 In addition, FZD7 expression was increased in 53% of adjacent dysplastic pre-cancerous regions in various transgenic murine models of HCC.9 These findings suggest that activation of the WNT3/FZD7-mediated β-catenin signaling may be an early event during hepatocarcinogenesis.
To examine this hypothesis, we studied the functional consequences of activation of the WNT3/FZD7-mediated signal in two cellular models of non-transformed hepatic cells. We found that stable ectopic expression of WNT3 and FZD7 genes will activate the canonical Wnt/β-catenin pathway in both the cell lines and contribute to the acquisition of tumorous properties as characterized by proliferation, migration, invasion and anchorage-independent growth. Features of epithelial–mesenchymal transition (EMT) were also observed such as a fibroblastoid-like morphology and expression of EMT markers, suggesting that the WNT3/FZD7 activation signal promotes the early stages of malignant transformation in hepatocyte-like cells.
Results
WNT3 and FZD7 expression induces activation of the Wnt/β-catenin pathway in non-transformed hepatic cells
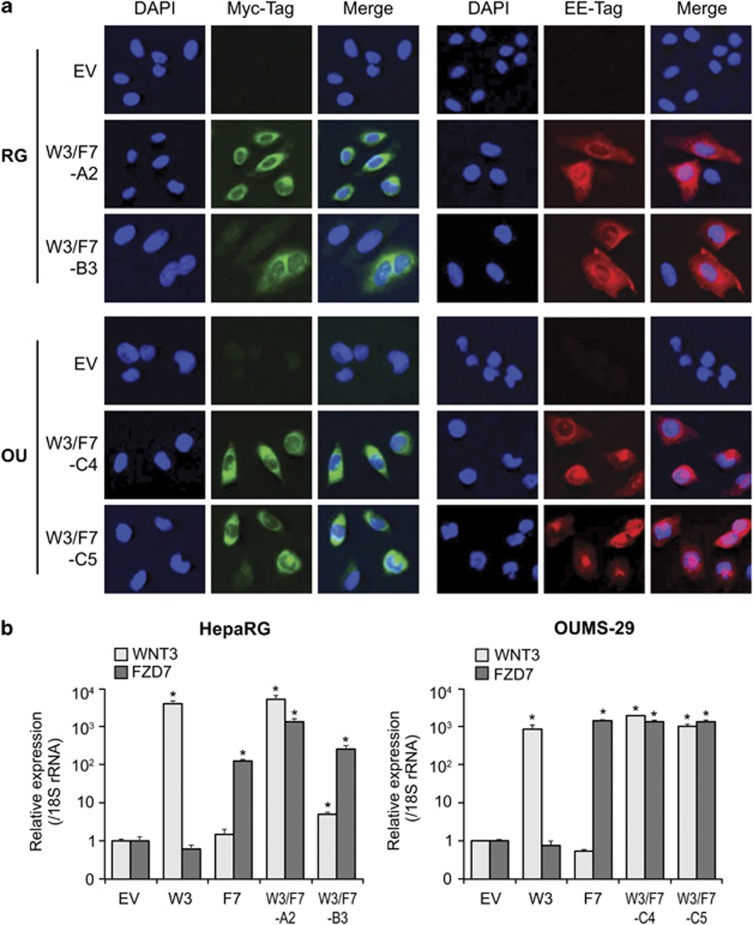
To study the effect of WNT3 and FZD7 overexpression on hepatic transformation, we used plasmids encoding the human WNT3 and FZD7 genes cloned into vectors containing G418 and hygromycin (hygro) resistance genes, respectively. Stable clones were generated that expressed the two proteins either alone or in combination by transfection into the OUMS-29 immortalized human fetal hepatocyte cell line (OU-W3, OU-F7 and OU-W3/F7 clones, respectively) or the HepaRG hepatic progenitor cell line (RG-W3, RG-F7 and RG-W3/F7 clones, respectively) followed by selection with G418, hygro or G418/hygro combination. We also generated G418/hygro-resistant clones as controls by stable transfection of the corresponding empty vectors (OU-EV and RG-EV). Expression of the two transgenes was confirmed by immunofluorescence staining using Myc-Tag (WNT3) and EE-Tag (FZD7) antibodies (Figure 1a) and qRT–PCR (Figure 1b) in OU-W3/F7-C4, OU-W3/F7-C5, RG-W3/F7-A2 and RG-W3/F7-B3 clones. The single stable clones expressing WNT3 or FZD7 alone were used as a positive control in the qRT–PCR experiment.
Figure 1.
Expression of WNT3-Myc and FZD7-EE in HepaRG and OUMS-29 cells. (a) Immunostaining showing protein expression of WNT3 (Myc-Tag, green) and FZD7 (EE-Tag, red) in RG-W3/F7- and OU-W3/F7-stable-expressing clones. RG-EV and OU-EV were used as negative controls ( × 400). (b) Expression of WNT3 and FZD7 mRNAs by qRT–PCR normalized to 18S rRNA expression. Bar graphs depict a ratio to the controls OU-EV or RG-EV. Stable clones expressing single WNT3 (W3) or FZD7 (F7) were used as positive controls. *P<0.01 compared with the negative controls OU-EV or RG-EV.
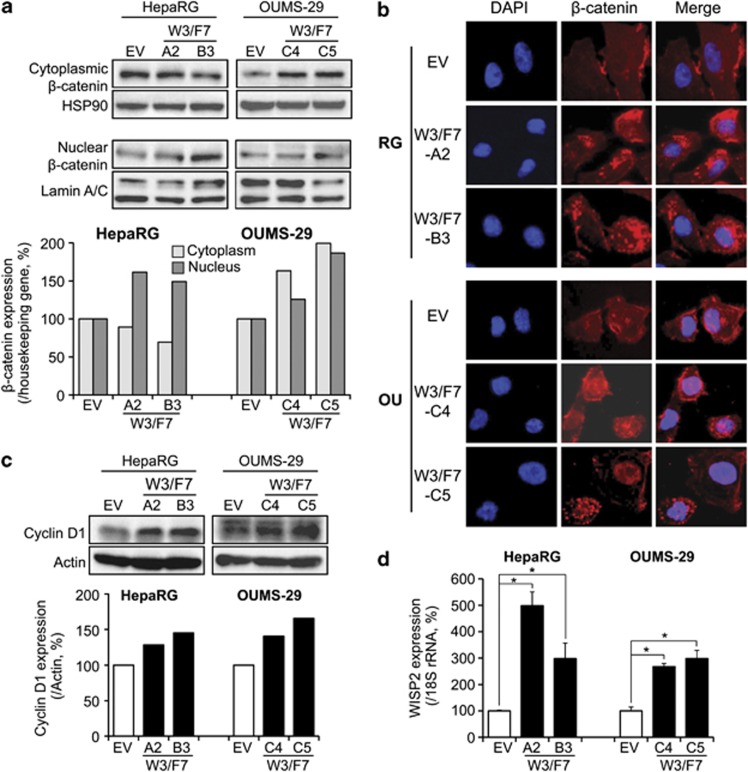
We examined the cellular levels of β-catenin to determine whether the expression of WNT3 and FZD7 can activate the canonical Wnt pathway. We observed an increase of β-catenin expression in the nucleus and/or cytoplasm by western blot analysis in the two cell types expressing WNT3 and FZD7 compared with EV-transfected control cells (Figure 2a). By immunofluorescence staining, β-catenin was expressed at the membrane and in the cytoplasm of the control cells (OU-EV and RG-EV, Figure 2b). RG-W3/F7 cells displayed a more intense cytoplasmic staining, suggesting an accumulation of β-catenin; nuclear staining was observed in OU-W3/F7 cells (Figure 2b). In the two cell lines, the stable clones expressing only WNT3 displayed weak accumulation of β-catenin compared with the clones expressing both WNT3 and FZD7, while no change was observed between FZD7-expressing cells and the control cells (data not shown). Consistent with the cellular accumulation of β-catenin, Cyclin D1 (Figure 2c, western blot) and WNT1-inducible signaling pathway protein 2 (WISP2) (Figure 2d, qRT–PCR), two known target genes of the Wnt/β-catenin pathway, were upregulated in HepaRG and OUMS-29 clones expressing both WNT3 and FZD7 proteins. Taken together, these results reveal that the β-catenin pathway was activated in two different non-transformed human hepatic cell lines by overexpression of both WNT3 and FZD7.
Figure 2.
Activation of the β-catenin pathway in cells expressing WNT3 and FZD7. (a) Top: Western blot analysis of β-catenin levels after subcellular fractionation in HepaRG and OUMS-29 clones. Bottom: β-catenin expression was quantified using ImageJ (NIH) and normalized to HSP90 expression (cytoplasmic fraction) or Lamin A/C (nuclear fraction). Bar graphs represent percentage to RG-EV or OU-EV control cells. (b) Immunostaining showing the subcellular localization of β-catenin (red) in HepaRG (top) and OUMS-29 (bottom) stable clones expressing WNT3 and FZD7 ( × 400). (c) Top: Expression of Cyclin D1 by western blot analysis. Bottom: Cyclin D1 expression level was normalized to Actin expression. Bar graphs represent percentage to RG-EV or OU-EV control cells. (d) Analysis of WISP2 expression by qRT–PCR, normalized to 18S rRNA expression. Bar graphs depict percentage to RG-EV or OU-EV control cells. *P<0.01 compared with the negative controls OU-EV or RG-EV.
WNT3/FZD7-mediated signaling promotes cell proliferation in non-transformed hepatic cells
To study the functional consequences of WNT3/FZD7/β-catenin-mediated signaling, we measured cellular proliferation of HepaRG and OUMS-29 cells. As shown in Figure 3a, cell proliferation rate was increased 1.5-fold in OU-W3/F7-C4 and twofold in OU-W3/F7-C5 compared with control cells as measured by the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium)/PMS (phenazine methosulfate) assay. Cell proliferation in RG-W3/F7 clones was assessed by western blot analysis of nuclear expression of proliferative cell nuclear antigen (PCNA), which was increased 2.5- and 1.5-fold in RG-W3/F7-A2 and RG-W3/F7-B3, respectively, compared with the control (Figure 3b). Consistent with these results, the proliferative index measured by the number of Ki-67-positive cells was increased in WNT3- and FZD7-overexpressing cells compared with RG-EV control (Supplementary Figure S1). Expression of WNT3 alone reduced cell proliferation rate compared with the control cells, whereas FZD7 expression enhanced the proliferation in both the cell lines (data not shown). Taken together these results suggest that WNT3/FZD7/β-catenin-mediated signaling promotes growth of both hepatocyte-derived cell lines.
Figure 3.
WNT3/FZD7-mediated signaling promotes hepatic cell proliferation. (a) The proliferation rate of OUMS-29 clones was measured by MTS assay over 7 days. (b) Left: western blot analysis of PCNA expression as proliferation marker in HepaRG clones using nuclear extracts. Right: PCNA levels were quantified and normalized to Lamin A/C expression. Bar graphs represent percentage to RG-EV control cells. *P< 0.01 compared with the negative controls OU-EV.
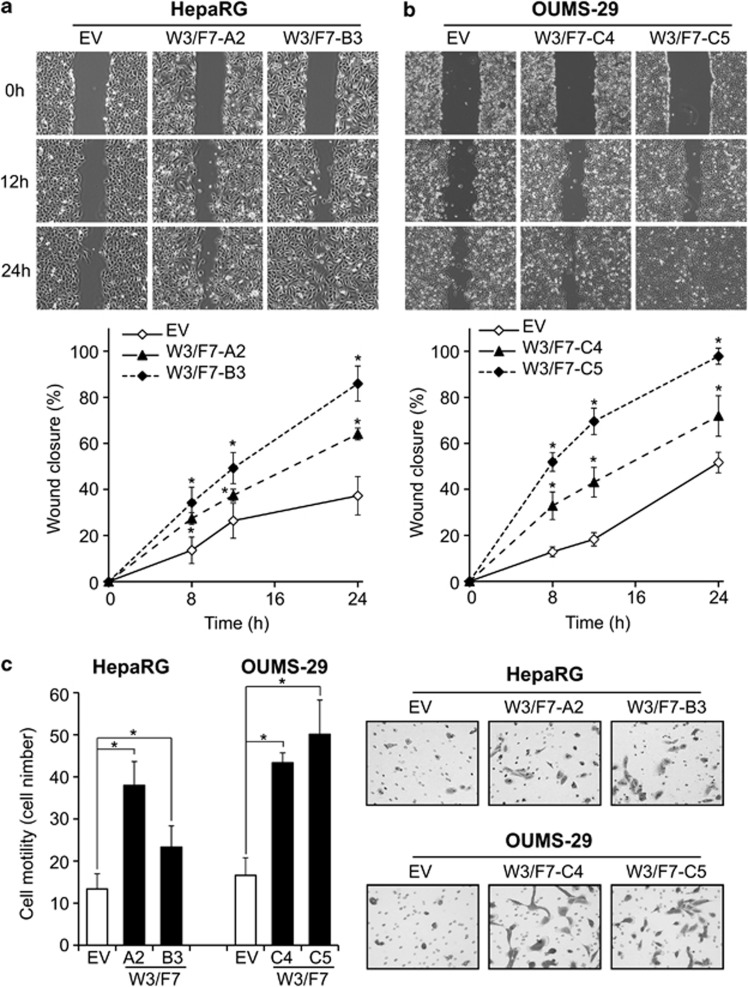
Cell migration properties are increased in cells expressing WNT3 and FZD7
The capability of hepatic cells overexpressing WNT3 and FZD7 to migrate was evaluated. A wound-healing assay revealed that after 24 h, the control RG-EV cells covered less than 40% of the wound, whereas RG-W3/F7-A2 and -B3 were able to close 65 and 85% of the wound, respectively (Figure 4a). Similar results were observed in OU-W3/F7-C4 (70%) and -C5 (98%) compared with OU-EV (50%) (Figure 4b). These observations were confirmed by a transwell cell migration assay as shown in Figure 4c. The number of migrating cells was increased 2.9- and 1.8-fold in RG-W3/F7-A2 and -B3, respectively, compared with RG-EV. A 2.6- and 3.1-fold increase was found in OU-W3/F7-C4 and -C5 clones, respectively (Figure 4c). Both a wound-healing and a transwell migration assays revealed that WNT3 expression alone promoted cell migration in only HepaRG but not in OUMS-29; FZD7 expression modestly increased cell migration in both cell lines (data not shown). Results from these two different assays demonstrate that enhanced WNT3 and FZD7 expression promotes cell migration.
Figure 4.
Activation of the WNT3/FZD7-mediated signaling promotes cell migration. (a, b) Analysis of cell migration in HepaRG (a) and OUMS-29 (b) expressing both WNT3 and FZD7 as measured by a wound-healing assay. Top: Representative photographs taken at 0 h, 12 h and 24 h post-wound ( × 40). Bottom: The wound closure was quantified at 8 h, 12 h and 24 h post-wound by measuring the remaining unmigrated area using ImageJ. (c) Transwell migration assay. Left: Quantification of the number of migratory cells using the transwell assay. Migratory cells were counted in 10 non-overlapping frames of the membrane using Stereologer (Dissector, Newton, MA, USA). Right: Representative photographs of stained cells attached to the bottom membrane of a transwell ( × 100). *P<0.01 compared with the negative controls OU-EV or RG-EV.
WNT3/FZD7 signaling promotes cell invasion and anchorage-independent growth.
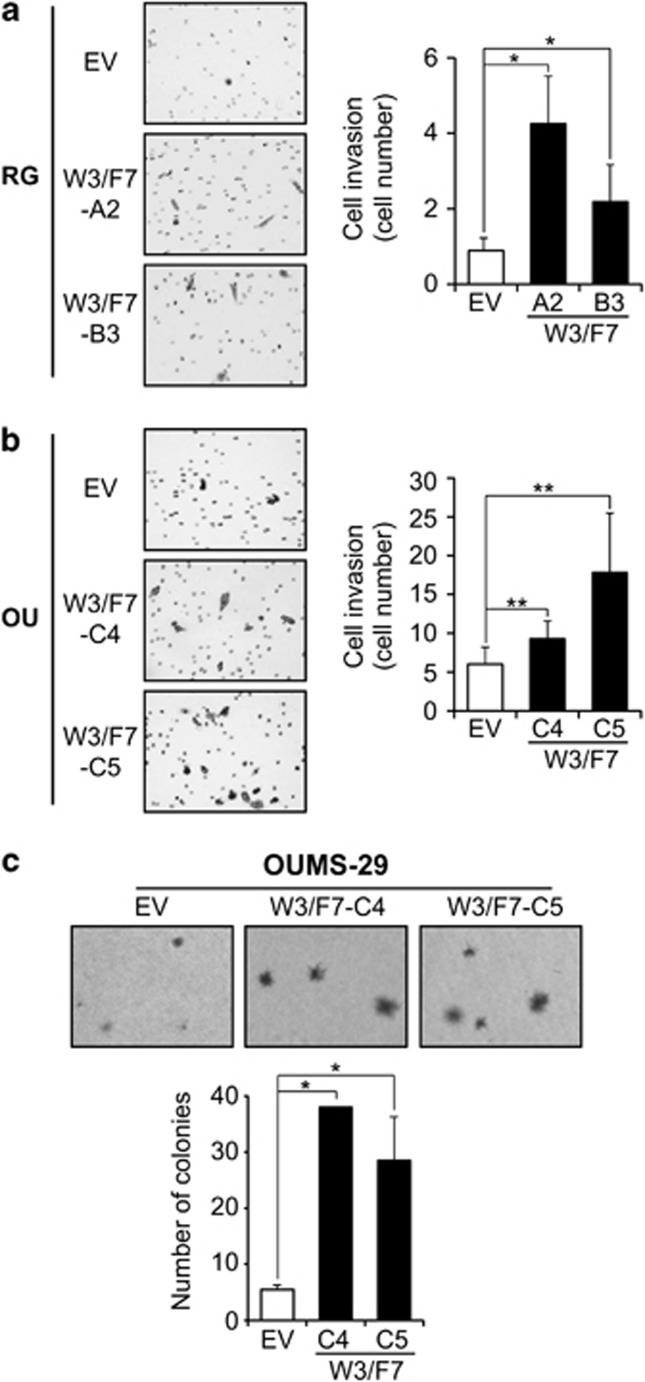
The invasive properties of W3/F7 clones were assessed by a matrigel-coated transwell assay. There was a low basal level of invasion in the control HepaRG cells (RG-EV). However, the rate was significantly increased 4.8-fold in RG-W3/F7-A2 and 2.5-fold in -B3 overexpressing both the WNT3 ligand and FDZ7 receptor (Figure 5a). A significant 1.5- and 3-fold increase was also observed with OU-W3/F7-C4- and -C5-expressing cells, respectively, compared with OU-EV (Figure 5b), demonstrating that the activation of WNT3/FZD7 signaling confers invasive properties to hepatocyte-derived parental cell lines. Both WNT3-expressing single clones (RG-W3 and OU-W3) were highly invasive, whereas FZD7-expressing single clones exhibited no difference compared with control cells (data not shown). Anchorage-independent growth was also assessed by a soft agar colony formation assay. In OUMS-29 cells, WNT3 and FZD7 expression led to the formation of larger colonies compared with the control cells (OU-EV) as reflected by the representative results presented in Figure 5c (upper panel). In addition, the number of colonies was increased 5- to 7-fold in the W3/F7 cells compared with the control cells (Figure 5c). These findings imply that activation of the WNT3/FZD7 signaling promotes invasion and anchorage-independent growth in non-transformed hepatic cell lines.
Figure 5.
WNT3/FZD7-mediated signaling promotes invasion and anchorage-independent growth. (a, b) Analysis of invasion using a matrigel-coated transwell assay in HepaRG (a) and OUMS-29 (b). Left: Representative photographs of stained cells ( × 100). Right: Quantification of invasive cells using Stereologer. (c) Results of soft agar colony formation assay with OUMS-29-derived clones. Top: Representative microscopic photographs of colony size and number ( × 40). Bottom: The number of colonies was calculated from macroscopic photographs of the wells. *P<0.01 and **P<0.05 compared with the negative controls OU-EV or RG-EV.
The transforming properties of WNT3/FZD7 pathway activation are associated with EMT
Accumulation of β-catenin has been correlated to EMT along with loss of E-cadherin expression in HCC.10 EMT is a reversible process that leads an epithelial cell to transition into a fibroblastoid phenotype with increased motility and invasive capacities and has a critical role in the development of HCC.11
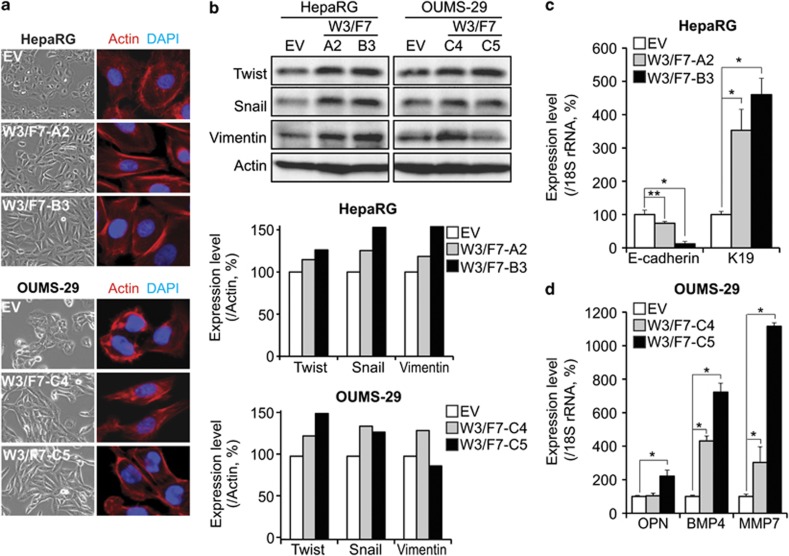
We observed that the control cells displayed a well-defined epithelial, polygonal shape, whereas W3/F7 cells exhibit a striking morphological change characterized by a spindle-like fibroblastoid appearance (Figure 6a, contrast phase). Actin staining revealed that the control cells displayed a cortical actin assembly and a diffuse distribution in the cytoplasm. By contrast, W3/F7 cells displayed large actin-rich protrusions around the periphery of the cell, with a few thin stress fibers located within the cell body (Figure 6a, immunofluorescence). Thus, these observations imply that WNT3/FZD7-expressing cells underwent EMT process. Furthermore, the expression levels of the three EMT markers Twist, Snail and Vimentin were increased in HepaRG and OUMS-29 cells expressing both WNT3 and FZD7 compared with the control cells (Figure 6b). Consistent with these findings, we observed a loss of E-cadherin expression with upregulation of keratin 19 (K19) in HepaRG cells expressing WNT3 and FZD7 as a hallmark of EMT (Figure 6c). We observed upregulation of the pro-migratory osteopontin (OPN) and bone morphogenetic protein-4 (BMP4) in OU-W3/F7 cells, as well as the matrix metalloproteinase-7 (MMP7), a well-known β-catenin target gene involved in cellular invasion (Figure 6d). These results suggest that in both hepatocyte-derived parental cell lines, the phenotypic changes induced by activation of the WNT3/FZD7/β-catenin signal might be associated with an EMT.
Figure 6.
WNT3/FZD7-mediated signaling induces epithelial–mesenchymal transition. (a) Phase-contrast photographs ( × 200) demonstrating the morphological appearance of HepaRG (top) and OUMS-29 (bottom) following phalloidin–rhodamin staining of F-Actin filaments as visualized by confocal microscopy (red, 630X). (b) Top: Expression of EMT markers Twist, Snail and Vimentin in HepaRG and OUMS-29 cells by western blot analysis. Middle and bottom: Protein expression level was normalized to Actin expression. Bar graphs represent percentage to EV cells. (c and d) Analysis of E-cadherin and K19 expression in HepaRG (c) and OPN, BMP4 and MMP7 expression in OUMS-29 (d) by qRT–PCR, and normalized to 18S rRNA expression. Data are expressed as percentage to the control cells RG-EV (c) or OU-EV (d). *P<0.01 and **P<0.05 compared with the negative controls OU-EV or RG-EV.
Discussion
Abnormal activation of Wnt/β-catenin signaling cascade has been observed in a large number of human tumors. We and other investigators have previously shown that this phenomenon can be attributable to the deregulated expression of the upstream components of the pathway. Among them, overexpression of the WNT3 ligand has been reported in the lung, gastric, breast and rectal cancers.12 More recently, WNT3 overexpression has been observed in a murine model of mammary tumors13 and was associated with the activation of the canonical Wnt cascade, as well as enhanced cell proliferation, which predicts a poor prognosis in non-small cell lung cancer.14 FZD7 is also overexpressed in esophageal and gastric tumors15, 16 and has been shown to regulate activation of the β-catenin pathway to promote a tumor phenotype in colorectal17, 18 or triple-negative breast tumors.19
We have previously shown that WNT3 and FZD7 are overexpressed in murine models as well as human HCCs6, 9, 20 and physically interact to activate the canonical Wnt/β-catenin pathway in HCC cell lines.8 Moreover, the inhibition of WNT3 by neutralizing antibodies or siRNA-mediated knockdown in various HCC cell lines led to an inhibition of β-catenin pathway activation and exhibited anti-migratory and proliferative effects.8 Similar results were observed with the inhibition of FZD7 expression by siRNA-mediated knockdown, or expression of a dominant-negative mutant as well as antagonizing peptide.7, 21, 22, 23 These findings suggest that the activation of the canonical WNT3/FZD7 pathway by overexpression of WNT ligand and FZD receptor may contribute to hepatic oncogenesis.
Interestingly, WNT3 and FZD7 expression was also increased in peri-tumoral tissues surrounding the HCC.6, 8, 9 This gradient of expression from the normal liver to developed tumor raises the hypothesis that overexpression of WNT3 and FZD7, resulting in activation of the β-catenin pathway, may be an early event in the multistep process of hepatocarcinogenesis and promotes the acquisition of a malignant phenotype. Similar to HCC, the upstream cell surface components of this pathway may also have a role in breast carcinogenesis, for example. Transgenic expression of WNT1 under the control of a mammary tumor virus promoter (MMTV) produced ductal hyperplasia and led to the development of adenocarcinomas in 50% of the female transgenic mice.24 In addition, MMTV-LRP6-generated transgenic mice displayed cellular accumulation of β-catenin and activation of downstream target genes in mammary glands.25 These events were accompanied by hyperplasia, supporting the idea that, in different tumor types, overexpression of upstream components of the Wnt/β-catenin pathway may act together to promote early stages of tumor progression.
In the present study, we addressed the question of whether expression of two upstream components of the β-catenin pathway, for example, WNT3 and FZD7, known to have a role in HCC, could induce the characteristics of a malignant phenotype in vitro. Using a cellular model of human fetal hepatocyte (OUMS-29) and human hepatic progenitors (HepaRG cells), we demonstrated that activation of WNT3/FZD7-mediated signal transduction induces cell proliferation, migration, invasion and anchorage-independent growth of these non-transformed hepatocyte-derived cell lines. The choice of the immortalized fetal hepatocytes (OUMS-29) was motivated by the hepatocyte-like characteristics of the cells, such as epithelial morphology, production of albumin, transferrin, α-antitrypsin and apolipoprotein A1 as well as functional cytochrome enzymes.26 The HepaRG cell line was isolated from a well-differentiated grade 1 hepatitis C virus-related HCC and cloned from a well-differentiated region resembling a ‘hepatocyte-like' subpopulation. The cells express various liver-specific enzymes27 and are capable, under specific in vitro culture conditions, to differentiate into two lineages, with characteristics of differentiated cholangiocytes and hepatocytes that mimic the behavior of hepatic progenitors.28 In vivo, HepaRG cells are able to repopulate damaged liver using the urokinase-type plasminogen activator-severe combined immunodeficiency mice animal model but fail to form subcutaneous tumors when injected into nude mice.29 Hepatocytic functionality was supported by human albumin expression in mice livers and human α-1-antitrypsin in sera.29
Our results using the clones expressing only WNT3 or FZD7 indicated that FZD7 may promote cell proliferation, whereas WNT3 enhances invasion of these two hepatocyte-derived cell lines. Expression of both proteins was required to induce the phenotype characterized by enhanced cell proliferation, migration, invasion and anchorage-independent growth. It has been suggested that the WNT3 ligand or FZD7 receptor may have different partners and thus different phenotypes may be driven by activation of alternative pathways. For instance, FZD7 has been shown to form a membrane complex with the receptor tyrosine kinase Ror2 upon WNT5A stimulation, leading to AP-1 pathway-mediated enhanced migration in mouse fibroblasts.30 Further studies are necessary to determine whether other cellular factors are activated by WNT3 and/or FZD7 in HepaRG and OUMS-29 cells.
EMT has been described as a critical factor for tumor progression and invasion of various carcinomas, including HCC.31 Deregulation of the upstream components of the WNT/β-catenin pathway have been shown to activate transcription factors involved in the EMT process and to include enhanced WNT1 expression in breast cancer.32 ERG (avian v-ets erythroblastosis virus E26 oncogene homolog)-induced FZD4 overexpression in prostate cancer was also revealed to be a mediator of EMT.33 There is consistent evidence to suggest that downregulation of this pathway by inhibitors such as secreted frizzled-related proteins or Dickkopfs will alter phenotypic changes similar to those observed during EMT.34, 35 In a model of experimental murine HCC where EMT was induced in cultured hepatocytes before transplantation, the resulting HCCs showed evidences of activation of the WNT/β-catenin cascade in association with markers of EMT. Conversely, inhibition of β-catenin signaling in this same murine model induced the acquisition of more differentiated epithelial hepatocyte markers.3
We found evidence of EMT in the two cell lines expressing WNT3 and FZD7. In HepaRG cells, WNT3/FZD7 signaling led to a decrease of E-cadherin expression accompanied by an upregulation of K19. E-cadherin is a component of adherent junctions and is frequently downregulated in aggressive HCC. The loss of E-cadherin expression is commonly associated with the upregulation of its transcriptional repressor Twist,36 as we observed in this study. K19 is a progenitor marker, which has been recently associated with a poor prognosis and more frequent vascular invasion in HCC expressing EMT and ‘stemness' cell markers.37 In addition, K19 expression has been found to be correlated with lymph node metastasis.38 On the other hand, in OUMS-29, EMT was associated with an increase of β-catenin target genes related to cell invasion and migration, such as MMP7, OPN and BMP4. These observations were consistent with the acquisition of motile and invasive abilities observed in the functional assays and with previous reports. MMP7 has been reported upregulated in the early stages of HCC development39 and expression of BMP4 was progressively increased from the evolution of cirrhosis to HCC in a murine model of HCC40 and also appears to promote tumor progression in human HCC.41 Expression of OPN has been associated with EMT makers in HCC42 and it has been recently proposed as a novel early marker for this tumor.43 Further studies will be necessary to determine the causes of the differences in the molecular alterations induced by WNT3 and FZD7 signal activation between the two cell lines. It may be due, in part, to a different origin and genetic cellular background. The functional consequences of pathway activation may also depend on the target genes activated in a particular cellular context and/or different cellular factors regulated by the WNT3/FZD7 signaling.
The canonical Wnt pathway is known to have a physiological role during normal hepatic regeneration and with chronic liver injury progressing to HCC.44, 45 WNT1 or active β-catenin (S45D mutant) expression after partial hepatectomy induces activation of this pathway and accelerates hepatocyte proliferation during the murine liver regeneration. However, expression of S45D mutant of β-catenin alone failed to promote tumorigenesis but increased the frequency of N-nitrosodiethylamine-induced HCC tumors.46 Several in vivo studies have demonstrated that expression of an active β-catenin mutant protein alone was not sufficient to induce hepatocarcinogenesis but may collaborate with other oncogenes such as MET (MNNG HOS transforming gene) or AKT/PKB (protein kinase B) to promote HCC.47, 48 Herein, we demonstrated that upstream activation of this pathway by WNT3 and FZD7 overexpression in hepatocyte-like cell lines induces hepatic transformation in vitro. These observations suggest that the WNT3/FZD7-dependent activation of the β-catenin pathway has important consequences and may participate in the early stages of hepatocyte transformation. However, the WNT3/FZD7 signaling is not sufficient to complete a cellular transformation as neither OUMS-29 nor HepaRG clones expressing WNT3 and FZD7 formed subcutaneous tumor in nude mice (data not shown). Further studies are necessary to identify a combination of genetic and/or epigenetic aberrations, including WNT3 and FZD7 overexpression, able to induce hepatic transformation in vivo. Finally, in addition to previous reports,49 our results raise the possibility that development of WNT3/FZD7 signal transduction inhibitors could be a viable therapeutic approach for HCC. Blocking this specific ligand-receptor interaction could disrupt hepatic oncogenesis.
Although the events leading to the aberrant expression of WNT3 and FZD7 in HCC are unknown, this study strengthens the previous results obtained from HCC cell lines and provides better understanding of a cellular mechanism promoting the early stages of HCC development associated with EMT. Moreover, these data reinforce the WNT3/FZD7 signaling as potential new molecular target for therapy of this devastating disease.
Materials and methods
Cells and establishment of stable transfected clones
Human immortalized fetal liver cells OUMS-2926 were maintained in DMEM (Dulbecco's modified Eagle's medium) medium (Lonza, Basel, Switzerland) supplemented with l-glutamine (200 m𝓂, Gibco, Carlsbad, CA), fetal bovine serum (10%, Lonza) and penicillin/streptomycin (100 μg/ml, Gibco). Human liver progenitors HepaRG cells27, 28 were maintained in William's medium (Lonza) supplemented with fetal bovine serum (10%), penicillin/streptomycin (100 μg/ml), bovine insulin (5 μg/ml, Sigma-Aldrich, Saint Louis, MO, USA) and hydrocortisone hemisuccinate (50 μ𝓂, Sigma-Aldrich). Both cell lines were transfected by WNT3-myc and FZD7-EE constructs cloned into pCDNA3.1 and pCDNA3.1/hygro (+), respectively (Invitrogen, Carlsbad, CA, USA). The corresponding empty vectors were used as negative controls. The transfections were performed using LT1 transfection reagent (Mirus Bio, Madison, WI, USA) according to the manufacturer's instructions. To obtain cells stably transfected with each plasmid, WNT3/FZD7-resistant colonies were selected by G418/hygromycin and expanded. OUMS-29 and HepaRG stable clones were further maintained in the original culture medium supplemented with hygromycin and G418 (0.4 mg/ml for OUMS-29 and 0.2 mg/ml for HepaRG cell lines).
Western blot analysis
All reagents for western blot analysis were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Total proteins were extracted using radioimmunoprecipitation buffer and cytosolic and nuclear fractions prepared with the NE-PER Nuclear and Cytoplasmic Extraction kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's instructions. Protein concentration was normalized using a BCA (bicinchoninic acid) assay and equal amount of protein was loaded on SDS–PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) gels. For immunoblot analysis, the following antibodies were used: β-catenin, Snail, Twist, Vimentin (Cell Signaling, Beverly, MA, USA), Cyclin D1, PCNA, HSP90, Lamin A/C, and Actin (Santa Cruz Biotechonolgy, Santa Cruz, CA, USA). Protein expression was quantified using ImageJ (NIH, Bethesda, MD, USA) and normalized to Actin (total lysates), HSP90 (cytoplasmic fractions) or Lamin A/C (nuclear fractions).
Real-time RT–PCR analysis
Total cellular RNAs were extracted using the TRIzol reagent (Invitrogen). Real time RT–PCR was performed as previously described7 using the following primers: FZD7, 5′-caacggcctgatgtactttaagg-3′ and 5′-catgtccaccaggtaggtgaga-3′ WNT3, 5′-acttcggcgtgttagtgtcc-3′ and 5′-catttgaggtgcatgtggtc-3′ WISP2, 5′-cctacacacacagcctatatc-3′ and 5′-ccttctcttcatcctaccc-3′ BMP4, 5′-cgctgagatcaggcagtcct-3′ and 5′-tgagtggatgggaacgtgtg-3′ OPN, 5′-gccacatggctaaaccctga-3′ and 5′-ggggctaggagattctgcttc-3′ MMP7, 5′-gctcatggggactcctaccc-3′ and 5′-ctaccatccgtccagcgttc-3′ E-cadherin, 5′-gggctggaccgagagagttt-3′ and 5′-tgggattgaagatcggagga-3′ K19, 5′-ccagccggactgaagaattg-3′ and 5′-acctcggacctgctcatctg-3′ and 18S rRNA, 5′-cgttgaaccccattcgtgat-3′ and 5′-tgggaattcctcgttcatgg-3′. 18S rRNA Ct (cycle threshold) values were used as standards.
Immunofluorescence studies
Cells were seeded in Labteck culture chamber slides (Thermo Fisher Scientific) and fixed by paraformaldehyde (4%) followed by Protein block serum-free solution (Dako, Glostrup, Denmark) with 0.1% Triton X100. The following antibodies were used: β-catenin (BD transduction Laboratories, Franklin Lakes, NJ, USA), Myc-Tag (Cell Signaling), and EE-Tag (Covance, Princeton, NJ, USA). After incubation with the appropriate secondary antibody (anti-mouse or -rabbit antibody conjugated with Alexa-594 or -488, Invitrogen), the chambers were removed and the coverslips were mounted using DAPI (4',6-diamidino-2-phenylindole)-containing anti-fade mounting medium (Vector Lab, Burlingame, CA, USA) and examined under an Olympus 1 × 70 microscope (Olympus America, Center Valley, PA, USA). For the F-Actin filament staining, fixed and permeabilized cells were incubated with Rhodamin–phalloidin (Invitrogen) directly following by mounting. The Zeiss LSM510 Confocal Laser Scanning Microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA) equipped with the user interface software Zen 2011 was used to visualize phalloidin staining.
Cell proliferation assay
Cell proliferation was measured by the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. Briefly, cells were seeded at low density in triplicate in 24-well plates and incubated for 1 h in culture medium containing MTS (0.3 mg/ml) every two days for 7 days. SpectraMax M5 (Molecular Devices, Sunnyvale, CA, USA) was used to measure the absorbance at 490 nm. Results are presented as the average absorbance of three wells per experiment.
Cell migration and invasion assays
A wound-healing assay was used as follows: a scratch was made on a uniform layer of cells using a sterile micropipette tip and cells were washed to remove debris. Photographs of the same area of the wound were taken after 8 h, 12 h and 24 h to measure the width of the wound. For the transwell migration assay, cells were resuspended in serum-free medium and seeded into the insert well of a 24-well plate (8-μm pores, BD biosciences, Franklin Lakes, NJ, USA) for 40 h. The culture medium containing 10% fetal bovine serum was used as a chemoattractant and placed in the bottom chamber. Cells were fixed in paraformaldehyde (4%) and stained in crystal violet (0.5% in 20% methanol). Remaining cells in the upper chamber (non-migratory cells) were washed with a cotton swab and the membrane was removed. Adherent cells to the bottom of the membrane (migratory cells) were counted under an Olympus 1 × 70 microscope (Olympus America). The invasion assay was performed under the same conditions using growth factor-reduced matrigel-coated insert wells (BD Biocoat, BD Biosciences).
Formation of colonies in soft agar
The soft agar colony formation assay was performed in 6-well plates. Noble agar (Sigma-Aldrich) was used for the base layer (0.8%) and for the top layer (0.4%) containing the cells. Cells were fed every four days by culture medium for 5 weeks and colonies were stained overnight with p-iodonitrotetrazolium violet (Sigma-Aldrich, 1 mg/ml in 50% ethanol). Macroscopic photographs of the wells were taken and the colony number was calculated using ImageJ. Microscopic representative photographs are displayed.
Statistical analysis
Data are expressed as mean±s.d. The Student's t test was performed using Excel and data were considered statistically significant when P<0.05.
Acknowledgments
We thank Dr Namba and Kobayashi (Okayama University, Japan) for OUMS-29 cells and Dr Jisu Li for HepaRG cells. This work was partially supported by R01-CA123544 from the National Institute of Health.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Severi T, van Malenstein H, Verslype C, van Pelt JF. Tumor initiation and progression in hepatocellular carcinoma: risk factors, classification, and therapeutic targets. Acta Pharmacol Sin. 2010;31:1409–1420. doi: 10.1038/aps.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulehner G, Mikula M, Schneller D, van Zijl F, Huber H, Sieghart W, et al. Nuclear beta-catenin induces an early liver progenitor phenotype in hepatocellular carcinoma and promotes tumor recurrence. Am J Pathol. 2010;176:472–481. doi: 10.2353/ajpath.2010.090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade AG, Monga SP. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow. Dev Dyn. 2011;240:486–500. doi: 10.1002/dvdy.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, et al. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengochea A, de Souza MM, Lefrancois L, Le Roux E, Galy O, Chemin I, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, et al. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, et al. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle P, Kim M, Herrmann M, Gupte A, Lefrancois L, Califano S, et al. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–862. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Yang MH, Chen CL, Chau GY, Chiou SH, Su CW, Chou TY, et al. Comprehensive analysis of the independent effect of twist and snail in promoting metastasis of hepatocellular carcinoma. Hepatology. 2009;50:1464–1474. doi: 10.1002/hep.23221. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh M. Molecular cloning and characterization of human WNT3. Int J Oncol. 2001;19:977–982. doi: 10.3892/ijo.19.5.977. [DOI] [PubMed] [Google Scholar]
- Yan L, Della Coletta L, Powell KL, Shen J, Thames H, Aldaz CM, et al. Activation of the canonical Wnt/beta-catenin pathway in ATF3-induced mammary tumors. PLoS One. 2011;6:e16515. doi: 10.1371/journal.pone.0016515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima N, Liu D, Huang CL, Ueno M, Zhang X, Yokomise H. Wnt3 gene expression promotes tumor progression in non-small cell lung cancer. Lung Cancer. 2011;76:228–234. doi: 10.1016/j.lungcan.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Akiyoshi T, Mori M, Wands JR, Sugimachi K. A novel frizzled gene identified in human esophageal carcinoma mediates APC/beta-catenin signals. Proc Natl Acad Sci USA. 1998;95:10164–10169. doi: 10.1073/pnas.95.17.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirikoshi H, Sekihara H, Katoh M. Up-regulation of Frizzled-7 (FZD7) in human gastric cancer. Int J Oncol. 2001;19:111–115. [PubMed] [Google Scholar]
- Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, et al. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincan E, Darcy PK, Farrelly CA, Faux MC, Brabletz T, Ramsay RG. Frizzled-7 dictates three-dimensional organization of colorectal cancer cell carcinoids. Oncogene. 2007;26:2340–2352. doi: 10.1038/sj.onc.1210026. [DOI] [PubMed] [Google Scholar]
- Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, et al. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- Longato L, de la Monte S, Kuzushita N, Horimoto M, Rogers AB, Slagle BL, et al. Overexpression of insulin receptor substrate-1 and hepatitis Bx genes causes premalignant alterations in the liver. Hepatology. 2009;49:1935–1943. doi: 10.1002/hep.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, et al. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, et al. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–299. doi: 10.1016/j.jhep.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So SK. Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer. 2011;10:16. doi: 10.1186/1476-4598-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Liu Q, Lu W, Bu G. Wnt signaling activation and mammary gland hyperplasia in MMTV-LRP6 transgenic mice: implication for breast cancer tumorigenesis. Oncogene. 2010;29:539–549. doi: 10.1038/onc.2009.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya K, Asahi S, Nagamori S, Sakaguchi M, Gao C, Miyazaki M, et al. Establishment of a human hepatocyte line (OUMS-29) having CYP 1A1 and 1A2 activities from fetal liver tissue by transfection of SV40 LT. In Vitro Cell Dev Biol Anim. 2001;37:266–269. doi: 10.1007/BF02577541. [DOI] [PubMed] [Google Scholar]
- Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D, Cannie I, et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc Natl Acad Sci USA. 2002;99:15655–15660. doi: 10.1073/pnas.232137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent R, Marion MJ, Furio L, Trepo C, Petit MA. Origin and characterization of a human bipotent liver progenitor cell line. Gastroenterology. 2004;126:1147–1156. doi: 10.1053/j.gastro.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Cerec V, Glaise D, Garnier D, Morosan S, Turlin B, Drenou B, et al. Transdifferentiation of hepatocyte-like cells from the human hepatoma HepaRG cell line through bipotent progenitor. Hepatology. 2007;45:957–967. doi: 10.1002/hep.21536. [DOI] [PubMed] [Google Scholar]
- Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, et al. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol. 2010;30:3610–3619. doi: 10.1128/MCB.00177-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, et al. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169–1179. doi: 10.2217/fon.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blavier L, Lazaryev A, Shi XH, Dorey FJ, Shackleford GM, DeClerck YA. Stromelysin-1 (MMP-3) is a target and a regulator of Wnt1-induced epithelial-mesenchymal transition (EMT) Cancer Biol Ther. 2010;10:198–208. doi: 10.4161/cbt.10.2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, et al. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- Qi L, Sun B, Liu Z, Li H, Gao J, Leng X. Dickkopf-1 inhibits epithelial-mesenchymal transition of colon cancer cells and contributes to colon cancer suppression. Cancer Sci. 2012;103:828–835. doi: 10.1111/j.1349-7006.2012.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauger KJ, Chenausky KL, Murray ME, Schneider SS. SFRP1 reduction results in an increased sensitivity to TGF-beta signaling. BMC Cancer. 2011;11:59. doi: 10.1186/1471-2407-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Poon RT, Yuen AP, Ling MT, Kwok WK, Wang XH, et al. Twist overexpression correlates with hepatocellular carcinoma metastasis through induction of epithelial-mesenchymal transition. Clin Cancer Res. 2006;12:5369–5376. doi: 10.1158/1078-0432.CCR-05-2722. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with "Stemness"-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–1717. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- Xiang ZL, Zeng ZC, Tang ZY, Fan J, Sun HC, Tan YS. Expression of cytokeratin 19 and matrix metalloproteinase 2 predicts lymph node metastasis in hepatocellular carcinoma. Mol Biol Rep. 2011;38:3531–3539. doi: 10.1007/s11033-010-0463-x. [DOI] [PubMed] [Google Scholar]
- Colombat M, Paradis V, Bieche I, Dargere D, Laurendeau I, Belghiti J, et al. Quantitative RT-PCR in cirrhotic nodules reveals gene expression changes associated with liver carcinogenesis. J Pathol. 2003;201:260–267. doi: 10.1002/path.1451. [DOI] [PubMed] [Google Scholar]
- Lu JW, Hsia Y, Yang WY, Lin YI, Li CC, Tsai TF, et al. Identification of the common regulators for hepatocellular carcinoma induced by hepatitis B virus X antigen in a mouse model. Carcinogenesis. 2012;33:209–219. doi: 10.1093/carcin/bgr224. [DOI] [PubMed] [Google Scholar]
- Maegdefrau U, Amann T, Winklmeier A, Braig S, Schubert T, Weiss TS, et al. Bone morphogenetic protein 4 is induced in hepatocellular carcinoma by hypoxia and promotes tumour progression. J Pathol. 2009;218:520–529. doi: 10.1002/path.2563. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SD, Mi Z, Kim VM, Guo H, Talbot LJ, Kuo PC. Osteopontin regulates epithelial mesenchymal transition-associated growth of hepatocellular cancer in a mouse xenograft model. Ann Surg. 2012;255:319–325. doi: 10.1097/SLA.0b013e31823e3a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang S, Plymoth A, Ge S, Feng Z, Rosen HR, Sangrajrang S, et al. Identification of osteopontin as a novel marker for early hepatocellular carcinoma. Hepatology. 2012;55:483–490. doi: 10.1002/hep.24703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte U, Singh S, Zeng G, Cieply B, Virji MA, Wu T, et al. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol. 2009;175:1056–1065. doi: 10.2353/ajpath.2009.080976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MD, Awuah P, Singh S, Monga SP. Disparate cellular basis of improved liver repair in beta-catenin-overexpressing mice after long-term exposure to 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Am J Pathol. 2010;177:1812–1822. doi: 10.2353/ajpath.2010.100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejak-Bowen KN, Thompson MD, Singh S, Bowen WC, Dar MJ, Khillan J, et al. Accelerated liver regeneration and hepatocarcinogenesis in mice overexpressing serine-45 mutant beta-catenin. Hepatology. 2010;51:1603–1613. doi: 10.1002/hep.23538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, et al. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA. 2007;104:14771–14776. doi: 10.1073/pnas.0706578104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer JK, Scarzello AJ, Andersen JB, De Kluyver RL, Back TC, Weiss JM, et al. Coactivation of AKT and beta-catenin in mice rapidly induces formation of lipogenic liver tumors. Cancer Res. 2011;71:2718–2727. doi: 10.1158/0008-5472.CAN-10-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambotin SB, Wands JR, Kim M. Points of therapeutic intervention along the Wnt signaling pathway in hepatocellular carcinoma. Anticancer Agents Med Chem. 2011;11:549–559. doi: 10.2174/187152011796011019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.